Abstract
In this research, optimization of the production medium to enhance tannase production by Bacillus gottheilii M2S2 in laboratory-scale packed bed reactor was studied. Amount of substrate Triphala, moisture content, aeration rate, and fermentation period was chosen for optimization study. During one variable at a time optimization, the highest tannase activity of 0.226 U/gds was shown with Triphala as a substrate at the fermentation period of 32 h. Furthermore, the optimum conditions predicted by response surface methodology (RSM) and genetic algorithm (GA) were found to be 11.532 g of substrate Triphala, 47.071% of the moisture content, and 1.188 L/min of an aeration rate with uppermost tannase activity of 0.262 U/gds. In addition, the single hidden layer feedforward neural network (SLFNN) and the radial basis function neural network (RBFNN) of an artificial neural network (ANN) were adopted to compare the prediction performances of the RSM and GA. It revealed that the ANN models (SLFNN, R2 = 0.9930; and RBFNN, R2 = 0.9949) were better predictors than the RSM (R2 = 0.9864). Finally, the validation experiment exhibited 0.265 U/gds of tannase activity at the optimized conditions, which is an 11-fold increase compared to unoptimized media in shake flask.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agricultural residues have been found to be feasible substrates for the production of value-added industrial enzymes (Pandey et al. 2001). Triphala is an agricultural residue which generally consists of equal proportions of three myrobalans, i.e., Emblica officinalis, Terminalia bellirica, and Terminalia chebula. The composition of tannic acid in these different solid substrates reported in the literature was (% w/w): E. officinalis, 28; T. bellirica, 17; and T. chebula, 30 (Bali et al. 2013). This mixed substrate Triphala has been used in our previous study for the production of a tannase (Subbalaxmi and Vytla 2017a, b), whereas individual substrates T. chebula and E. officinalis have been used independently for the synthesis of tannase (Prasanna et al. 2012; Selwal et al. 2010).
Tannin acyl hydrolase (E.C. 3.1.1.20) is commonly known as tannase, which is one of the most important industrial enzymes which acts on tannic acid and hydrolyzes to glucose and gallic acid. Tannase have found various applications in different industrial sectors such as food (fruit and vegetable juice clarification, deprivation of plant phenolics, and preparation of coffee flavor cold drinks and instant tea), chemical (treatment of effluents of textile and tannery), pharmaceutical (the products of tannase gallic acid and propyl gallate used in the synthesis of an antibacterial drug called trimethoprim and antioxidants, respectively), and beverage (removal of chill haze formation in the preparation of beer and wine) industries (Aithal and Belur 2013; Aguilar et al. 2007; Rout and Banerjee 2006).
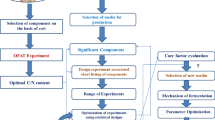
A variety of bacteria are capable of producing enzyme tannase. Bacillus species are well known for their efficient production of a varied range of industrial microbial enzymes (Pandey et al. 2001). A wide variety of agricultural residues have been previously studied in both submerged fermentation (SmF) and solid-state fermentation (SSF) processes for economic production of industrial products. Among these processes, SSF has been found to be the most suitable system for the exploitation of agricultural residues in the synthesis of industrially important enzymes (Pandey et al. 2001). The SSF process of agricultural residues brings about commercial and manufacturing advantages over SmF. These consist of a high yield of product, cost-effective upstream and downstream processes, the minimum amount of effluent generation, and lower capital investment (Pandey et al. 2000). Culture conditions such as moisture content, inoculum size, temperature, and aeration rate significantly affect the SSF process, as well as consecutively affect the fermentation products (Pandey et al. 1999). Though quite a lot of studies have been carried out using synthetic tannic acids in the production of tannase by means of SmF process (Mukesh et al. 2015; Vikas et al. 2013), whereas limited attempts have been made to produce tannase using agricultural residues as substrate in the SSF process (Natarajan and Rajendran 2012; Sabu et al. 2006). Then again, most of the research on bacterial tannase production under SSF has been carried in shake flasks, and very little study has been made in the bioreactors for the production of tannase in SSF (Mata-Gómez et al. 2015; Rodriguez-Duran et al. 2011; Sabu et al. 2006). To the best of the authors’ knowledge, no reports on tannase production by Bacillus gottheilii M2S2 in packed bed reactors using Triphala as a substrate have been published. The present study reports on the bioconversion of Triphala a cost-effective tannin substrate for the production of tannase in laboratory-scale packed bed reactor by B. gottheilii M2S2. In this study, one variable at a time and response surface methodology (RSM) techniques were used to study the interaction effects of three independent variables, including the initial moisture content, inoculum volume, and the aeration rate on tannase production, and to determine the most favorable levels of these variables to achieve the maximum yield of tannase. In addition, the optimization tool, genetic algorithm (GA), was used with RSM to predict and validate the process conditions for the production of tannase in PBR. Furthermore, two types of artificial neural network (ANN) models are also implemented to predict the tannase activity for input variable values not used in the experimentation.
