Abstract
The tolerance mechanism of chemical pesticide is necessary to combat the pest infestation challenges. This study intended to analyze the responses of enzymatic activity and expression level of an antioxidant gene to organophosphate pesticide stress. The alteration of anti-oxidative correlated with pesticide treatment in eggplant (S. melongena L.cv. Longai) using varying concentrations (0, 50, 100, 150 and 200 ppm) of malathion (PM) and tatafen (PTF) each. The enzyme activities of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) were observed to be elevated with pesticide treatment in eggplant seedling. FeSOD (iron SOD), CAT and APX genes associated in defense mechanisms were significantly expressed under PM and PTF stress which contributed to stress tolerance to the plant. The different concentration of both pesticide stresses altered the expression level of mRNA, FeSOD, CAT and APX genes in comparison to the non-treated plant. While mRNA level of three antioxidant genes were evaluated and found to be APX gene expression was more potent than the CAT and FeSOD gene subjected to different concentrations of PM and PTF in eggplant. The current experiment highlights the presence of minimum level of pesticide concentration impacted positively towards the plant growth and metabolism, while high level of pesticide concentration impacted negatively. In summary, antioxidant enzymes activity responded to both pesticide stresses at an early stage of exposure and their gene expression profiles provided more details about their complex interaction and effectively scavenge reactive oxygen species. This allows the plant to maintain growth under pesticide stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eggplant (Solanum melongena L., 2n = 24) is generally termed as brinjal, aubergine or Guinea squash which is mostly benign in the warm-temperate region, cultivated in Asian tropical and sub-tropical regions across the globe (Taher et al. 2017). It is an abatement-growing perennial solanaceous crop, behaves as an annual crop that can be prolonged under preserved cultivation. Eggplant is contemplated as a day-neutral plant with a height of 50 to 150 cm and flowering starts at the 6th to 10th leaf phase lasting for an extended period, its fruits bear in different shapes, sizes, and skin color as well as weight ranging from 200 to 500 gm (Taher et al. 2017). It is considered as one of the vegetables with the highest capacity of antioxidant (Hurtado et al. 2012). Its extracted tissue has been used in traditional medicine treatment of several diseases, such as bronchitis, asthma, dysuria, cholera as well as curing diabetes (Quamruzzaman et al. 2020).
Eggplant is contemplated as a native of the Indian sub-continent and a credible center of origin, whereas China, Japan and Indo-Burma is the inferior center of origin (Kumar et al. 2020). In Assam, eggplant is propagated to a considerable extent, but it curtails productivity of crops in many countries. Its production affords an imperative source of livelihood, especially in poor resource farmers (Hautea et al. 2016). The preeminent constraint to the fruits of eggplant throughout Asia, is chronic and extensive infestation of the noxious pest, fruit and shoot borer (FSB, Leucinodes orbonalis Guenee) (Hautea et al. 2016). The larvae are catastrophic in yield and quality production of fruits by boring into the leaves of midrib and petiole, tender shoots resulting in desiccation and wilting of the stem and it has been active in moderate humidity throughout the year (Javed et al. 2017). Till date, its management practices to combat insect and pest are still restraint to frequent sprays of various pesticides which are detrimental to human health, environment and livestock (Challa et al. 2021). The survey of eggplant cultivators in the major cultivation area of India (Rai 2015) revealed that the yield loss ranges from 11 to 93% of total production due to the attack of pests. Almost all farmers use chemical pesticides, such as malathion, cypermethrin, carbyl, fenvalerate, and imidacloprid, etc., for the elimination of EFSB damaged fruits. Residues of these pesticides were distinguished in the harvested crops as well as eggplant farms soil (Hautea et al. 2016).
Like many other toxic chemicals, pesticides persuade the production and accumulation of free radicals viz. hydroxyl radicals, singlet oxygen, hydrogen peroxide and superoxide (Ara et al. 2013). Reactive Oxygen Species (ROS) are mostly provoked via photorespiration in peroxisomes, photosynthesis in chloroplasts and aerobic respiration in mitochondria (Zaid and Wani 2019). The enormous production of ROS scavenging can result in irreversible oxidation of proteins, lipids, nucleic acid and chloroplastic pigments (Li et al. 2012). Injury in plants caused by excess ROS production is termed “oxidative stress”. Plants are incorporated with an enzymatic and non-enzymatic antioxidant defense mechanism to cope and detoxify the enormous generation of ROS. This system consists of numerous enzymatic antioxidants, such as Superoxide dismutase (SOD, EC 1.15.1.1), Ascorbate peroxidase (APX, EC 1.11.1.11), Dehydroascorbate reductase (DHAR), Guaiacol peroxidase (GPX, EC 1.11.1.7), Catalase (CAT, EC 1.11.1.6), Monodehydroascorbate (MDHAR), and Glutathione Reductase (GR), where non-antioxidants such as reduced glutathione, proline and Ascorbic Acid (ASA) work in cascade to sustain the sub-cellular redox homeostasis by scavenging various ROS production (Wani et al. 2018). The antioxidant enzymes were detected in eggplant subjected to organophosphate pesticides and levels of the enzymes were dependent on the concentration of pesticides. SOD is considered as the first line of defense against the production of ROS, which converts O2− free radicals to H2O2. Then H2O2 reduced to H2O and molecular O2 by enzyme APX, POD and CAT, thus averting further damage to the plant cell membrane (Semida et al., 2021). Understanding the involvement of both antioxidant activity and gene expression levels in pesticide tolerance is necessary for the detection of the main antioxidant defense system. The analyses of antioxidant genes in several plant species will increase the comprehensive insight of their specific roles in the defense of plant mechanisms. In this study, the functions of FeSOD, CAT and APX gene expression levels in the defense system of eggplant exposed to various concentrations of PM and PTF. The expression of mRNA levels of FeSOD, CAT and APX genes was established by qRT-PCR in a pesticide treated plants.
