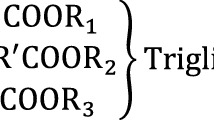Abstract
Biodiesel production catalyzed by immobilized lipases offers the possibility of easy reuse of the catalyst, which is very important to minimize costs and to make this process economically feasible. In this study, the reuse of three commercial immobilized lipases (Novozym 435, Lipozyme RM IM, and Lipozyme TL IM) was investigated in ethanolysis of soybean oil. The effect of the use of solvents (ethanol, butanol, and hexane) to wash the immobilized lipases before the enzyme reuse was evaluated, as well as the lipase reuse without solvent washing. The washing with butanol and ethanol led to the lowest decrease in ester yield after the first batch and allowed the highest glycerol removal (>85 %) from biocatalysts. The biocatalysts were incubated at 50 °C for 2 h in these three solvents. Esterification activities of the enzyme preparations, scanning electron microscopy (SEM) analyses of the beads, and protein content in organic phase were evaluated before and after incubation in the solvent. SEM analysis showed a significant change in beads morphology of Novozym 435 after contact with hexane. For Lipozyme TL IM lipase, this effect was visualized with ethanol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, there are many ongoing studies about the use of lipases as alternative biocatalysts for biodiesel production. These biocatalysts can be often immobilized on a solid support before use, which facilitates both their use in continuous operations and their recovery from the product mixture [1, 2]. However, the high cost of commercial enzymes when compared to the chemical catalyst is a great disadvantage of this route, together with the longer reaction time. In this context, much effort have been done to reduce the production costs of the biocatalysts and to maximize their reutilization. From an economic point of view, the reuse of the biocatalyst is very important, since the higher the lifetime of the biocatalyst is, the lower their contribution in process costs is [3].
Moreover in enzymatic transesterification reactions, the glycerol by-product that is hydrophilic and insoluble in oil causes the inactivation of the biocatalyst, which is one of the main problems related to this process. Glycerol produced can easily get adsorbed on the surface of the immobilized enzyme leading to a decrease in lipase activity and operational stability [3, 4]. The enzyme washing with solvent has been described as an efficient method to minimize the negative effects of glycerol on the lipase activity and to improve the operational stability of the biocatalyst [5].
The choice of the solvent used for lipase washing should consider some aspects, such as capacity of glycerol removal that is adsorbed on the support of the biocatalyst, denaturation of the enzyme, and interactions of the solvent with the support [6], which are directly related to the polarity-hydrophobicity of the solvent. One of the most important parameters of the polarity-hydrophobicity of an organic solvent for enzyme-catalyzed reaction is the log P [logarithm of the partition coefficient (P) of the solvent between 1-octanol and water] [7]. In general, enzyme activity and stability are lower in hydrophilic solvents (log P < 2), moderate in solvents with log P between 2 and 4, and higher in more hydrophobic solvents (log P > 4). On the other hand, the best performance of hydrophilic solvents to remove glycerol adsorbed on the immobilized lipase should be considered. Solvents reported for washing lipases before its reuse include hexane [8–11], chloroform [4], acetone [12–14], water [9, 10, 16], isopropyl alcohol [15], ethanol [9, 17], tert-butanol [14, 18], propanol [9, 16], phosphate-buffered saline [19], Triton X-100 and 2-propanol in Tris–HCl buffer [20], and dimethylcarbonate [21].
The aim of this work was to study the reuse of three commercial immobilized lipases (Novozym 435, Lipozyme RM IM, and Lipozyme TL IM) in ethanolysis of soybean oil. Soybean oil was chosen, because soybean crop is already established in Brazil and soybean oil is the main oil used for biodiesel production in this country. The current production of biodiesel in Brazil is about 300,000 m3/month, of which about 70 % are obtained from soybean oil [22]. Moreover, the literature reports the use of this oil as raw material for enzymatic biodiesel synthesis [4, 9, 15].The effect of lipase reuse without solvent washing and the use of three solvents (ethanol, butanol, and hexane) to wash the lipases in ester yield were studied. The glycerol removal from the biocatalyst by the solvents after the reactions was also investigated. The effect of the incubation of beads in ethanol, butanol, and hexane on the surface morphology of Lipozyme RM IM, Lipozyme TL IM, and Novozym 435 was studied. The effects of the solvent in esterification activity and the desorption of protein due to incubation of the biocatalysts with the three solvents were also studied.