Materials and methods
Reagents
All the chemicals and reagents used were of analytical grade and were procured from Hi-Media and Merck.
Culture conditions and inoculum preparation
The isolated strain B. gottheilii M2S2 (MTCC 12554 and Accession number no. KU866380) from the tannery effluent soil sample as described in our previous report (Subbalaxmi and Vytla 2016) is used in this study and was maintained on nutrient agar slants and preserved at 4 °C.
The inoculum of B. gottheilii M2S2 was developed in a 50 mL nutrient broth and incubated at 32 °C for 20 h. It was established that the total number of viable cell count was 4 × 1012 CFU/mL by means of the colony count technique (Subbalaxmi and Vytla 2016).
Solid substrate
An inexpensive crude tannin substrate Triphala was obtained from the native market place in Manipal, India. The formulation of Triphala generally consists of equal proportions of pericarps of the three myrobalans: T. bellirica, E. officinalis, and T. chebula (Bali et al. 2013).
Estimation of tannins: qualitative and quantitative methods
The presence of tannins in Triphala was quantified qualitatively by taking a sample of 0.5 g and distilled water of 20 mL in a test tube and boiled for 10 min. Further with the Whatman filter, the mixture was filtered and to the filtrate, few drops of 0.1% FeCl3 were added, and the color change was observed. Development of blue–black or brownish green color points out the existence of tannins Fig. 1 (Evans and Trease 1989).
Protein precipitation method was adopted to quantify the tannin content in crude substrate Triphala (Ann-Hagerman and Larry-Butler 1978). The protein bovine serum albumin precipitates tannins present in Triphala and forms tannin-protein complex. This precipitate is dissolved in sodium dodecyl sulfate-triethanolamine, and spectrophotometrically, the colored solution formed with ferric chloride reagent was measured. It was observed that the Triphala consisted of 7% (w/v) of tannic acid.
Moistening media
A mineral solution containing (% w/v) tannic acid, 1.9; sucrose, 0.5; NH4NO3, 0.5; KH2PO4, 0.1; MgSO4, 0.1; CaCl2·2H2O, 0.05; and NaCl, 0.1 having a pH of 5.0.
Experimental setup and solid-state fermentation
The reactor is of 20 cm height with 2.5 cm internal diameter, made up of glass, and a rubber cork with a 0.3 cm diameter hovel was placed at the bottom of the reactor to support glass beads, an air passage, and substrate Triphala. Further for the uniform distribution of air, the reactor was filled with glass beads up to 5 cm in length (Fig. 2). The production medium comprised of Triphala moistened with an optimized mineral solution and it was prepared in the reactor itself by adjusting to the required moisture content as described by Shaligram et al. (2008) and sterilized in an autoclave at 121 °C for 20 min at 15 psi. After sterilization, it was inoculated aseptically with 1 mL of inoculum with 5 g Triphala powder. The airflow through the reactor was the first filter sterilized using a sterile polytetrafluoroethylene membrane filter of size 0.2 µm (Millipore, USA) and its flow was controlled with the control valve. Furthermore, the filtered air was humidified by passing them to the humidification bottle and the resultant humidified air move into the PBR at the bottom and go out at the top. The PBR was kept for incubation for 32 h at 32 °C. To each experiment, the air flow rate, amount of substrate, and moisture content were adjusted to levels as shown in the experimental design matrix (Table 2). The air flow rate of various levels was set by following the principle of water displacement method as described elsewhere (Subbalaxmi and Vytla 2018; Derakhti et al. 2012; Qureshi et al. 2005):
Cell-free extract
After fermentation, 1 g of the fermented substrate was taken and cell-free supernatant was extracted by adding 20 mL of 0.05 M citrate buffer (pH 5.0). The flasks were then kept in a rotatory shaker for 15 min at 180 rpm and the cells were separated by cold centrifuged at 8500g and 4 °C for 15 min. The cell-free extract was collected in ampoules and well preserved for further examination. Experimental tests were carried out independently in duplicates and tannase activities were determined.