Materials and methods
Experimental design
The experimental materials were cultivars that are currently cultivated in Karimghanj, Assam, India. Due to the pesticides stress trigger a wide variety of plant responses ranging from altered antioxidant metabolism and gene expression to changes in growth rate and crop yield. Under normal growth conditions, the ROS production in cell is very low while stress conditions, elevate the levels of ROS in plant cells. It is highly lethal and can oxidize most of nucleic acids, lipids and protein subsequently causing cell death due to lipid peroxidation and inactivation of antioxidant enzymes as well as membrane damage. Thus, to cope with oxidative stress, plants develop a complex antioxidative defense mechanism consisting SOD, APX, CAT and POD etc., that scavenge peroxide and free radicals (Gill and Tuteja 2010; Shakir and Rehman 2018). Among the key antioxidant enzymes, SOD leads the frontline defense in the antioxidant defense system by O2•− into H2O2 and reducing the possibility of •OH formation. Then, APX and POD are associated in the scavenging of H2O2 (Gajewska and Skłodowska 2007). CAT is tertrameric heme-containing enzyme for detoxification of ROS which converts more than 25 million H2O2 molecules into H2O in 60 s. Peroxidase mainly oxidizes PhOH for producing phenoxyl radical (PhO•), where H2O2 accepts electron and is converted to H2O (Gill and Tuteja 2010; Hasanuzzaman et al. 2020). These changes in the activity of these antioxidant enzymes indicate redox alterations related to oxidative stress.
Pesticide treatment
The eggplant seedlings were propagated in plastic pots filled with farm manure about 2 kg of soil per pot. The primary characteristic of the soils is sand particle 30.2%, silt 39.4%, and clay 30.4%. Seedlings were grown outdoors in polybag placed under a defensive condition. Four pesticide treatments via 0, 50, 100, 150 and 200 ppm each for PM (Malathion, EC-50%; accessed from ASAMAL-5, Assam Chemical Industries, Bongaigaon, Assam) and PTF (Tatafen, 10%; Rallis India Limited, Mumbai, India) were selected for the treatment of plants with control samples (without pesticides leveled as 0 ppm). Stock solutions of the PM and PTF were assembled with DMSO due to their insolubility in water (concentrations ranging between 50 and 200 ppm) and stored in the dark condition at 4 °C. Treatment concentration of PM and PTF was standardized following related studies conducted earlier studies by Shakir et al. (2018). Treated and control seedling was uprooted at 20 days of growth and analyzed for the antioxidant assay and gene expression analysis.
Determination of antioxidant stress markers
Frozen leaf was ground to a fine powder with ice-cold NaPO4 (pH 7.0) (50 mM) containing polyvinylpyrrolidone (1%, w/v) (2:1 buffer volume/FW) and EDTA (0.1 mM). The homogenate was centrifuged for 20 min at 4 ºC at 10,000 rpm and the supernatant was used for spectrophotometric determination of antioxidant activity (Kaur et al. 2016). SOD activity: The activity was evaluated according to Vuleta et al. (2016) based on the measurement of inhibition of the photochemical reduction of nitroblue tetrazolium (NBT; HiMedia) spectrophotometrically at 560 nm. The cocktail contained PO4 (50 mM) (pH 7.4), EDTA (0.1 mM) to which molecular O2 generating system containing NBT (82.5 µM), riboflavin (2.2 µM) and methionine (14.3 mM) and required amount of crude enzyme/sample extract (25 µl). The reaction was initiated by adding riboflavin and placing the tubes under the light source (below 30 cm) (40 W fluorescent tube-light: Philips, Kolkata, India) for 30 min. A complete reaction mixture without enzyme which gave the maximal color served as blank. A non-pesticides, treated complete reaction mixture served as a control. One unit of SOD enzyme is inhibited by 50% of maximum reduction of NBT under specific assay conditions as recorded at 560 nm. The CAT enzymatic assay was determined according to Azarabadi et al. (2017) with a slight modification. To determine the H2O2 decomposition by monitoring the decrease in absorbance was recorded at 240 nm.
APX enzyme assay: The supernatant subjected to analyze for spectrophotometric determination of enzymatic activity was termed by the process of Moura et al. (2018). It was assayed from the decrease in absorbance as ascorbate was oxidized.
Total RNA isolation and complementary DNA (cDNA) synthesis
The possible variation among the antioxidant genes encoding some scavenger enzymes that is necessary for the direct detoxification of ROS by three well-documented genes, such as FeSOD, CAT and APX. The steps are as follow: Total RNA was isolated from eggplant leaves treated with different concentration of PM and PTF using Trizol (Life Technologies, USA) protocol followed by RNA BR (Broad-Range) Assay Kit (Qubit®) following the manufacturer’s directives. To abolish genomic DNA, the extracted RNA was treated with RNase-free DNase I supplemented provided with the assay kit. To evaluate the RNA purity and quality was measured by Qubit® 2.0 Fluorometer (Life Technologies, USA) and gel electrophoresis which contains agarose (1.5%) as described by Sambrook et al. (1989). The synthesis of the first-strand cDNA was based on reverse transcription reactions and which was achieved with total RNA (2 µg) using 5 mM oligo (dT)18 primers and reverse transcriptase (M-MLV Reverse Transcriptase) 1 mM dNTP solution, 20 µl 3 mM Mg2+, 4 µl 5X FS Buffer and 0.1 M DTT according to the manufacturer’s directives (Invitrogen, USA). The thermo-cycling conditions were carried out at 65 °C for 5 min, 37 °C for 60 min and 70 °C for 10 min.