Materials and Methods
Raw Materials and Reagents
Soybean oil was provided by Piraquê S. A. (Rio de Janeiro, Brazil) and presented the following compositions in fatty acids (wt.%): 0.1 % lauric acid, 11.3 % palmitic acid, 0.1 % palmitoleic acid, 4.3 % stearic acid, 25.8 oleic acid, 52.1 % linoleic acid, 5.1 % linolenic acid, 0.7 % arachidonic acid, 0.3 % behenic acid, and 0.2 % linoelaidic acid. The composition was determined by gas chromatography following the standard method AOCS Ce 1f-96 [23]. A gas chromatograph equipped with a flame ionization detector (FID) and a BPX70 capillary column (120 m × 0.25 mm × 0.25 μm) was used. The detector and injector were set at 250 °C and the oven temperature was 205 °C. The acid value of the soybean oil was about 0.08 mg KOH per gram of oil. The commercial immobilized lipases used were Lipozyme RM IM (lipase from Rhizomucor miehei, immobilized on macroporous anion exchange resin), Lipozyme TL IM (lipase from Thermomyces lanuginosus, immobilized in hydrophilic support), and Novozym 435 (lipase from Candida antarctica, immobilized on acrylic macroporous resin), all kindly donated by Novozymes Latin America Ltda. (Araucária, Brazil). Ethanol (pro analysi (p.a.)), butanol (p.a.), acetone (p.a.), sodium hydroxide, and hexane (99 %) were obtained from Vetec Química Fina Ltda (Rio de Janeiro, Brazil). Oleic acid (extra pure) was purchased from Merck (Darmstadt, Germany). Methyl heptadecanoate (a chromatographic standard) was obtained from Sigma/Aldrich (St. Louis, USA).
Measurement of Esterification and Transesterification Activities
Esterification activity was measured by the consumption of oleic acid in esterification reactions with butanol (oleic acid:butanol molar ratio of 1) with an enzyme amount of 3 wt.% at 45 °C. The reactions were carried out in closed 15-mL batch reactors magnetically stirred and thermostated. At fixed intervals, triplicates samples of 100 μL were removed from the medium, dissolved at acetone/ethanol 1:1 (40 mL), and residual free fatty acids (FFAs) were analyzed by titration with NaOH 0.04 mol.L−1. One esterification unit (U) was defined as the enzyme amount that consumes 1 μmol of fatty acid per minute under the experimental conditions [24]. The esterification activities of commercial lipases, Lipozyme RM IM, Lipozyme TL IM, and Novozym 435 were 1880, 720, and 3554 (μmol of acid · min−1 g−1), respectively.
Transesterification activity was measured by the consumption of trilaurin in the transesterification reactions with ethanol (trilaurin:ethanol molar ratio of 1:3) with an enzyme amount of 5 wt.% at 50 °C. The trilaurin consumption was measured by gaseous phase chromatography according to described in Langone and Sant’Anna [11]. One transesterification unit (U) was defined as the enzyme amount that consumes 1 μmol of triglyceride per minute under the experimental conditions. The transesterification activities of commercial lipases, Lipozyme RM IM, Lipozyme TL IM, and Novozym 435 were 650, 3220, and 3980 (U g−1), respectively.
Transesterification Reaction
Transesterification reactions were performed in a closed 15-mL batch reactor magnetically stirred and coupled to a condenser in order to avoid alcohol loss. Water circulating in the condenser was cooled by a thermostatic bath. Reaction medium temperature was kept constant at 50 °C by circulating hot ethylene glycol through the reactor jacket. A thermostatic bath allowed a close control over the process temperature. The mass of soybean oil used was 8 g in all experiments. This mass corresponds at 0.01 mol of triglycerides and is based on the molar mass of the oil, calculated from the oil composition (801 g mol−1). The reactions were carried out in a solvent-free medium during 4 h, using 5 wt.% of commercial lipase, and a ratio ethanol:oil of 3:1, with the alcohol added at the beginning of the reaction or in three steps (T 0 = 1/3, T 30 min = 1/3, and T 60 min = 1/3) at 50 °C. The biodiesel content was determined by gas chromatography.
