Abstract
Immobilization of recombinant Thermomyces lanuginosus lipase (designated as rPichia/lip) was carried out by moisture capacity impregnation of mesoporous silica granules followed by drying, and forcible adsorption of enzyme occurred. Eventually prepared lipase-active heterogeneous biocatalysts were systematically studied for enzymatic esterification performed at ambient conditions (20 ± 2 °C, 1 bar) in unconventional anhydrous media of organic solvents such as hexane and diethyl ether. The saturated fatty acids differing in the number of carbon atoms (C2–C10, C18), and aliphatic alcohols differing in the structure of the molecules, namely both the number of carbon atoms (C2–12, C16), and the isomerism of the carbon skeleton (n- and iso-), and OH-group position (prim-, sec-, tert-) were studied as substrates for enzymatic esterification. The specificity of the heterogeneous enzymatic esterification was determined by comparing the reaction rates for various pairs of substrates; and the matrix of relative units of activities was composed. The immobilized on silica rPichia/lip was found to have sufficiently wide specificity toward saturated fatty acids and aliphatic alcohols. High reaction rates were measured in esterification of fatty acids and primary n- and iso-aliphatic alcohols possessing more than four carbon atoms in the molecules. The enanthic acid (heptanoic, C7:0) reacted with butanol (C4) with the highest rate; and the kinetic parameters such as Michaelis constant (KM) for acid and maximal reaction rate (Vmax) were determined under the studied conditions of esterification. Substrates containing aromatic residues did not participate in esterification. The lipase-active heterogeneous biocatalysts possessed considerably high operational stability, and the catalytic activity was completely retained for several tens of reaction cycles in a periodic batch process of low-temperature synthesis of various fatty acid esters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, enzymatic esterification is considered as a competitive alternative to the chemical organic synthesis of various esters which are valuable commercial products commonly used in manufacturing flavors, fragrances, emollients, lubricants, antimicrobial agents and non-toxic surfactants for food, cosmetic and pharmaceutical industries. The requirements of consumers for such natural products is constantly increasing. Compared with organic synthesis using strong liquid and solid acids as catalysts and temperature above 100 °C (usually, at 120–150 °C), enzymatic esterification is currently of great commercial interest since this method of ester production proceeds efficiently at a low temperature (usually, at 20–40 °C) with 100%-selectivity without formation of any by-products and with very high specificity toward substrates. The heterogeneous biocatalysts for the low-temperature ester synthesis were prepared by the immobilization of the unique enzymes such as lipases on solid supports. The heterogeneous biocatalytical processes were realized in periodic or continuous modes and fully satisfy the requirements of “green” chemistry, so they are promising for implementation into the organic synthesis industry [1,2,3].
Nowadays, the lipase-active heterogeneous biocatalysts are intensively studied for enzymatic esterification of different organic acids including saturated and unsaturated fatty acids (substrate S1). Substances containing OH-group(s) (substrate S2) are commonly used in a molar excess. The S2 are aliphatic alcohols [4,5,6,7,8,9,10,11,12], and carbohydrates (glucose, fructose, mannose, lactose) [13,14,15,16,17], and ascorbic acid [18], and polyols, in particular glycerol [19, 20] and trimethylol propane (2-(hydroxymethyl)-2-ethylpropane-1,3-diol) [21,22,23,24]. Some parameters of ester synthesis were described in literature. For example, the productivity of Staphylococcus warneri lipase immobilized on silica was 230 mmol of hexyl decanoate for 24 h-long reaction cycle [4]. The highest activity of the biocatalyst prepared by immobilization of Candida antarctica lipase B inside core–shell polystyrene particles was observed to be 800 μmol min−1 g−1 in the esterification of oleic acid (18:1) with ethanol at 40 °C [6]. Using commercial NOVOZYMES biocatalysts (Novozym®, Lypozyme® types) production of esters of caprylic acid (C10:0) with C4–C16 aliphatic alcohols reached 0.8 mmol for 3 h [9]. The modulation of the regio-selectivity of T. lanuginosus lipase via engineering of heterogeneous biocatalysts was described in [7], and alteration of regio-specificity toward position of acyl groups in vegetable oils’ triglycerides depending on hydrophobicity of the supports was observed. In [25], it was shown that the specific activity and regio-specificity of lipase changed upon immobilization on hydrophobic (octadecyl) methacrylate beads The best biocatalyst produced diacylglycerols enriched in linolenic acid (C18:3) with a ratio of regio-isomers sn-1,3/sn-1,2 equal to 21.8; the highest conversion of conjugated linolenic acid was observed to be 54% for 3 h at 40 °C [25].