Analysis of quantitative real time-polymerase chain reaction (qRT-PCR)
The experiment includes three biological and technical replicates each for PM and PTF treated and non-treated plants sample. The primer sequences employed in the experiment are shown (Table 1). The primer sequences of the target antioxidant genes are FeSOD (GenBank Accession No: KU240391.1), CAT (GenBank Accession No: X71653.1), APX (GenBank Accession No: AB828071.1) and housekeeping or internal control gene actin (ACT, GenBank Accession No: GU984779.1) as reference genes used for normalization in eggplant. The primers were designed based on the eggplant gene sequences available in the Genebank database (https://www.ncbi.nlm.nig.gov/genbank). The transcription levels of all the antioxidant genes were performed using the qRT-PCR with Thermo Fisher Scientific QuantStudio-5–384 Real-time PCR (Applied Biosystems). The PCR reaction mixtures consisted of 1 µl of each primer, 1 µl of cDNA and ddH2O to final volume of 20 µl and amplification product were observed via SYBR Green dye which is an intercalator-based method. The reaction mixture was initially denatured for 2 min at 94 °C was set to 1cycle and then, denaturation at 94 °C for 30 s followed by 40 cycles and annealing for the 30 s, extension at 60 °C for 30 s were allowed for completion of polymerization. The relative expression levels of antioxidant genes were calculated using the log10 function (Gébelin et al. 2013) and normalized to the relative transcript level of the ACT gene. Each primer was composed of about 20 nucleotides with the melting temperatures around 60 °C. Melt-curve analysis was performed to confirm the specificity of the selected primers and primer–dimer absorbance. For quantification, data collection was done during the stage of annealing as well as copy numbers of transcript FeSOD, APX, CAT and ACT genes under PM and PTF stress treatment was established using amplification curves.
Statistical analysis
All the assays were observed with three independent replicates and were analyzed using the SPSS Statistical Software (IBM SPSS 21.0, IBM Corporation) windows version 16.0 and Microsoft Excel 2007. The data are presented as mean ± SE of three replicates. Differences between each pesticide treatment were analyzed by Least Significant Difference (LSD) (p ≤ 0.05).
Results
Activities of enzymatic antioxidant
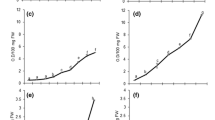
The antioxidant enzyme activities were measured to evaluate the mechanisms of pesticide tolerance in the leaves of the pesticide-treated S. melongena. The activity of SOD in PM and PTF treated samples was significantly higher than those of the control plants. The activity of SOD increased up to 40.93 U/mg protein at 100 ppm and suddenly decreased as the treatment concentration increased to 150 ppm (31.56 U/mg protein) and 200 ppm (24.12 U/mg proteins) of PM treatment (Fig. 1a). Similarly, SOD activity was found to be gradually increased at 50 ppm (26.35 U/mg protein) and 150 ppm (35.01 U/mg protein) but a sudden decrease occurred at 100 ppm (23.2 U/mg protein) and 200 ppm (7.32 U/mg protein) of PTF exposure compared with the control plant (12.32 U/mg protein). As shown in Fig. 2a, the activity of CAT was higher in the PM-treated plants and peaked at 200 ppm (16.58 U/mg protein) followed by 100 ppm (7.98 U/mg protein), 150 ppm (7.22 U/mg protein) and 50 ppm (6.59 U/mg protein). In PTF treated plant, the maximum increase was found in 150 ppm treatment (13.43 U/mg protein) followed by 50 ppm (4.87 U/mg protein), whereas activities of CAT significantly decreased at 100 ppm (2.3 U/mg protein) and 200 ppm (1.7 U/mg protein) as compared to the control plant (3.79 U/mg protein). The activity of APX showed a maximum increase under pesticides stress and peaked at 200 ppm (6.04 U/mg protein) followed by 100 ppm (4.47 U/mg protein), 50 ppm (2.5 U/mg protein) and 150 ppm (2.45 U/mg protein) of PM treatment, whereas in PTF treated plant showed maximum activity of APX at 150 ppm (4.66 U/mg protein) followed by 100 ppm (3.3 U/mg protein), 50 ppm (3.24 U/mg protein). When the treatment concentration raised to 200 ppm the APX activity was maximum compared to the control plant (0.90 U/mg protein) (Fig. 3a).
Effect of different concentration of PM and PTF stress on the activity and transcript expression level of superoxide dismutase (SOD) enzymes in eggplant leaves. a SOD activity in leaf. b Relative expression of antioxidant gene FeSOD transcript established through qRT-PCR in PM and PTF at 50, 100, 150 and 200 ppm using ACT as housekeeping gene. The values are mean ± SD (n = 3)
Effect of different concentration of PM and PTF stress on the activity and transcript expression level of catalase (CAT) in eggplant leaves. a CAT activity in leaf. b Relative expression of antioxidant gene CAT transcript established through qRT-PCR in PM and PTF at 50, 100, 150 and 200 ppm using housekeeping ACT gene. The values are mean ± SD (n = 3)
Effect of different concentration of PM and PTF stress on the activity and transcript expression level of ascorbate peroxidase (APX) in eggplant leaves. a APX activity in leaf. b Relative expression of antioxidant gene APX transcript established through qRT-PCR in PM and PTF at 50, 100, 150 and 200 ppm using housekeeping ACT gene. The values are mean ± SD (n = 3)
Gene expression
The expression levels of FeSOD, CAT and APX genes were evaluated by qRT-PCR (Thermo Fisher QuantStudio-5-384 qRT-PCR platform, Applied Biosystems) in eggplant treated with different concentrations of PM and PTF. The qRT-PCR data are normalized with the ACT as an internal control gene. To analyze the stability results of the transcript level of FeSOD, CAT, APX and ACT of two pesticides samples were measured 3 times for each concentration exposure. Concerning the control and to each other, different expression levels were recorded in all the concentrations (50, 100, 150 and 200 ppm) of PM and PTF exposure.