Biocatalyst Reuse Without Solvent Washing and with Solvent Washing
Novozym 435, Lipozyme RM IM, and Lipozyme TL IM reuses were evaluated in ethanolysis of soybean oil. Reactions were carried out for 4 h, and after that, two different methods were tested for lipase reuse. In the first one (with no solvent washing), the enzyme was separated by decantation; the medium was withdrawn and replaced by a new one (direct reuse). This methodology was repeated three times. The second method was to wash the biocatalyst with a solvent (ethanol, butanol, or hexane), in which the lipase was separated from reaction medium by decantation and washed with 30 mL of solvent under magnetic stirring during 5 min. After that, it was vacuum filtered and placed in a desiccator for 24 h. After this treatment, the enzyme was reused in a new batch. The effect of the solvent washing on lipase reuse was studied in two consecutive transesterification reactions.
Chromatography Analysis (Biodiesel Yield)
The fatty acid ethyl ester (FAEE) content in the reaction medium was analyzed on Varian gas chromatograph (CP–3380 model), equipped with a flame ionization detector (FID), a CP WAX 52 CB capillary column 30 m × 0.25 mm × 0.25 mm, and a split injection system with a 1:20 ratio. Injector and detector temperatures were kept at 280 and 300 °C, respectively. The oven was initially maintained at 200 °C for 4.5 min, then heated up to 250 °C at 20 °C min−1 rate, and kept constant at the final temperature for 5 min. Hydrogen was used as the carrier gas at 2 mL min−1 flow rate; column pressure was set at 20 psi. The FAEE content in the medium was calculated according to Eq. (1).
Analysis of the Glycerol Adsorbed on the Lipase
The glycerol content was determined in the obtained filtrate (supernatant) after washing the enzyme preparation with solvent (ethanol, butanol, and hexane). Of the water, 30 mL was added to the biocatalysts in order to verify if there was still glycerol adsorbed on the support of the commercial lipases. After vacuum filtration, the glycerol content was also determined in the filtrate. The concentration of glycerol was performed according to the modified Soloni method [25], using HACH spectrophotometer, model DR/4000U, at 410 nm. The glycerol percentage removed by the solvent was calculated from the total glycerol adsorbed on lipase. The total glycerol adsorbed on lipase (100 %) was obtained by the sum of the concentrations of glycerol in the solvent and in the water.
Treatment of the Immobilized Lipases with the Solvents
The effect of the solvents used for lipase washing (ethanol, butanol, and hexane) on the esterification activity of Novozym 435, Lipozyme RM IM, and Lipozyme TL IM was studied. The immobilized lipases (0.3 g) were incubated in 3 mL of the solvent during 2 h at 25 °C and 50 °C, in a closed 15-mL batch reactor magnetically stirred. The experiments were performed in triplicate. After 2 h, the suspension was centrifuged and the supernatant (solvent) was used for protein quantification, while the solid (immobilized lipase) was used for analysis of the esterification activity and scanning electron microscopy (SEM) analysis. Esterification activity was chosen because it is a simpler analytical method and provides a faster response in relation to the method of determining transesterification activity.
Protein Quantification After Treatment with Solvent
The amount of protein present in the solvent after lipase incubation was determined according to biuret method [26]. In this context, the absorbance of the supernatant solution remaining after the treatment with the three solvents was studied at 550 nm. The absorbance was correlated with the concentration of protein by means of a calibration curve performed with several concentrations of the casein (0–10 mg mL−1).