Comparing two methods of synthesis of valuable market products such as fatty acid esters, namely traditional organic and biocatalytic synthesis, the following main features of enzymatic esterification can be noted: (1) 100%-selectivity of reactions performed at very low reaction temperatures (20–40 °C) without by-products and pollutants, as mentioned above (2) a possibility to produce esters which are difficult or impossible to obtain by conventional methods of organic chemistry, for example, esters with carbohydrates and polyols; (3) no need in special apparatus in order to remove water formed during esterification from the reaction medium, like in organic chemistry, for enhancement of the product yield by shifting the equilibrium toward ester synthesis. Relatively low concentrations of substrates (acid and alcohol) and, consequently, product (ester) in an organic solvent (less than 2.0 M) is regarded as the main disadvantage of the enzymatic esterification, since this makes the procedure of purification of the target product more time-consuming per unit of weight of the purified substance. But this problem can be solved by optimizing technology.
It was found that the specificity of microbial lipase depended on the taxonomy of microorganisms from which the enzyme was isolated. The hydrolysis of various substrates by various microbial lipases was studied in [26], and authors explained the observed differences in specificity by differences in the structure of active sites of enzyme. For example, R. miehei, T. lanuginosus and Fusarium oxysporum lipases have wide alcohol binding cleft but a narrow acyl binding cleft, whereas C. antarctica and C. rugosa lipases have a narrow tunnel for accommodation of acyl group but wider alcohol binding site [26]. It was found that Candida sp. lipases had a wider specificity toward substrates than R. miehei and T. lanuginosus lipases; the latter lipases hydrolyzed esters of aromatic/cyclic acids with aromatic/cyclic alcohol at a very low rate [26]. In reactions of esterification, microbial lipases exhibited wide specificity for substrates S1 and S2 with 4–16 carbon atoms [9, 27]. The study of esterification specificity of recombinant T. lanuginosus lipase immobilized on mesoporous silica was started by us, and matrix of relative units of the catalytic activities began to be composed [28, 31].
In this work the systematic comparative study on the specificity of recombinant T. lanuginosus lipase immobilized onto mesoporous silica via forcible adsorption was continued; the all obtained results were summarized. The esterification of saturated fatty acids with aliphatic alcohols differing in the structure of the molecules, namely both the number of carbon atoms (C2–C18), and the isomerism of the carbon skeleton of alcohols (n-, iso-), and position of OH-group (prim-, sec-, tert-alcohols), and the presence of aromatic or cyclic residues was performed in anhydrous media of organic solvents (hexane and diethyl ether) at ambient conditions. By comparing the reaction rates, a matrix of relative units of enzymatic activity was fully composed. The kinetic parameters such as Michaelis constant (KM ) and maximal rate (Vmax) were determined for the ester synthesis occurred with the highest reaction rate, namely, for the n-butyl heptanate—ester of enanthic acid (C7:0) and n-butanol (C4). The catalytic properties of rPichia/lip lipase immobilized by impregnation onto mesoporous silica, such as enzymatic activity and operational stability, were studied in the periodic process of low-temperature synthesis of valuable esters differing by molecular structure.