In the first treatment (50 ppm), the expression level of FeSOD was increased under both PM and PTF. Subsequently, the maximum FeSOD expression level was found up-regulated in 100 ppm of PM, which was recorded as a 1.44-fold change followed by 200 ppm (1.071 fold change) and 50 ppm (1.036 fold change) compared to the control level. A decrease was observed again at 150 ppm (0.474 fold change). Then, the expression was shown slightly up-regulated at a concentration of 200 ppm (1.071 fold change) PM treatment. At PTF stress, it was observed that the expression level of the FeSOD gene was moderately up-regulated in 50 ppm (0.578 fold change) and 150 ppm (0.686 fold change) and slightly decreases in 100 ppm (0.3 fold change) treated plants. At 200 ppm treated eggplant FeSOD expression level was moderately down-regulated in PTF stress, which was a −0.79-fold change decrease compared to the control (Fig. 1b). During the increased concentration of pesticide, plasmolysis started in eggplant leaves. The expression levels of the FeSOD gene were under control levels in all the concentrations of PM treatment, but slightly similar in exposed to 150 ppm of both PM and PTF treatment. Expression of CAT was up-regulated in all the concentrations of PM treated eggplant, the highest expression level was found in 100 ppm which was 1.599 fold change, but at the treatment of 200 ppm was observed 1.572 fold change followed by 50 ppm (1.124 fold change) and slightly decreased in 150 ppm (0.983 fold change) as compared to control (1.0 fold change). The expression of the CAT gene was not affected by different concentrations of PM treatment. In PTF stress treatment, CAT gene expression was observed up-regulated in 150 ppm which was 1.283 fold change in 50 ppm it was 0.966 fold change and it was slightly down-regulated at 100 ppm (− 0.11 fold change) and in 200 ppm (− 0.23 fold change) compared to control (1.0 fold change), but the level of CAT gene was not expressed under the concentration of 100 ppm and 200 ppm of PTF treatment (Fig. 2b). The expression of APX was observed highly up-regulated in all concentrations of PM stress. But, the highest expression levels were found in eggplant exposed to 200 ppm PM concentration which was a 2.21-fold change followed by 100 ppm (2.14 fold change) and 50 ppm (1.58 fold change), whereas slightly decreased in 150 ppm with 1.14 fold change as compared to control. During PTF treatment, the expression level of the APX gene was highly up-regulated in 50 ppm, which was 2.21 fold change and followed by 150 ppm (1.66 fold change) and subsequently suppressed the expression level at 100 ppm (0.83 fold change). Down-regulated APX was observed in 200 ppm PTF which 1.15-fold change in treating eggplant (Fig. 3b).
Discussion
Plants respond to different insects/pests through several biological, morphological and molecular mechanisms to combat the effects of the attack (Zaid et al. 2021). The mechanisms of defense against pest attacks are wide-ranging, highly dynamic and mediated by direct and indirect defenses. In addition, to attract the natural enemies of the pest/insect, plants also liberate volatile organic compounds, either act in conjugation or independently with each other. Therefore, this defensive mechanism is still limited and could be developed as an essential implement for pest management to curtail the amounts of chemical insecticides/pesticides used for pest control (War et al. 2012). Under oxidative stress conditions, uncontrolled free radicals, enhance, and plants use enzymatic and non-enzymatic antioxidants decrease oxidants and regulate cellular homeostasis (Kamran et al. 2020). Several stress factors are continuously exposed to plants which affect their yield production. ROS accumulation in plants can cause severe oxidative stress (Wani et al. 2018). To resist the excess ROS production in plants, the enzymatic antioxidant enzymes cooperate thereby protecting the functions and structures of cellular components. In general, the activity of more than one antioxidant enzyme in plants increases under different abiotic stress conditions, such as pesticides, salt, etc. (Zaid et al. 2020, 2019; Zaid and Mohammad 2018). This increase in enzymatic antioxidant activity correlates with increased stress tolerance (Heidari et al. 2021). The enzymatic antioxidant activity was differently altered in both the pesticide treatment and control samples. At the lower concentration of pesticide, the SOD activity was elevated in both PM and PTF treated plants, whereas decreased with the pesticide increases. The results are inconsistent with Sharma et al. (2012) that enzymatic antioxidant activity such as SOD, CAT, APX, MDHAR, and GPX increased after chlorpyrifos (CPF) (0.02%, 0.04%, and 0.06%) and 24-epibrassinolide (EBL) (10–11, 10–9 and 10–7 M) treatments alone and in combination with Indica rice variety Pusa Basmati-1. Accumulation of ROS can cause severe oxidative damage as well as toxic molecules found in various subcellular compartments of plants (Ahmad et al. 2019). Detoxification of ROS is a most vital role in any defense mechanism, this is why plants possess a complex antioxidant system that includes non-enzymatic molecules (such as ascorbate, phenols, proline, tocopherols), and antioxidant enzymatic components (such as ascorbate peroxidase, catalase, superoxide dismutase) and components of the ascorbate–glutathione cycle (Apel and Hirt 2004). Therefore, the equilibrium between ROS production and detoxification is sustained by different enzymatic and non-enzymatic antioxidants (Caverzan et al. 2016; Ahmad et al. 2019). The results are also in the same line with Bashir et al. (2007), where the enzymatic antioxidants, activity of SOD, APX and GR increased significantly markedly in relation to increasing concentration of deltamethrin exposed I Glycine max (L.) Merr (soybean). Their results indicated that a higher concentration of deltamethrin produces oxidative damage in the soybean. These changes observed were dose-dependent, showing a strong correlation with the concentration of treatment. Singh and Roy (2017) also studied Allium cepa were treated with different concentration of malathion (50, 125, 250 and 375 ppm) under different periods (3, 9 and 18 h) showed significant (p ≤ 0.05) elevated levels of SOD and CAT than the control sample, while the activity of POD was significantly (p ≤ 0.05) low at 375 ppm. Their finding suggested that antioxidant enzyme activity could serve as the useful indicators for monitoring the effects of malathion exposures in plants. SOD and CAT enzyme play an important role in the antioxidant defense mechanisms under malathion toxicity.