SEM Analysis
In order to investigate the surface morphology of the commercial immobilized lipases Novozym 435, Lipozyme RM IM, and Lipozyme TL IM) before and after contacting with solvent (butanol, ethanol, and hexane), scanning electron microscopy analysis was performed. The analyses were carried out in the Centro de Caracterização em Nanotecnologia (CENANO) located in National Institute of Technology (Instituto Nacional de Tecnologia, INT). Samples were prepared by drying and covering with a conductive gold layer for 2 min at 20 mA. After plating, the samples were analyzed in the microscope (FEI INSPECT S50), at ×50, ×200, ×400, ×600, and ×2000 magnifications.
Results and Discussion
Biodiesel Synthesis
The effect of the stepwise addition of ethanol was studied with the commercial lipases Lipozyme RM IM, Lipozyme TL IM, and Novozym 435 in biodiesel synthesis using a 3:1 alcohol:oil molar ratio and 5 wt.% of enzyme. The results are shown in Fig. 1.
As can be seen in Fig. 1, the addition of ethanol in steps improved the ester yields for the three enzymes, as expected. This effect was previously described by literature due to the denaturation of the lipase by contact with high concentrations of the more polar solvent [27–29] and was more pronounced with Lipozyme TL IM. The biodiesel content obtained after 4 h of the reaction carried out with single addition of ethanol was 12 % and with stepwise addition of alcohol was 42 %. Considering these results, further experiments of biodiesel synthesis were carried out with stepwise addition of ethanol.
Biocatalyst Reuse Without Solvent Washing
The reuse of the three biocatalysts (Novozym 435, Lipozyme RM IM, and Lipozyme TL IM) in ethanolysis of soybean oil was performed without solvent washing before reutilization. Novozym 435 could be reused for three reaction cycles, while Lipozyme RM IM was reused for only two consecutive reactions. In the first use of Novozym 435, the FAEE was 36 %, and after the third batch reuse, the yield was reduced by ten times (3 %). A reduction in biodiesel yields, from 29 to 9 %, was also observed with Lipozyme RM IM in the second reaction (Fig. 2). The support of Lipozyme TL IM dissolves in the reaction medium, so the reuse of the biocatalyst was jeopardized. It is noticeable that without the previous washing of the immobilized lipase, the enzymatic activity suffers a drastic reduction after few cycles [9, 14]. As previously reported by several authors, this reduction in enzymatic activity can be explained due to a deposition of the glycerol by-product over the pores of catalyst, which blocks the access of substrates to active sites of lipase [30]. To avoid this problem, it is common to wash the lipase before using it in another batch in order to remove the glycerol adsorbed on support. Polar solvents such as ethanol and acetone have the advantage of better removal of this hydrophilic by-product. On the other hand, some authors report that hydrophobic solvents, such as hexane, are most interesting, since they better remove the non-polar components (substrates and products) that may be adsorbed on the enzyme [9, 10].
Relative ester yield attained in 4 h after reuse of Novozym 435 and Lipozyme RM IM without washing of the enzymes before the reuse. Reactions conditions: 5 wt.% of commercial lipase, 8 g of soybean oil, and a molar ratio ethanol:oil of 3:1 added in three steps (T 0 = 1/3, T 30 min = 1/3, and T 60 min = 1/3), at 50 °C
Effects of the Solvents in the Esterification Activity and Morphology of the Immobilized Biocatalysts
In the present work, three solvents were used to wash lipase before its reuse. Ethanol (log P = −0.30) was chosen since it is produced on a large scale from renewable sources [1] and it is itself a reagent for the studied reaction. Hexane (log P = 3.5) is a hydrophobic solvent and was chosen due to previous results obtained in the literature using this solvent for lipase washing [9, 10]. Butanol is another hydrophilic solvent (log P = 0.79) that is less polar than ethanol and can also be used as reagent in transesterification reactions.
In order to obtain a better evaluation of the effects of these washing solvents on the three commercial immobilized biocatalysts, Novozym 435, Lipozyme RM IM, and Lipozyme TL IM were incubated in ethanol, butanol, or hexane for 2 h under magnetic stirring, at 25 and 50 °C. The results of relative esterification activities obtained after the contact with the solvents at 50 °C for the three biocatalysts are shown in Fig. 3. It can be seen that the contact with ethanol for 2 h promoted the highest decrease in esterification activity for Novozym 435. This result is due to the high polarity of this solvent compared with butanol and hexane. José et al. [6] observed that the secondary structure of C. antarctica B lipase (Novozym 435) has changed after the contact with ethanol, with an increase in the contribution of β-sheet structure. This can be explained since these polar solvents can strip water from the surface of the protein and compete for hydrogen bonds between the atoms of the protein, denaturing the structure to a largely unfolded state.