Tannase activity assay
The enzyme tannase was quantified using the spectrophotometric method with substrate methyl gallate as described elsewhere (Sharma et al. 2000). One unit of tannase is defined as one micromole of gallic acid formed per minute, under experimental conditions.
Experimental design
Screening of significant parameters by one variable at a time technique
Optimum physicochemical and nutrient parameters required for maximum tannase production from B. gottheilii M2S2 in PBR using Triphala as substrate were determined for the initial moisture content (40–70%), amount of substrate (5–25 g), and aeration rate (0–2 L/min). The protocol adopted for optimization of process parameters was to evaluate the effect of an individual parameter and to incorporate it at the optimized level in the experiment before optimizing the next parameter. After optimizing all parameters, a time-course experiment was conducted incorporating all the optimized parameters. All experiments were carried out in duplicate and the mean values were reported with standard deviation.
Optimization of critical parameters using central composite design
A 23 factorial central composite experimental design with four start points (α = 2) and six replicates at the central point, resulting in 20 experiments were used to optimize the screened variables (Minitab 17.0). The screened variables with experimental range and levels were used in CCD for tannase production from B. gottheilii M2S2, which are shown in Table 1, and the experimental designs for tannase production using PBR are shown in Table 2.
Statistical model
The linear, quadratic, and linear interactions of each parameter with their regression coefficients were fitted to a second-order polynomial equation (Eq. 1):
where Z is the predicted tannase activity (response); λ0 is the intercept; λi and λii are the linear and quadratic coefficients for the variable i, respectively, whereas λij is the interaction coefficients between variables i and j. The fitness of the second-order polynomial model equation was tested based on the coefficient of determination R2 and F test from analysis of variance (ANOVA). This model equation can be validated by carrying out the SSF process with Triphala in duplicates at optimal conditions in PBR and then quantifying the enzyme tannase (Subbalaxmi and Vytla 2016).
Optimization by genetic algorithm (GA)
Genetic algorithms are a type of optimization algorithm, used to find the optimal solution(s) to a given computational problem that maximizes or minimizes a particular function. GA represents one branch of the field of study called evolutionary computation in that they imitate the biological processes of reproduction and natural selection to solve for the ‘fittest’ solutions (Kinnear 1994; Goldberg 1989). Like in evolution, many of genetic algorithm’s processes are random; however, this optimization technique allows one to set the level of randomization and the level of control (Goldberg 1989). These algorithms are far more powerful and efficient than random search and exhaustive search algorithms (Kinnear 1994); yet require no extra information about the given problem. This feature allows them to find solutions to problems that other optimization methods cannot handle due to a lack of continuity, derivatives, linearity, or other features. In this study, the second-order polynomial equation obtained from the RSM was used as the fitness function to carry out GA. For the production of tannase, the functions selected to carry out the GA are rank scaling function, constrain-dependent uniform creation function, scattered crossover function, stochastic uniform selection function, and non-linear constraint algorithm (Augmented Lagrangian). The parameters used in the computational optimization by GA were chromosome length (50), population size (50), crossover fraction (0.8), and the number of generations (100). The optimization toolbox of MATLAB R2015b was used for the GA studies. The objective function can be given as follows:
where \( f\left( y \right) \) is the objective function obtained from the RSM studies, y is an input variable, and z represents the response obtained. The symbols \( y_{i}^{\text{L}} \) and \( y_{i}^{\text{U}} \) designate the upper and lower levels of \( y_{i}^{\text{U}} \).
Artificial neural networks
Artificial neural networks (ANNs) are a class of machine learning techniques which are capable of mapping between sets of non-linear input and output variables. The use of supervised feedforward ANNs is popular in fitting an equation for the case of non-linear mapping between multiple input–output pairs. Thus, they can be used to predict the output of a system for a given set of new inputs. The ANN can be implemented in two stages—training and testing. In the training phase, the biases and the weights on the synaptic connections are initially set to very small random values. The forward pass of training essentially consists of propagating the input data for training from the input layer through the hidden layer to the output layer. The ANN outputs are obtained at the output layer, which is not the same as the desired outputs. In the backward pass of training, the training error (the difference between ANN output and desired output) is back-propagated in such a way that the synaptic weights and biases are updated (modified). One forward and backward pass constitutes an epoch. The epochs are repeated in an iterative way during which the training error continuously decreases. After the completion of a number of epochs, the training error attains a very small value, and the ANN is said to be trained. In the testing phase, the inputs not used in training are provided to the trained ANN. The corresponding ANN output obtained is found to be similar to the desired outputs (Haykin 1999; Anil 1996).