CAT activity had significantly increased in PM treated plants but at PTF treated plants showed decreased activity at the higher concentration of the stress. CAT is considered the main H2O2 scavenging an enzyme is also widely involved in plant immunity from various abiotic stresses (Yan et al. 2021). Therefore, a various pattern of the antioxidant activity was shown in the intermediate concentration. It has been reported that APX plays a vital role in combating various biotic and abiotic stresses in plants (Koussevitzky et al. 2012). In particular, APX is one of the important enzymes in the defense mechanism of plants that can detoxify H2O2 into H2O and O2 using ascorbic acid as an electron donor (Foyer and Noctor 2005). The activity of APX in PM treated plants was gradually increased with increasing concentration of pesticides, whereas PTF treated plants showed gradual increases at 150 ppm and then decreased at 200 ppm compared to the control plant.
APX is also an important enzyme that is involved in the diverse developmental and physiological processes and stress responses by scavenging H2O2 in plants (Xiao et al. 2021). It is considered an important ROS-scavenging enzyme, which catalyzes the removal of H2O2 to prevent oxidative damage (Kaur et al. 2021). At lower concentrations, the activities of SOD, CAT, and APX have gradually elevated in PM-treated plants and were similar to PTF treated plants and decreased the activity at the higher concentration. From the overall results, the activity of SOD showed maximum fold change of expression among the entire enzymatic antioxidant assayed at 100 ppm PM followed by 150 ppm PTF treated eggplant. A similar result was reported by Mishra et al. (2009) on effect of dimethoate and UV-B on antioxidant response of Momordica charantia L. seedling. ROS accumulated considerably in leaves due to high concentration of dimethoate and UV-B. Furthermore, the stresses alone and together also caused the increase activity of SOD, CAT and POD. Thus, high concentration of dimethoate and UV-B accelerated the ROS accumulation particularly H2O2 in leaves, causing heavy damage to photosynthetic pigments and growth of bitter gourd seedlings.
Shakir et al. (2018a) also reported that the excessive application of pesticides, such as emamectin benzoate, alpha-cypermethrin, and imidacloprid, can adversely affect the antioxidant activities of SOD, GR, CAT, POD, APX and proline compared with non-treated plants. At higher doses of pesticide application, a significant enhancement in antioxidant levels was found and their results strongly suggested that the above recommended dose of pesticide application can provoke the state of oxidative stress and can damage in non-target host plants. Wang et al. (2014) reported that the various levels of pesticides such as IPP in plant growth and physiological condition of a wheat plant showed a significantly delayed decrease, whereas the antioxidant activity of SOD, CAT, PO, PPO and MDA was increased. Wu et al. (2010) also reported that the effect of fluroxypyr (0–0.8 mg l−1) triggers oxidative damage in Oryza sativa plants after 6 days of exposure by producing hydrogen peroxide and superoxide radical. The fluroxypyr-induced oxidative stress activated significant changes in activity of SOD, CAT APX and POD. These antioxidant enzymes activities show a general increase at low fluroxypyr concentrations while a decrease at high fluroxypyr levels (except for POD). Wang et al. (2014) also studied that effect of pesticide 1-[6-chloro-3-methyl-pyridyl-8-nitro-7-methyl-1 2 3 5 6 7-hexahydro imidazo (1,2a)]-pyridine (IPP) to wheat plants. At lower dose rate caused stress effects and modified the activities of SOD, CAT, POD and PPO. Thus, different patterns of biomarker responses were observed by an increase in SOD and MDA and differential effects for antioxidant enzymes with a decrease in CAT, POD and PPO.