Similar results of relative activity were observed in experiments conducted with Lipozyme RM IM. This result can also be attributed to the effect of ethanol on the lipase support. Lipozyme RM IM is a R. miehei lipase produced by submerged fermentation of the microorganism Aspergillus oryzae and is immobilized on macroporous ion exchange resin (a hydrophilic support Duolite ES 562) [31]. Thus, ethanol may dissolve its support causing enzyme desorption. Lipozyme TL IM maintained similar activities after the contact with ethanol or butanol, which were lower than with hexane. Lipozyme RM IM and Lipozyme TL IM maintained about 80–85 % of its initial activity after contact with hexane. Similar results were obtained at 25 °C. Relative esterification activities above 80 % were attained after enzyme incubation in hexane, while the contact with ethanol or butanol resulted in higher loss of enzyme activity.
The effect of the solvents in the surface morphology of the three commercial biocatalysts was also investigated. As can be seen in Fig. 4, in the presence of hexane, the support morphology of Novozym 435 was changed; however, no modification was visualized after enzyme incubation in ethanol and butanol under studied conditions. This result can be attributed to the hydrophobic nature of hexane, which could dissolve the macroporous resin (support). Novozym 435 is a commercial immobilized lipase B from C. antarctica (CALB) produced by submerged fermentation of a genetically modified Aspergillus microorganism. This lipase is adsorbed on a macroporous resin called Lewatit VP OC, that is a polymer of metacrylic acid cross-linked with divinylbenzene (DVB) and has certain hydrophobic nature [6, 32].
However, the results of esterification activity and protein content did not show that the lipase was desorbed from the support when incubated in hexane. The largest amount of protein in the solvent after incubation of Novozym 435 was found when ethanol was used. José et al. [6] observed that ethanol dissolves the resin that constitutes the support of the C. antarctica lipase (poly methyl methacrylate (PMMA)). Furthermore, the authors found that the interaction of ethanol with Novozym 435 occurs not only on the surface but also into the biocatalyst’s bead. Ethanol diffuses into the biocatalyst’s beads remaining strongly adsorbed, and this diffusion alters the inner texture of the beads generating channels and increasing the roughness of the polymeric material [6]. The resin dissolution caused by the solvents leads to the migration of the polymer and other substances used as additives in lipase immobilization process toward the liquid phase [6, 33]. Thus, although hexane has promoted the apparent dissolution of Novozym 435 support, it seems that the lipase remained adsorbed on the macroporous resin after the treatment with this solvent.
The SEM images (Fig. 5) of beads Lipozyme RM IM show no significant change in the structure of immobilized biocatalyst after incubation in the solvents tested. Lipozyme RM IM is a lipase from R. miehei produced by submerged fermentation of A. oryzae and immobilized on an anionic resin. The support of the immobilized enzyme is Duolite ES 562, a weak anion exchange resin based on phenol–formaldehyde copolymers [31]. The analysis of protein content after incubation in hexane showed that the amount of dispersed proteins in the medium was low compared to the amount of protein obtained after incubation in ethanol.
Lipozyme TL IM is a T. lanuginosus lipase produced by a genetically modified strain of A. oryzae. This lipase is immobilized via ionic adsorption on a hydrophilic support (a gel of granulated silica) [34, 35]. As can be seen in Fig. 6, when this biocatalyst was incubated in a polar solvent (ethanol and butanol), a significant change occurred in the morphology of the support. The proteins dispersed in the medium were also high after incubation in ethanol. This result shows that polar solvents such as ethanol and butanol are able to dissolve the lipase support, and this interaction with the support also affects the lipase activity (Fig. 3). This result also corroborates with those attained in the reuse of Lipozyme TL IM without washing, in which was observed the dissolution of the lipase support in the reaction medium.