Experimental
Materials and methods
Commercial silica KSK-G® type (Karpov manufacturing plant, Russia) with granules of 1–2 mm diameter was used as support for immobilizing recombinant T. lanuginosus lipase and preparing lipase-active heterogeneous biocatalyst. The texture parameters measured by Hg-intrusion porosimetry using ASAP 2400 V3.07 and AUTO-PORE IV 9500 V1.09 devices (Micrometrics Instrument Corporation, USA) were following: the specific surface area was 157 m2 g−1; total pore volumes (VΣ) was 0.76 cm3 g−1, average pore diameter was 19.4 nm, porosity was 58%.
Reagents such as substrates (fatty acids, alcohols), solvents (hexane, diethyl ether) with analytical grade purity were produced in Russia. Bovine serum albumin and Coomassie G-250 dye were purchased from Sigma.
The studied recombinant lipase designated as rPichia/lip was produced by the methylotrophic yeast Pichia pastoris X-33 strain specially constructed by the following genetic engineering manipulations such as (1) design and synthesis of the nucleotide sequence for the gene of a mature T. lanuginosus lipase (Protein Data Bank, PDB-database), (2) cloning of the synthesized gene into a plasmid vector and the production of the constructed recombinant plasmid in E. coli cells, (3) transformation of competent P. pastoris cells with the obtained plasmid and selection of recombinant yeast clones, (4) analysis of the selected clones for the ability to produce and secrete recombinant T. lanuginosus lipase into the culture medium. Finally, conditions for the cultivation and intensive growth of the strain-producer were optimized in order to increase the medium concentration of the target lipase to 2 g L−1. The lab-scale production of rPichia/lip lipase was preformed in a 10-L BIOK gas–vortex bioreactor (ZAO Sayany, Russia). Partial purification of enzyme was carried out by precipitation of secreted recombinant lipase with ammonium sulfate (up to 75% saturation) at 4 °C for 16 h. The precipitates were dissolved in distilled water, and further dialysis against a 25 mM acetate buffer pH 4.0 was carried out. The samples of dialyzed and lyophilized rPichia/lip were used for the study.
Immobilization of rPichia/lip and preparation of lipase-active heterogeneous catalysts were carried out by two type of adsorption such as spontaneous and forcible types. Spontaneous adsorption of recombinant lipase was carried out by physical adsorption of lipase from its solutions under condition described in [29]. Forcible adsorption was performed by impregnation of dry granules of silica with lipase solutions. The lipase concentration in these solutions was measured by modified method based on Coomassie G-250 dye-peptide bond interaction [30], and bovine serum albumin was used as standard for calibration curve. The immobilization by impregnation was carried out as follows. At first, granules of mesoporous silica were dried at 115 °C for 4 h. Then, the support samples were impregnated by moisture capacity with the buffered (phosphate buffer pH 7.0) solutions containing lipase at various concentrations, from 7.7 till to 17.4 mg mL−1, and were stayed in a closed container for 5 h at room temperature (20 ± 2 °C). After finishing impregnation, the prepared biocatalysts were dried at ambient conditions for 1 day to a dry-air state. During drying, the lipase molecules lost their hydrated shell, and forcible adsorption of the enzyme on the silica surface occurred. The amount of the immobilized lipase was calculated per 1 g of silica (mg g−1) taking into account the total pore volume of silica (0.8 cm3 g−1) and the lipase concentrations in impregnating solutions.