Gene expression
At the molecular level, the expression profiling of FeSOD, CAT and APX genes was specific to each pesticide PM and PTF concentration was found to be correlated with the equivalent enzyme activity. Our results indicated that the initial concentration of pesticide treatment, the SOD activity were increased with the increasing concentration of PM and PTF exposure. In PM-treated plants, the expression of the FeSOD gene was highly up-regulated in 100 ppm. With the increasing concentration of PTF stress, the expression of FeSOD was observed to be down-regulated at 200 ppm compared to the non-treated plants. The current experiment suggested that the enzyme activity response of eggplant due to PM and PTF treatment could be reflected as a change in gene transcripts. The changes in enzyme activity and FeSOD gene transcript in eggplant under stress are shown (Fig. 1b). Similar results were reported by Sharma et al. (2015) that elite rice variety Pusa Basmati-1 was exposed under different salt and pesticides (chlorpyrifos and imidacloprid) stress condition which results in reduction in lipid peroxidation, reduced ROS accumulation and enhanced the enzyme activity as well as expression of antioxidative defense gene. Seedlings treated with IMI stress manifested a pronounced enhancement in the FeSOD transcript level in comparison to stress conditions alone. In case of APX gene, stress resulted in a selective up-regulation (1.5 fold) under the effect of IMI samples. The expression of GR and CAT was also enhanced under the effect of IMI stress. Their results suggested that increased activity and expression of chloroplastic genes Cu/Zn-SOD, APX and GR under various stresses points toward increased ROS production in chloroplasts under these stresses. Aydin et al. (2014) that activity of SOD in tomato plants could be reflected as alters in gene transcripts, but SOD gene expression patterns revealed no positive correlation with antioxidant activity. Consequently, stressful conditions led to treatment in tomato plants and triggered the levels of the SOD gene expression. The silencing and over-expression of the SOD gene in tomatoes under PEG and NaCl treatment remain to be identified in further studies. The gene expression analysis patterns during PM and PTF treatments illustrated a complex profile. Therefore, the expression level of the FeSOD genes in the treated samples was exposed to 100 ppm PM stress showed the maximum among all the pesticide concentrations. These results are also inconsistent with Wu et al. (2017) that the effects of malathion on activity of total SOD and MnSOD their transcriptional levels in Oxya chinensis showed the highest at the concentration of 0.8 µg µL−1 malathion treatment and elevated significantly about 1.81 and 2.48 fold compared with the control, while CuZnSOD activity has no significant changes after malathion treatments. The increased mRNA expression of ecCuZnSOD1, MnSOD and ecCuZnSOD2 were observed after malathion treatment showed the change of MnSOD transcript was similar to the profiles of MnSOD activity. The up-regulation expression of MnSOD transcript led to the elevate of MnSOD activity to eliminate the excessive ROS caused by malathion. The results suggested that MnSOD exerted a vital role in defense oxidative stress caused by malathion.
During PM treatment, APX activity was elevated gradually at the higher concentration of pesticide treatment, whereas the expression of the APX gene was up-regulated gradually in three concentrations (50, 100, 200 ppm) but it was slightly decreased at 150 ppm. Likewise, in PTF treated eggplant, the APX gene was observed up-regulated, but highly down-regulated at 200 ppm as compared to the control. The CAT activity also increases with an increased concentration in both pesticide treatments, but gene expression of CAT showed gradual up-regulated in all two concentrations (50 and 100 ppm) under PM stress, but it was slightly down-regulated in two concentrations (100 ppm and 200 ppm) of PTF treatment.Harb et al. (2015) also studied the presence of abiotic stress treatment of Rum and Yarmouk genotype, CAT2, SOD, and APX gene was up-regulated after drought stress in Yarmoukk genotype after 2 days. Therefore, under the same drought conditions, each genotype employs various biochemical and molecular responses (Harb et al. 2015). This can be elucidated by Sharma et al. (2013) that the expression of some important antioxidant genes such as FeSOD, Cu/ ZnSOD, MnSOD, CAT, APX and GR were investigated under the various treatments of IMI (imidacloprid) and EBL alone showed an up-regulation in the expression of most of the genes. Though, transcriptional changes for both EBL and IMI treated pusa basmati-1 seedlings indicate their interactions and this could have a potential connection in offering EBL induced IMI stress tolerance in rice.
Conclusions
To improve crop yield under PM and PTF treatments, it is vital to analyze the antioxidant enzyme and gene expression studies to understand the molecular mechanisms of different stress responses in eggplants. Therefore, to reduce the detrimental impacts of pesticide-induced oxidative stress, the results indicated a different antioxidants pattern, where stimulated the activity of SOD, APX and CAT, which was observed to be more efficient in PM than PTF stress. The expression of FeSOD, CAT, and APX genes were observed to have differential expression pattern. The selected antioxidant genes might be induced to stress-resistant capacity and developed several mechanisms to cope with abiotic stress in plant and they contribute in the increase activity of related enzymes activity of eggplant. Therefore, an in-depth mechanistic approach based on molecular breeding and crop improvement of eggplant for tolerance to pesticide stress. The high throughput sequencing method can also be used to expand insight into stress-responsive antioxidant genes.