Biocatalyst Reuse with Solvent Washing
In order to improve the reusability of the immobilized biocatalysts in soybean oil ethanolysis, Novozym 435 and Lipozyme RM IM were washed with ethanol, butanol, and hexane before their reuse. Figure 7 shows the relative ester yield attained after the lipase washing with the three solvents. According to the Fig. 7, the relative ester yield attained with butanol washing was higher that the two other solvents for Novozym 435. After washing with butanol, the ester yields were about 70–80 % for both enzymes. For the washing with ethanol, the ester yield obtained in the second batch using Novozym 435 was 57 % of that attained in the first reaction. It can be observed in Fig. 7 that 100 % of relative ester yield was attained when Lipozyme RM IM was washed with ethanol. After washing with hexane, the yield was only 8 and 16 % of that obtained in the first reaction for Novozym 435 and Lipozyme RM IM, respectively. This result can be attributed to a better glycerol removal adsorbed on the lipase support by the polar solvents.
As reported by several authors, glycerol adsorption on the biocatalyst support leads to significant loss of activity [4]. Therefore, solvent washing of commercial immobilized lipase has been tested and the glycerol content was analyzed. The results showed that butanol and ethanol allowed higher glycerol removal (>85 %) compared to hexane for both enzymes. Hexane was able to remove about 40 % of the total glycerol adsorbed on Lipozyme RM IM and Novozym 435. These results are expected, considering that glycerol is a polar solvent, and therefore its more miscible into ethanol (log P = −0.30) and butanol (log P = 0.79). Hexane showed the lowest values of glycerol removal because it is a non-polar molecule (log P = 3.5).
Conclusion
In this study, the lipase reuse without solvent washing as well as the use of three solvents (ethanol, butanol, and hexane) for washing commercial immobilized lipases were investigated. The reuse of the commercial lipases without solvent washing was investigated in order to reduce costs. However, the results showed a great reduction in ester yield after the reuse. The best results for lipase reuse were obtained when commercial enzymes Novozym 435 and Lipozyme RM IM were washed with polar solvents (ethanol and butanol). These solvents were more efficient to remove the glycerol produced in the transesterification reaction. The effects of the solvents in the esterification activity and in the structure of the beads were analyzed after the incubation of the enzymes in these solvents. The contact with hexane provided a lower decay in enzyme activity. The results obtained by SEM showed that the morphology of Novozym 435 was changed after contact with hexane. Moreover, the structure of Lipozyme TL IM was altered when the enzyme was in contact with ethanol. These results show that the choice of the solvent to wash the immobilized lipase should be taken into account the effects of the solvent on the lipase structure, as well as its effects on the immobilization support and its capacity to remove the glycerol by-product adsorbed on the biocatalyst. These combined effects will contribute to the overall performance of the biocatalyst in successive reuses, and further work can be carried out to better assess the changes observed in lipase supports.
References
Robles-Medina, A., González-Moreno, P. A., Esteban-Cerdán, L., & Molina-Grima, E. (2009). Biocatalysis: towards ever greener biodiesel production. Biotechnology Advances, 27, 398–408.
Ranganathan, S. V., Narasimhan, S. L., & Muthukumar, K. (2008). An overview of enzymatic production of biodiesel. Bioresource Techonology, 99, 3975–3981.
Gog, A., Roman, M., Tosa, M., Paizs, C., & Irimie, F. D. (2012). Biodiesel production using enzymatic transesterification—current state and perspectives. Renewable Energy, 39, 10–16.
Hernández-Martín, E., & Otero, C. (2008). Different enzyme requirements for the synthesis of biodiesel: Novozym-35 and Lipozyme-TL IM. Bioresource Technology, 99, 277–286.
Al-Zuhair, S. (2007). Production of biodiesel: possibilities and challenges. Biofuels, Bioproducts & Biorefining, 1, 57–66.