For the work carried out in this article, amounts of Triphala (X1), moisture content (X2), and the aeration rate (X3) are considered as the ANN inputs, and the Tannase activity (t) acts as the output. 20 pairs of input–output values are available for training. Thus, the number of nodes in the input layer is three and that at the output layer is one. Figure 3 shows the schematic of the ANN used for modeling the Tannase production experiment. aj (j = 1 to L) are the biases of the hidden layer. wji (i = 1 to 3; j = 1 to L) are the weights on the synaptic connections between the nodes of the input layer and the hidden layer. bk is the biases of the output layer and vjk are the weights on the synaptic connections between the nodes of the hidden layer and the output layer. O1 is the output of the ANN obtained from the single node of the output layer.
The output of the jth node of the hidden layer is given by the following equation:
Each hidden node output hj is passed through a hyperbolic tangent sigmoid transfer function to get the transfer function output as yj:
The output of the single node of the output layer is given as follows:
The performance of the ANN is measured in terms of the prediction accuracy, mean relative error, and the R2 coefficient of regression value. These parameters are computed using the ANN output (O) and the desired output (t).
The percentage mean relative error (MRE) for training and testing are computed using the following equations:
The prediction accuracies of the ANN on the training and the test data have been computed using the following equations:
where qTrg is the number of training pairs that are mispredicted out of a total number of QTrg training pairs, and qTst is the number of test pairs mispredicted out of a total number of QTst test pairs. For a given pair of input–output, if the MRE between the ANN output and the desired output, for any output node is greater than ± 5, it is considered as a misprediction.
To know how best the model has fit a curve to the data under consideration, the statistical parameter R2 given in Eq. (10) is used:
where SST is the sum squared total and SSE is the sum squared error (Montgomery 2005).
In this work, two types of ANNs are used, viz., single hidden layer feedforward neural network (SLFNN) and the radial basis function neural network (RBFNN). The configurations of both SLFNN and RBFNN are the same, except that, in an RBFNN, the nodes of the hidden layer are replaced by RBF units. Each RBF unit is specified by its center and width (spread parameter). Both the ANNs are implemented using the MATLAB Deep Learning Toolbox (Beale et al. 2018).
Results and discussion
Screening of significant parameters by one variable at a time technique
The influence of moisture content
The maximum tannase activity of 0.176 ± 0.01 U/gds was exhibited by B. gottheilii M2S2 at an optimum moisture content of 50%, and whereas other parameters such as aeration rate, amount of substrate, and fermentation time were maintained at 1 L/min, 10 g, and 32 h, respectively, as shown in Fig. 4a. Away from 50% moisture content, the tannase activities were found to be decreased. The enzyme production and growth of microorganism majorly depends on the moisture content of the substrate bed (Pandey et al. 1999). Fungi require widespread series of moisture content say 20–70% to support enhanced growth and metabolism, whereas bacteria can show higher productivity simply at a higher moisture content. However, in this study, at 60 and 70% moisture content, the tannase activities were observed to be decreased, which could be because the substrate Triphala was compacted and due to an external aeration of 1 L/min for the solid bed was not fixed at the place and moved towards the bottom of the packed bed reactor over the time of fermentation time. At 40% moisture content, the entire solid bed was not soaked with required mineral solution thereby showed decreased tannase activity and oxygen penetration. Hence, in PBR, maintaining an optimum moisture content plays an important role.
The influence of the amount of substrate Triphala
The effect of substrate Triphala was studied in PBR in a different range from 5 to 25 g at 1 L/min of aeration rate, 50% of the moisture content, and 32 h of fermentation time. As the amount of substrate Triphala increased, the tannase activity has also been increased and an optimum amount of substrate was found to be 15 g with tannase activity of 0.195 ± 0.006 U/gds (Fig. 4b). Whereas at higher substrates of 20 and 25 g, the tannase activity was found to be decreased; the reason could be that the increased bed height in the PBR led to the inadequate distribution of moistening media and air flow, thereby resulting in incomplete hydrolysis of substrate Triphala (Kar et al. 1999). Hence, in PBR, sustaining an optimum amount of substrate plays a significant role in the tannase production.