References
Ahmad B, Zaid A, Sadiq Y, Bashir S, Wani SH (2019) Role of selective exogenous elicitors in plant responses to abiotic stress tolerance. Plant Abiotic Stress Tolerance. https://doi.org/10.1007/978-3-030-06118-0_12
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Ara N, Nakkanong K, Lv W, Yang J, Hu Z, Zhang M (2013) Antioxidant enzymatic activities and gene expression associated with heat tolerance in the stems and roots of two cucurbit species (“Cucurbita maxima” and “Cucurbita moschata”) and their interspecific inbred line “Maxchata”. Int J Mol Sci 14:24008–24028. https://doi.org/10.3390/ijms141224008
Aydin S, Büyük I, Aras ES (2014) Expression of SOD gene and evaluating its role in stress tolerance in NaCl and PEG stressed Lycopersicum esculentum. Turk J Botany 38:89–98. https://doi.org/10.3906/bot-1305-1
Azarabadi S, Abdollahi H, Torabi M, Salehi Z, Nasiri J (2017) ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in response to Erwinia amylovora in pear (Pyrus communis L). Eur J Plant Pathol 147:279–294. https://doi.org/10.1007/s10658-016-1000-0
Bashir F, Mahmooduzzafar STO, Iqbal M (2007) The antioxidative response system in Glycine max (L.) Merr. exposed to Deltamethrin, a synthetic pyrethroid insecticide. Environ Pollut 147:94–100. https://doi.org/10.1016/j.envpol.2006.08.013
Caverzan A, Casassola A, Brammer SP (2016) Antioxidant responses of wheat plants under stress. Genet Mol Biol 39:1–6
Challa N, Singh M, Bharadwaj RK, Sharma R, Gaikwad MB, Thakur P (2021) Characterization of eggplant genotypes for different resistance mechanisms against Leucinodes orbonalis. Neotrop Entomol. https://doi.org/10.1007/s13744-021-00888-w
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875. https://doi.org/10.1105/tpc.105.033589
Gajewska E, Skłodowska M (2007) Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals 20:27–36. https://doi.org/10.1007/s10534-006-9011-5
Gébelin V, Leclercq J, Hu S, Tang C, Montoro P (2013) Regulation of MIR genes in response to abiotic stress in Hevea brasiliensis. Int J Mol Sci 14:19587–19604. https://doi.org/10.3390/ijms141019587
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Harb A, Awad D, Samarah N (2015) Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare l.) under controlled severe drought. J Plant Interact 10:109–116. https://doi.org/10.1080/17429145.2015.1033023
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:1–52. https://doi.org/10.3390/antiox9080681
Hautea DM, Taylo LD, Masanga APL, Sison MLJ, Narciso JO, Quiloy RB, Hautea RA, Shotkoski FA, Shelton AM (2016) Field performance of Bt eggplants (Solanum melongena L.) in the Philippines: Cry1Ac expression and control of the eggplant fruit and shoot borer (Leucinodes orbonalis Guenée). PLoS ONE 11:1–22. https://doi.org/10.1371/journal.pone.0157498
Heidari P, Amerian MR, Barcaccia G (2021) Hormone profiles and antioxidant activity of cultivated and wild tomato seedlings under low-temperature stress. Agronomy 11(1146):1–16. https://doi.org/10.3390/agronomy11061146
Hurtado M, Vilanova S, Plazas M, Gramazio P, Fonseka HH, Fonseka R, Prohens J (2012) Diversity and relationships of eggplants from three geographically distant secondary centers of diversity. PLoS ONE. https://doi.org/10.1371/journal.pone.0041748
Javed H, Mukhtar T, Javed K, Mohsin A (2017) Management of eggplant shoot and fruit borer (Leucinodes orbonalis Guenee) by integrating different non-chemical approaches. Pak J Agric Sci 54:65–70. https://doi.org/10.21162/PAKJAS/17.5282
Kamran M, Parveen A, Ahmar S, Malik Z, Hussain S, Chattha MS, Saleem MH, Adil M, Heidari P, Chen JT (2020) An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int J Mol Sci 21(148):1–27. https://doi.org/10.3390/ijms21010148
Kaur N, Dhawan M, Sharma I, Pati PK (2016) Interdependency of reactive oxygen species generating and scavenging system in salt sensitive and salt tolerant cultivars of rice. BMC Plant Biol 16:1–13. https://doi.org/10.1186/s12870-016-0824-2
Kaur S, Prakash P, Bak D, Hong SH, Cho C, Chung M, Kim J, Lee S, Bai H, Lee SY, Chung BY, Lee SS (2021) Regulation of dual activity of ascorbate peroxidase 1 From Arabidopsis thaliana by conformational changes and posttranslational modifications. Front Plant Sci 14(12):1–12. https://doi.org/10.3389/fpls.2021.678111
Kumar A, Sharma V, Jain BT, Kaushik P (2020) Heterosis breeding in eggplant (Solanum melongena L.): gains and provocations. Plants 9:1–16. https://doi.org/10.3390/plants9030403
Li H, Luo H, Li D, Hu T, Fu J (2012) Antioxidant enzyme activity and gene expression in response to lead stress in perennial ryegrass. J Am Soc Hortic Sci 137:80–85. https://doi.org/10.21273/jashs.137.2.80
Mishra V, Srivastava G, Prasad SM (2009) Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UV-B irradiation. Sci Hortic (amsterdam) 120:373–378. https://doi.org/10.1016/j.scienta.2008.11.024
Quamruzzaman AKM, Khatun A, Islam F (2020) Nutritional content and health benefits of bangladeshi eggplant ccultivars. Eur J Agric Food Sci 2:1–7. https://doi.org/10.24018/ejfood.2020.2.4.76
Rai AB (2015) Integrated pest management of vegetable crops. Improv Prod Technol Veg Crop 59:150–169
Rotino GL, Perri E, Acciarri N, Sunseri F, Arpaia S (1997) Development of eggplant varietal resistance to insects and diseases via plant breeding. Adv Hortic Sci 11:193–201
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning 2 cold. Springer
Sękara A, Cebula S, Kunicki E (2007) Cultivated eggplants – origin, breeding objectives and genetic resources, a review. World 19:97–114