José, C., Bonetto, R. D., Gambaro, L. A., Torres, M. P. G., Foresti, M. L., Ferreira, M. L., & Briand, L. E. (2011). Investigation of the causes of deactivation–degradation of the commercial biocatalyst Novozym® 435 in ethanol and ethanol–aqueous media. Journal of Molecular Catalysis B: Enzymatic, 71, 95–107.
Koskinen, A. M. P., & Klibanov, A. M. (1996). Enzymatic reactions in organic media (1st ed.). New York: Blackie Academic and Professional.
Lai, C., Zullaikah, S., Vali, S. R., & Ju, Y. (2005). Lipase-catalyzed production of biodiesel from rice bran oil. Journal of Chemical Technology & Biotechnology, 80, 331–337.
Rodrigues, R. C., Volpato, G., Wada, K., & Ayub, M. A. Z. (2008). Enzymatic synthesis of biodiesel from transesterification reactions of vegetable oils and short chain alcohols. Journal of American Oil Chemistry Society, 85, 925–930.
Martins, A. B., Graebin, N. G., Lorenzoni, A. S. G., Fernandez-Lafuente, R., Ayub, M. A. Z., & Rodrigues, R. C. (2011). Rapid and high yields of synthesis of butyl acetate catalyzed by Novozym 435: reaction optimization by response surface methodology. Process Biochemistry, 46, 2311–2316.
Langone, M. A. P., & Sant’Anna, G. L., Jr. (2002). Process development for production of medium chain triglycerides using immobilized lipase in a solvent-free system. Applied Biochemistry and Biotechnology, 98–100, 997–1008.
Shah, S., & Gupta, M. N. (2007). Lipase catalyzed preparation of biodiesel from Jatropha oil in a solvent free system. Process Biochemistry, 42, 409–414.
Soumanou, M. M., & Bornscheuer, U. T. (2003). Improvement in lipase-catalyzed synthesis of fatty acid methyl esters from sunflower oil. Enzyme and Microbial Technology, 33, 97–103.
Azócar, L., Ciudad, G., Heipieper, H. J., Muñoz, R., & Navia, R. (2011). Lipase-catalyzed process in an anhydrous medium with enzyme reutilization to produce biodiesel with low acid value. Journal of Bioscience and Bioengineering, 112, 583–589.
Lee, D. H., Kim, J. M., Shin, H. Y., Kang, S. W., & Kim, S. W. (2006). Biodiesel production using a mixture of immobilized Rhizopus oryzae and Candida rugosa lipases. Biotechnology and Bioprocess Engineering, 11, 522–525.
Ho, L. J., Lee, D. H., Lim, J. S., Um, B.-H., Park, C., Kang, S. W., & Kim, S. W. (2008). Optimization of the process for biodiesel production using a mixture of immobilized Rhizopus oryzae and Candida rugosa lipases. Journal of Microbiology and Biotechnology, 18, 1927–1931.
Aguieiras, E. C. G., Cavalcanti-Oliveira, E. D., de Castro, A. M., Langone, M. A. P., & Freire, D. M. G. (2014). Biodiesel production from Acrocomia aculeata acid oil by (enzyme/enzyme) hydroesterification process: use of vegetable lipase and fermented solid as low-cost biocatalysts. Fuel, 135, 315–321.
Chen, J., & Wu, W. (2003). Regeneration of immobilized Candida antarctica lipase for transesterification. Journal of Bioscience and Bioengineering, 95, 466–469.
Ye, P., Xu, Z.-K., Che, A.-F., Wu, J., & Seta, P. (2005). Chitosan-tethered poly(acrylonitrile-co-maleic acid) hollow fiber membrane for lipase immobilization. Biomaterials, 26, 6394–6403.
Yi, S.-S., Noh, J.-M., & Lee, Y.-S. (2009). Amino acid modified chitosan beads: improved polymer supports for immobilization of lipase from Candida rugosa. Journal of Molecular Catalysis B: Enzymatic, 57, 123–129.
Zhang, L., Sun, S., Xin, Z., Sheng, B., & Liu, Q. (2010). Synthesis and component confirmation of biodiesel from palm oil and dimethyl carbonate catalyzed by immobilized-lipase in solvent-free system. Fuel, 89, 3960–3965.