Enzymatic activity of the prepared biocatalysts was measured in the two-substrate reaction of esterification; the products of this reaction were an ester and water. The first substrate (S1) was saturated fatty acid with a number of carbon atoms in molecules from 2 to 18. The second substrate (S2) was aliphatic alcohol with a number of carbon atoms in molecules from 2 to 16, and with different isomerism of the molecular carbon skeleton, i.e. n- and iso-alcohols, and with the various position of OH-group, i.e. primary, secondary and tertiary alcohols. A binary mixture of organic solvents such as hexane and diethyl ether in the volume ratio of 1 : 1 was used for dissolving substrates. The concentration of enanthic acid (S1) was varied from 0.05 M till 1.5 M. Alcohols (S2) were used in double molar excess; the concentration of alcohol was varied from 0.1 M till 3.0 M. The biocatalyst (0.5 g) was immersed into reaction medium (3.0 mL) containing S1, i.e. the content of the biocatalyst was 20.8 wt%. The reaction was started by adding substrate S2, namely, liquid (C2–C11) or solid (C16) alcohols. Periodic process of esterification was carried out in a batch reactor at room temperature (20 ± 2 °C). Aliquots of reaction medium were taken periodically. The initial and current concentrations of organic acids were measured by titration; NaOH solution with known concentration was used. The one reaction cycle was carried out till full conversion of fatty acid occurred, and then samples of biocatalyst were washed by solvent for 20 h. Analysis of esterification products were performed by both a thin layer chromatography (TLC) and a two-dimensional gas chromatography using an Agilent 7890A Series GC System chromatograph equipped with a flame ionization detector and an Agilent flow modulator gas as described [31], as well as a gas chromatography with mass-spectrometry using an Agilent 7000B GC/MS Series; a column ZB-WAX, 30 m × 0.25 mm × 0.25 μm was used. The carrier gas was high purity helium with flow rate 1.0 mL min−1. Oven program was 8°/min from 50 to 260 °C, electron ionization is 70 eV, scan is 40–500 m/z. The results of titrimetric and chromatographic analyzes agreed within experimental error of less than 10%.
The rate of the esterification (in µmol L−1 s−1) was determined using initial linear segment of kinetic curve of S1 concentration decrease in time. Taking into account reaction rate, enzymatic activity of the biocatalysts was calculated and expressed in an amount of international Units of activity (1 U = µmol min−1) per 1 g of dry biocatalyst (in U g−1). The specific activity of adsorbed rPichia/lip lipase was expressed in U mg−1.
All the experiments were carried out at least by triplicate. Standard deviations of replicates were calculated using statistics model based on normal Gauss–Laplace probability distribution. Experimental results were represented as \(\bar{x} \pm \frac{{t{\kern 1pt} s}}{\sqrt n }\), where \(\bar{x}\) is the mean; t is the Student coefficient, for example, equal to 4.30 for 3 measurements; s—dispersion calculated according to the formula, \(s = \sqrt {\frac{{\sum {(x_{i} - \bar{x})^{2} } }}{n - 1}}\); n is the number of measurements, n = 3–6. Relative experimental error (Sr) was estimated trice using 5–6 identical measurements; Sr was estimated to be less than 10% (min 6%).
Results and discussion
Impregnation versus physical adsorption of lipase on mesoporous silica
As described in “Experimental” section, the lipase-active biocatalysts were prepared by both spontaneous and forcible adsorption using rPichia/lip lipase solutions at the same concentration. The adsorption was close in magnitude and equal to 4.2 and 5.4 mg g−1 respectively. It was found that under exactly the same conditions of the synthesis of n-butyl heptanate, the stationary activity of the spontaneously adsorbed lipase was twofold less than the activity of forcibly adsorbed enzyme, for example, 2.4 vs. 5.0 U g−1. This method of impregnation was characterized not only by comparatively high enzymatic activity, but simplicity of its implementation and economical enzyme consumption. For example, a minimal volume of lipase solution used for spontaneous adsorption on 1 g of silica was 3.0 mL, whereas for forcible adsorption was 0.8 mL equal to VΣ of silica.
From the results obtained it may be concluded that the forcible adsorption by impregnation was appropriate and promising method for preparing the most active heterogeneous lipase-active biocatalysts. All results described below were obtained for forcibly adsorbed recombinant lipase.
Texture parameters of the lipase-active biocatalysts
The texture parameters of the biocatalyst with maximal content of forcibly adsorbed lipase, equal to 14.0 mg g−1, were following: surface area was 143 m2 g−1 (vs. 157 m2 g−1 for initial silica), the total pore volume was 0.67 cm3 g−1 (vs. 0.76 cm3 g−1), the average pore diameter was 18.8 nm (vs. 19.4 nm), the porosity was 56% (vs. 58%). One can see that texture parameters decreased insignificantly after immobilization of rPichia/lip on silica.