Semida WM, Abdelkhalik A, Mohamed GF, El-Mageed TAA, El-Mageed SAA, Rady MM, Ali EF (2021) Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 10(421):1–17. https://doi.org/10.3390/plants10020421
Shakir SK, Irfan S, Akhtar B, Rehman S, Daud MK, Taimur N, Azizullah A (2018) Pesticide-induced oxidative stress and antioxidant responses in tomato (Solanum lycopersicum) seedlings. Ecotoxicology 27:919–935
Sharma I, Bhardwaj R, Pati PK (2012) Mitigation of adverse effects of chlorpyrifos by 24-epibrassinolide and analysis of stress markers in a rice variety Pusa Basmati-1. Ecotoxicol Environ Saf 85:72–81. https://doi.org/10.1016/j.ecoenv.2012.07.003
Sharma I, Bhardwaj R, Pati PK (2013) Stress modulation response of 24-epibrassinolide against imidacloprid in an elite indica rice variety Pusa Basmati-1. Pestic Biochem Physiol 105:144–153. https://doi.org/10.1016/j.pestbp.2013.01.004
Sharma I, Bhardwaj R, Pati PK (2015) Exogenous application of 28-homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety pusa basmati-1. J Plant Growth Regul 34:509–518. https://doi.org/10.1007/s00344-015-9486-9
Singh D, Roy BK (2017) Evaluation of malathion-induced cytogenetical effects and oxidative stress in plants using Allium test. Acta Physiol Plant. https://doi.org/10.1007/s11738-017-2391-z
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270. https://doi.org/10.1111/j.1365-3040.2011.02336.x
Taher D, Wu T, Prohens J, Chou Y, Rakha M, Wu T (2017) World vegetable center eggplant collection: origin, composition, seed dissemination and utilization in breeding. Front Plant Sci 8:1–12. https://doi.org/10.3389/fpls.2017.01484
Vijayanandraj S, Brinda R, Kannan K, Adhithya R, Vinothini S, Senthil K, Chinta RR, Paranidharan V, Velazhahan R (2014) Detoxification of aflatoxin B1 by an aqueous extract from leaves of Adhatoda vasica Nees. Microbiol Res 169:294–300. https://doi.org/10.1016/j.micres.2013.07.008
Vuleta A, Manitašević Jovanović S, Tucić B (2016) Adaptive flexibility of enzymatic antioxidants SOD, APX and CAT to high light stress: the clonal perennial monocot Iris pumila as a study case. Plant Physiol Biochem 100:166–173. https://doi.org/10.1016/j.plaphy.2016.01.011
Wang P, Yang X, Huang WW, Zhang M, Lu WH, Zhao HT, Wang J, Liu HL, Dong AJ, Zhang H, Xu RB, Zou P, Cheng CL, Zhang YC, Jing J (2014) Effect of pesticide 1-[6-chloro-3-methyl-pyridyl-8-nitro-7-methyl-1 2 3 5 6 7-hexahydro imidazo (1, 2a)]-pyridine when responding to a wheat plant’s antioxidant defense system. Food Chem 146:569–576
Wani W, Masoodi KZ, Zaid A, Wani SH, Shah F, Meena VJ, Wani SA, Mosa KA (2018) Engineering plants for heavy metal stress tolerance. Rend Lincei 29:709–723. https://doi.org/10.1007/s12210-018-0702-y
War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306–1320. https://doi.org/10.4161/psb.21663
Wu GL, Cui J, Tao L, Yang H (2010) Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 19:124–132. https://doi.org/10.1007/s10646-009-0396-0
Wu H, Zhang Y, Shi X, Zhang J, Ma E (2017) Chemosphere overexpression of Mn-superoxide dismutase in Oxya chinensis mediates increased malathion tolerance. Chemosphere 181:352–359. https://doi.org/10.1016/j.chemosphere.2017.04.087
Xiao L, Jiang G, Yan H, Lai H, Su X, Jiang Y, Duan X (2021) Methionine sulfoxide reductase b regulates the activity of ascorbate peroxidase of banana fruit. Antioxidants 10:1–18. https://doi.org/10.3390/antiox10020310
Yan Y, Wang P, Wei Y, Bai Y, Lu Y, Zeng H, Liu G, Reiter RJ, He C, Shi H (2021) The dual interplay of RAV5 in activating nitrate reductases and repressing catalase activity to improve disease resistance in cassava. Plant Biotechnol J 19:785–800. https://doi.org/10.1111/pbi.13505
Zaid A, Mohammad F (2018) Methyl jasmonate and nitrogen interact to alleviate cadmium stress in mentha arvensis by regulating physio-biochemical damages and ROS detoxification. J Plant Growth Regul 37:1331–1348. https://doi.org/10.1007/s00344-018-9854-3
Zaid A, Wani SH (2019) Reactive oxygen species generation, scavenging and signaling in plant defense responses. Bioactive Molecules in Plant Defense. Springer, pp 111–132
Zaid A, Mohammad F, Wani SH, Siddique KMH (2019) Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. Ecotoxicol Environ Saf 180:575–587. https://doi.org/10.1016/j.ecoenv.2019.05.042
Zaid A, Mohammad F, Fariduddin Q (2020) Plant growth regulators improve growth, photosynthesis, mineral nutrient and antioxidant system under cadmium stress in menthol mint (Mentha arvensis L.). Physiol Mol Biol Plants 26:25–39. https://doi.org/10.1007/s12298-019-00715-y
Zaid A, Ahmad B, Wani SH (2021) Medicinal and aromatic plants under abiotic stress: a crosstalk on phytohormones’ perspective. Plant Growth Regul. https://doi.org/10.1007/978-3-030-61153-8_5
Acknowledgements
PY acknowledge Plant Molecular Biology and Biotechnology Lab, Assam University, Silchar, Assam, India for providing infrastructural facility to carry out the present work and farmers of two villages, Ambarkhana and Darakuna, Karimganj district of Assam, India, for providing all the necessary seed throughout the experiment.
Author information
Authors and Affiliations
Contributions
PBM and PY designed the experiment. PY produced the Solanum melongena L.cv. Longai seeds, analyzed the data, and drafted the manuscript. PBM supervised the research study. All authors have read and approved manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Yengkokpam, P., Mazumder, P.B. Antioxidant enzymatic activities and profiling of gene expression associated with organophosphate stress tolerance in Solanum melongena L.cv. Longai. 3 Biotech 11, 510 (2021). https://doi.org/10.1007/s13205-021-03061-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-03061-7