Agência Nacional do Petróleo, Gás Natural e Biocombustíveis. Boletim mensal de biodiesel. Available from: www.anp.gov.br. Accessed 11 Jan 2006.
AOCS. (1996). Official methods and recommended practices of the American Oil Chemists’ Society. Determination of cis and trans-fatty acids in hydrogenated and refined oils and fats by capillary GLC. In D. Firestone (Ed.), AOCS official method Ce 1f-96 (2002). Champaign: American Oil Chemists’ Society Press.
Souza, M. S., Aguieiras, E. C. G., Silva, M. A. P., & Langone, M. A. P. (2009). Biodiesel synthesis via esterification of feedstock with high content of free fatty acids. Applied Biochemistry and Biotechnology, 154, 253–267.
Soloni, F. G. (1971). Simplified manual micromethod for determination of serum triglycerides. Clinical Chemistry, 17, 529–534.
Gornall, A. G., Bardawill, C. J., & David, M. M. (1949). Determination of serum proteins by means of the biuret reaction. The Journal of Biological Chemistry, 177, 751–766.
Shimada, Y., Watanabe, Y., Samukawa, T., Sugihara, A., Noda, H., Fukuda, H., & Tominaga, Y. (1999). Conversion of vegetable oil to biodiesel using immobilized Candida antarctica lipase. Journal of American Oil Chemistry Society, 76, 789–793.
Watanabe, Y., Shimada, Y., Sugihara, A., Noda, H., Fukuda, H., & Tominaga, Y. (2000). Continuous production of biodiesel fuel from vegetable oil using immobilized Candida antarctica lipase. Journal of American Oil Chemistry Society, 77, 355–360.
Bernardes, O. L., Bevilaqua, J. V., Leal, M. C. M. R., Freire, D. M. G., & Langone, M. A. P. (2007). Biodiesel fuel production by the transesterification reaction of soybean oil using immobilized lipase. Applied Biochemistry and Biotechnology, 136–140, 105–114.
Rodrigues, R. C., & Ayub, M. A. Z. (2011). Effects of the combined use of Thermomyces lanuginosus and Rhizomucor miehei lipases for the transesterification and hydrolysis of soybean oil. Process Biochemistry, 46, 682–688.
Rodrigues, R. C., & Fernandez-Lafuente, R. (2010). Lipase from Rhizomucor miehei as a biocatalyst in fats and oils modification. Journal of Molecular Catalysis B: Enzymatic, 66, 15–32.
Ganesan, A., Moore, B. D., Kelly, S. M., Price, N. C., Rolinski, O. J., Birch, D. J. S., Dunkin, I. R., & Halling, P. J. (2009). Optical spectroscopic methods for probing the conformational stability of immobilised enzymes. ChemPhysChem Special Issue Biophysics, 10, 1492–1499.
Zhao, H., & Song, Z. (2010). Migration of reactive trace compounds from Novozym® 435 into organic solvents and ionic liquids. Biochemical Engineering Journal, 49, 113–118.
Wang, W., Li, T., Ning, Z., Wang, Y., Yang, B., & Yang, X. (2011). Production of extremely pure diacylglycerol from soybean oil by lipase-catalyzed glycerolysis. Enzyme and Microbial Technology, 49, 192–196.
Fernandez-Lafuente, R. (2010). Lipase from thermomyces lanuginosus: uses and prospects as an industrial biocatalyst. Journal of Molecular Catalysis B: Enzymatic, 62, 197–212.
Acknowledgments
This research received financial support from FAPERJ and CNPq. The authors are also grateful to Fabiana M. T. MENDES from National Institute of Technology (Instituto Nacional de Tecnologia, INT) for kindly providing the SEM analyses.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Aguieiras, E.C.G., Ribeiro, D.S., Couteiro, P.P. et al. Investigation of the Reuse of Immobilized Lipases in Biodiesel Synthesis: Influence of Different Solvents in Lipase Activity. Appl Biochem Biotechnol 179, 485–496 (2016). https://doi.org/10.1007/s12010-016-2008-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2008-9