Peculiarities of biocatalysis by immobilized lipase in anhydrous media
The stage of considerable increase of activity (in 2–4 times) of dried biocatalysts during the first 1–3 reaction cycles, named pre-conditioning stage, was observed in all experiments. It was found that the greater the activity of the biocatalyst, the shorter the pre-conditioning stage. This stage was probably due to the ongoing accumulation of formed product—water, in the vicinity of the adsorbed lipase inside the silica-based biocatalyst. Calculation showed that upon full conversion of fatty acid (in average 85%), a volume of 0.1 mL of water was formed under studied conditions. Since the total pore volume of this supports (VΣ) was multi-fold greater than the volume of the water formed inside for one reaction cycle, this water was firmly held inside KSK-G® type silica applied as a dehumidifier for industrial gases. Therefore, during esterification the favorable aqua microenvironment was created for adsorbed lipase; and the activities of the dried biocatalysts increased in several times.
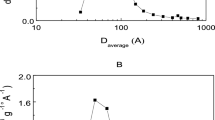
It was found, that after the pre-conditioning stage the catalytic activity (named as stationary activity) of the biocatalysts was fluctuated slightly in magnitude during the next 38 cycles (~ 900 h) of the periodic esterification process (Fig. 1), i.e. the operational stability was a sufficiently high. Also, the prepared biocatalysts possessed a high long-term stability under storage in the used solvent (hexane and diethyl ether) at 20 ± 2 °C, as well as under storage in dried state in a refrigerator for 9 months.
Operational stability of the biocatalysts in periodic esterification of various fatty acids such as butyric (C4:0), enanthic (C7:0), capric (C10:0), stearic (C18:0) with n-butanol as a function of enzymatic activities in dependence of a number of reaction cycle. The content of rPichia/lip on silica was 14.0 mg g−1. Concentrations of acid and alcohol were 0.25 and 0.50 M. Ambient conditions: 20 ± 2 °C, 1 bar
Specificity of esterification by immobilized recombinant T. lanuginosus lipase
In order to determine the specificity of immobilized recombinant T. lanuginosus lipase, the matrix of relative catalytic activities was composed by comparing the rates of esterification of various pairs of substrates (Table 1). The following observations were drawn from the experimental data: (1) the rate of esterification of acetic acid (C2) was very low, (2) the rate of synthesis of ethyl esters of fatty acids using ethanol (C2) was 1.3–2 times lower than the rate of the synthesis of esters with alcohols possessing more than 3 carbon atoms, (3) the more the number of carbon atoms in the molecule of fatty acid, the more the rate of esterification; thus, the rates of esterification of C7, C10 and C18 fatty acids were in 2–5 times higher than ones for the C4–C6 fatty acids, (4) the highest rate was observed in the esterification of enanthic acid C7:0 with C3, C4-alcohols (Table 1). The maximum ratio of relative difference in reaction rates for various fatty acids was 5.9, whereas for alcohols it was 1.8 only (Fig. 2). Consequently, the immobilized on silica rPichia/lip was more sensitive to the molecular structure of fatty acid (S1) than to the structure of aliphatic alcohol (S2).
The activities of the biocatalysts in esterification of enanthic acid with aliphatic alcohols differing in carbon skeleton isomerism and OH-group position were presented in Table 2. One can see, that the rates of esterification of enathic acid with of iso-butanol and iso-pentanol were insignificantly higher (in 1.1 times) than ones with n-isomers. It should be noted that the most pronounced specificity was observed with respect to the position of the OH-group in the alcohol molecule. Thus, the rates of esterification of fatty acids with secondary alcohols were two orders of magnitude lower than those of primary alcohols (Table 2). The esterification of fatty acids with tert-butanol did not occur; the reaction rate was zero. The biocatalysts tested in the esterification of fatty acids with sec- and tert-butanol were washed with a solvent and then examined in the esterification of enanthic acid with n-butanol. It was found that the 91% of initial catalytic activity was restored.