The influence of aeration rate
The influence of aeration rate was understood by carrying out the experiments in the different range from 0.5 to 2 L/min at 50% moisture content, 15 g substrate Triphala, and 32 h of fermentation time. The maximum tannase activity of 0.238 ± 0.016 U/gds was shown at 1.2 L/min (Fig. 4c), and beyond this point, there was a decline in activity which was noticed. The reason may possibly be that the air flow through the substrate bed has vaporized moisture during the course of fermentation (Derakhti et al. 2012).
The influence of fermentation time
At last, to understand the combined effect of exogenous variables such as moisture content, aeration rate, and amount of substrate Triphala, a time-course study was carried out at their optimum levels. The experiments were conducted including all the optimized variables. The maximum tannase activity of 0.226 ± 0.015 U/gds was exhibited at 32 h (Fig. 4d). Thereafter, the decrease in tannase activity was noticed may be due to moisture loss producing an unfavorable environment for growth and metabolism. An additional reason may possibly be the tannase which further builds glucose and gallic acid available to the microorganism. Gallic acid released over time can also inhibit the tannase action competitively, since tannic acid is composed of gallic acid units (Kumar et al. 1999; Kar et al. 1999).
Statistical optimization of significant parameters using central composite design
The significant parameters such as moisture content, aeration rate, and amount of substrate Triphala were further optimized using a statistical central composite design (CCD) with 20 different experimental runs for tannase production in PBR by B. gottheilii M2S2. The experimental design matrix with coded and real values of the above-mentioned variables is shown in Tables 1 and 2 with tannase activities. This study showed a varied range of tannase activities from 0.051 ± 0.005 U/gds (Run 6) to 0.258 ± 0.031 U/gds (Run 19) by B. gottheilii M2S2. The results of the experimental design matrix were fitted with a second-order and polynomial model as a function of three parameters with coded values and are shown as Eq. (1) for tannase production in PBR:
where Y is tannase activity (U/gds); (X1) amount of substrate Triphala, g; (X2) moisture content, %; (X3) aeration rate, L/min.
The results of the statistical analysis of variance (ANOVA) obtained in the present study for the production of tannase from B. gottheilii M2S2 with Triphala are shown in Table 3. The results are in good agreement with the general facts of higher F value, predicted 2 values, and lower PRESS values which specify a better fit. P values < 0.05 indicate that the model terms were significant. In this study, all the linear, square and interactive terms of X1, X2, and X3 were significant for tannase production from B. gottheilii M2S2 in PBR (Table 3).
The three-dimensional surface plots have been used to visualize the interaction effects among individual parameter on tannase production by B. gottheilii M2S2 (Fig. 5). The surface plot of the parameters such as the amount of substrate Triphala, moisture content, and aeration rate indicated the prominent interaction and as maximum tannase activities at their hold values. The same phenomena are numerically shown in Table 3 ( < 0.05: the presence of interaction and > 0.05: no interaction). The regression model was solved for maximum tannase production using the response optimizer tool in MINITAB 17.0 and the optimal levels of individual parameter in real units were as follows: amount of substrate Triphala = 11.465 g, moisture content = 47.071%, and aeration rate = 1.188 L/min; all of them were found within the experimental levels. The predicted tannase activity under these optimal environments was 0.262 U/gds.
Surface plots showing the interaction effects of variables on tannase production by B. gottheilii M2S2 with Triphala in PBR with the remaining factors held constant at the middle level of the CCD. a Amount of Triphala and moisture content, b amount of Triphala and aeration rate, and c moisture content and aeration rate
To validate the results, experiments were done in duplicates at the optimized values as mentioned above. Under these optimized conditions, 0.262 U/gds and 0.265 U/gds were the predicted and experimental values of tannase activities, respectively. The good correlation between the predicted observed values approves the competence of the model. This two-step optimization approach led to the improvement in tannase production by B. gottheilii M2S2 from 0.029 U/gds (unoptimized medium, flask scale) to 0.265 U/gds (optimized medium, PBR), an 11-fold increase. This value is comparatively higher than reported for different bacterial strains (Subbalaxmi and Vytla 2017a, b; Prasanna et al. 2009, 2010, 2012).