It was found that the esterification of acids and alcohol possessing cyclic or aromatic residues occurred with very low rates. For example, the rate of esterification of benzoic acid with n-butanol was equal to zero; and any traces of the ester on TLC-plates were not observed. The rate of esterification of enanthic acid with methyl cyclohexanol was lower in 240 times than the rate of esterification of enanthic acid with n-butanol; and the catalytic activities were equal 0.07 and 16.9 U g−1 respectively under the same conditions. Also, the full conversion of enanthic acid did not exceed 50% when S2 was methyl cyclohexanol, whereas the conversion of enanthic acid reached 90% when S2 was n-butanol. The biocatalysts tested in the esterification of aromatic acid and cyclic alcohol were washed with a solvent and then examined in the esterification of enanthic acid with n-butanol. It was found that the ~ 80% of initial biocatalytic activity was restored. Thus, these substrates were neither substrates nor irreversible inhibitors of immobilized rPichia/lip lipase. These results are consistent with the literature data described in [26].
Kinetics of esterification of enanthic acid with n-butanol
The esterification reaction of enanthic acid with a double excess of n-butanol obeyed the kinetic equation for the reaction of the 1st order, C = C0 exp (− 2.3 × 10−4 × t), where C0 and C are the initial and current concentration of acid, mol L−1, t is the time of reaction, s. According this equation, the rate constant was 2.3 × 10−4 s−1. The maximum conversion of fatty acid, in average 85%, was achieved after 2–4 h of esterification at ambient conditions (20 ± 2 °C, 1 bar).
The selectivity of enzymatic esterification was 100%, and only one product, an ester n-butyl heptanate, was detected by gas chromatography (Fig. 3). Chromatographic peak 3 corresponding to the ester increased in height with increasing acid conversion in time, while the chromatographic peaks 2 and 4 corresponding to n-butanol and enanthic acid decreased (Fig. 3). Purification of the produced ester, for example, iso-pentyl decanoate, using conventional vacuum distillation, was quite a suitable method, and the content of the main substance was 99.0% [28, 31].
Chromatogram of the reaction medium with the identification of main components and their content in dependence of conversion of enanthic acid: 1—hexane and diethyl ether as solvent, 2, 3, 4—conversion 21.9%, *2,*3,*43—conversion 61.9%. Contents of main compomemts in reaction medium, in wt%: 2,*2—n-butanol (42.8 → 36.5), 3,*3—n-butyl heptanate (29.3 → 47.8), 4,*4—heptanoic acid (15.7 → 7.3 wt%)
The biocatalyst with lipase content of 6.2 mg g−1 was used for determining kinetic parameters such as Michaelis constant (KM) for enanthic acid and maximal reaction rate (Vmax) under studied conditions of the esterification of enanthic acid with a double excess of n-butanol. The kinetic curve (Fig. 4) was satisfactory described by the classic hyperbolic equation at an acid concentration lower than 1.0 mol L−1, \({\text{V}} = \frac{{{\text{V}}_{\text{max} } \cdot {\text{C}}_{0} }}{{{\text{K}}_{\text{M}} + {\text{C}}_{0} }}\), where V and Vmax were the initial observed and maximum reaction rates μmol L−1 s−1; C0 was the initial substrate concentration, mol L−1, KM was the Michaelis constant, mol L−1. Then, at a concentration of enanthic acid above 1.0 mol L−1 the reaction rate decreased perhaps due to inactivation of enzyme by high concentration of n-butanol as described previously for esterification of capric acid with iso-pentanol [29]. The values of KM and Vmax were determined using Lineweaver–Burke linear approximation and the regression of hyperbolic equation by ORIGIN programs. These parameters were equal to 0.22 ± 0.05 mol L−1 and 66.7 ± 4.0 μmol L−1 s−1, respectively. The values of KM and Vmax estimated from the full Michaelis–Menten kinetic curve presented in Fig. 4 were close to these magnitudes. Comparison of the obtained results with data published earlier in [29, 31] showed that the Michaelis constant for enanthic acid (heptanoic, C7:0) was 3 times less than KM for capric acid (decanoic, C10:0) for esterification with C4–C5 aliphatic alcohols. This means, that the affinity of immobilized on silica recombinant lipase to heptanoic acid was higher, and the maximum reaction rate Vmax was achieved at lower initial substrate S1 concentrations.
It was found that the enzymatic activity of the biocatalysts was directly proportional to the content of immobilized lipase. The maximum biocatalytic activity (Amax) was calculated using Vmax value (Fig. 4). For the biocatalysts with content of adsorbed lipase 6.2 mg g−1 Amax was equal to 22.3 ± 2.0 U g−1. The specific activity of forcibly adsorbed rPichia/lip lipase was 3.3 ± 0.3 U per 1 mg of lipase. From the data in Fig. 1, the maximal observed activity (Amax) of the biocatalyst with adsorption of 14.0 mg g−1 was measured to be 50.4 ± 4.0 U g−1, and the specific activity of immobilized lipase was 3.6 ± 0.3 U mg−1. From the results above, the specific activity of immobilized lipase was the same for both biocatalysts and independent on amount of adsorbed enzyme. Comparison with literature data showed that maximum reaction rate (Vmax) measured here with forcibly adsorbed recombinant T. lanuginosus lipase in the synthesis of fruit flavor esters (n-butyl acetate, n-propyl acetate) was in 3–4 times higher than Vmax measured with Rhizopus oligosporus lipase immobilized on activated silica [32].
Conclusions
The lipase-active heterogeneous biocatalysts were prepared by impregnating the moisture capacity of dry silica granules with recombinant T. lanuginosus lipase solutions; and forcible adsorption of enzyme occurred. During the research of these biocatalysts in esterification of different pairs of substrates—saturated fatty acids and aliphatic alcohols differing in the structure of the molecules, namely both the number of carbon atoms (C2–18) and the isomerism of the alcohols’ carbon skeleton (n- and iso-) the matrix of relative enzymatic activity was composed. According to this matrix, the forcibly adsorbed on silica recombinant lipase demonstrated the relatively wide specificity. The high reaction rates were observed when saturated fatty acids with 7 (or more) carbon atoms were esterified with primary n- and iso-alcohols with more than 3 carbon atoms; and the highest reaction rate was observed in the synthesis of ester of enanthic (heptanoic, C7:0) acid and n- or iso-butanol in a batch reactor at ambient conditions (20 ± 2 °C, 1 bar). The Michaelis constant for enanthic acid was determined to be equal to 0.22 ± 0.05 mol L−1 in the reaction of n-butyl heptanate synthesis. It was found that the esterification of fatty acids with tert-alcohol did not occur, and substrates with aromatic and cyclic restudies reacted with very low rates.
The lipase-active heterogeneous biocatalysts had a considerably high operational stability. Thus, catalytic activity did not change during 38 reaction cycles (900 h) under conditions of low-temperature synthesis of various esters in unconventional anhydrous media of organic solvents (hexane and diethyl ether mixture).
Analyzing our own and literature data, it was concluded that due to the prominent catalytic properties, such as enzymatic activity and operational stability, in combination with simplicity of the immobilization method, the biocatalysts prepared by forcible adsorption of recombinant lipase onto mesoporous silica are promising for practical implementation for reactions of organic synthesis, in particular for production of fatty acid esters.
References
Grunwald P (2009) Biocatalysis. Imperial College Press, London
Tao J, Kazlauskas R (2011) Biocatalysis for green chemistry and chemical process development. Wiley, Hoboken
Sun J, Lee L, Liu S (2014) Aust J Chem 67:1373–1381
Talon R, Montel M, Berdague J (1996) Enzym Microb Technol 19:620–6228
Hsu A, Foglia T, Siya S (2000) Biotechnol Appl Biochem 31:179–183
Pinto M, Freire D, Pinto J (2014) Molecules 19:12509–12530
Silveira E, Moreno-Perez S, Basso A, Serban S, Mamede R, Tardioli P, Farinas C, Rocha-Martin J, Fernandez-Lorente G, Guisan J (2017) BMC Biotechnol 17:88–101
Mulalee S, Srisuwan P, Phisalaphong M (2015) Chin J Chem Eng 23:1851–1856
Gandhi N, Mukherjee K (2001) J Am Oil Chem Soc 78:161–165
Lopresto C, Calabro V, Woodley J, Tufvesson P (2014) J Mol Catal B 110:64–71
Raita M, Kiatkittipong W, Laosiripojana N, Champreda V (2015) Chem Eng J 278:19–23
Pang J, Zhou G, Liu R, Li T (2016) Mater Sci Eng C 59:35–42
Chang S, Shaw J (2009) New Biotechnol 26:109–116
Sutili F, Ruela H, Leite S, Miranda L, Leal I, Souza R (2013) J Mol Catal B 85–86:37–42
Gumel A, Annuar M, Heidelberg T, Chisti Y (2011) Process Biochem 46:2079–2090
Enayati M, Gong Y, Goddard J, Abbaspourrad A (2018) Food Chem 266:508–513
Jia C, Wang H, Zhang W, Zhang X, Feng B (2018) Process Biochem 66:28–32
Bernal C, Escobar S, Wilson L, Illanes A, Mesa M (2014) Carbon 74:96–103
Wang Z, Du W, Dai L, Liu D (2016) J Mol Catal B 127:11–17
Guebara S, Ract J, Vitolo M (2018) Arab J Sci Eng 43:3631–3637
Cavalcanti E, Aguieiras É, da Silva P, Duarte J, Cipolatti E, Fernandez-Lafuente R, da Silva J, Freire D (2018) Fuel 215:705–713
Fernandes K, Papadaki A, da Silva J, Fernandez-Lafuente R, Koutinas A, Freire D (2018) Ind Crops Product 116:90–96
Akerman C, Hagström A, Mollaahmad M, Karlsson S (2011) Hatti-Kaul. Process Biochem 46:2225–2231
Kim H, Choi N, Kim Y, Kim H, Lee J, Kim I (2019) Renew Energy 130:489–494
Verdasco-Martin C, Garcia-Verdugo E, Porcar R, Fernandez-Lafuente R, Otero C (2018) Food Chem 245:39–46
Naik S, Basu A, Saikia R, Madan B, Paul P, Chaterjee R, Brask J, Svendsen A (2010) J Mol Catal B 65:18–23
Arsan J, Parkin K (2000) Biotechnol Bioeng 69:222–226
Perminova L, Kovalenko G, Chukanov N, Patrushev Y (2017) Rus Chem Bull 11:2194–2197
Kovalenko G, Perminova L, Chuenko T, Rudina N (2016) Appl Biochem Microbiol 52:582–588
Bearden J (1978) Biochim Biophys Acta 533:525–529
Kovalenko G, Perminova L, Beklemishev A, Mamaev A, Patrushev Y (2018) Catal Ind 10:68–74
Mahapatra P, Kumari A, Garlapati V, Banerjee R, Nag A (2009) J Mol Catal B 60:57–63
Acknowledgements
Authors are grateful to Maria B. Pykhtina for cultivation of the rPichia/lip strain-producer for this research. This work was conducted within the framework of the budget Project No. AAAA-A17-117,041,710,075-0.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kovalenko, G.A., Perminova, L.V. & Beklemishev, A.B. Catalytic properties of recombinant Thermomyces lanuginosus lipase immobilized by impregnation into mesoporous silica in the enzymatic esterification of saturated fatty acids with aliphatic alcohols. Reac Kinet Mech Cat 128, 479–491 (2019). https://doi.org/10.1007/s11144-019-01648-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-019-01648-z