To the best of our knowledge, no report on bacterial tannase production in packed bed reactors using Triphala as crude tannin substrate under SSF has been published. Several researchers have used the laboratory-scale packed bed reactors to study the production of various industrial enzymes. Couto et al. (2000) reported the production of ligninolytic enzyme and decolorization of dye Poly R-478 from Phanerochaete chrysosporium BKM-F-1767 in packed bed reactor with PUF. Aeration was supplied to the reactor at 0.5 vvm and exhibited maximum lignin peroxidase of 197 U/L on the 7th day of fermentation and whereas 30% biological degradation of dye was observed. Abdeshahian et al. (2010a, b) reported β-mannanase and β-glucosidase production in a glass column reactor with palm kernel cake as a substrate from Aspergillus niger FTCC 5003. They used central composite design to optimize the culture conditions, and therefore, maximum β-mannanase and β-glucosidase activities of 2117.89 U/g and 52.06 U/g were obtained, respectively. Moreira et al. (1997) reported the continuous production of manganese peroxidase production in a packed bed reactor with PUF from P. chrysosporium. Variables such as nutrient feed rates, manganese concentration, use of air or oxygen, hydraulic retention time, and recycling flow were optimized for production of manganese peroxidase and thereby showed the maximum activity of 250 U/L. The success of the production process in a packed bed reactor majorly depends upon the correct choice of the solid medium. However, very limited studies have been carried out with packed bed reactor for the production of tannase and were predominantly from fungal source with inert support PUF (Mata-Gómez et al. 2015; Rodriguez-Duran et al. 2011; Van de Lagemaat and Pyle 2001, 2004).
Optimization and validation based on GA
GA produces global results, whereas local results by RSM. To get the global results, optimization based on GA was repeatedly carried out numerous times for accuracy. The optimal conditions identified by GA based on RSM was found to be 11.532 g of substrate Triphala, 47.071% moisture content, and 1.188 L/min aeration rate. The tannase yield (U/g) under optimized conditions determined by GA based on RSM was 0.263 U/g, which was almost similar to 0.262 which was predicted by RSM under optimal conditions. The optimal results obtained by both GA and RSM were found to be comparable, and hence, the model proposed (Eq. 11) can be used to predict the tannase production for given conditions. The best fitness value and the corresponding best individual are shown in Fig. 6. The negative sign in the best fitness plot was because of the inclusion of a negative sign on the regression equation. Moisture content was found to be the best individual in GA. To confirm the predicted result, a validation experiment was carried out under optimized conditions predicted by RSM-GA model. It exhibited a tannase activity of 0.265 U/g.
Comparison of the RSM and ANN models
Table 4 shows the values of the tannase production obtained from an experiment in the third column and the values predicted by the RSM model, SLFNN model, and the RBFNN model, respectively, in the fourth, fifth, and sixth columns. It can be observed that the prediction of the ANN models is relatively closer to the experimental values. However, the prediction performances of the three models can be statistically compared by computing their prediction performance parameters given in Eqs. (6), (7), (8), (9), and (10). The computed values are shown in Table 5.
It is clear from Table 5 that the prediction performance of the SLFNN is superior to those of RBFNN and RSM. It can also be seen that the RBFNN performs better than the RSM model. Figure 7a–c shows the residual chart for the RSM, RBFNN, and SLFNN models, respectively. Again, it is clear from the charts that the SLFNN model has the least residuals.
Conclusions
The results confirmed the inexpensive agricultural residue Triphala as a prominent substrate for production of tannase. The optimized and validated models had good agreement with each other. The critical values of process parameters predicted by RSM and GA were found to be 11.532 g of substrate Triphala, 47.071% moisture content, and 1.188 L/min aeration rate with highest tannase activity of 0.265 U/gds. In addition, a comparison of the prediction performances of the RSM and the ANN models SLFNN and RBFNN reveals that the ANN models (R2 = 0.9949) are better predictors than the RSM (R2 = 0.9864), and, furthermore, to understand the behavior of the strain, need to carry out the kinetic studies, and develop a suitable kinetic model.
References
Abdeshahian P, Samat N, Hamid AA et al (2010a) Utilization of palm kernel cake for production of β-mannanase by Aspergillus niger FTCC 5003 in solid substrate fermentation using an aerated column bioreactor. J Ind Microbiol Biotechnol 37(1):103
Abdeshahian P, Samat N, Wan Y (2010b) Utilization of palm kernel cake for production of β-glucosidase by Aspergillus niger FTCC 5003 in solid substrate fermentation using an aerated column bioreactor. Biotechnology 9(1):17–24
Aguilar CN, Rodriguez R, Gutierrez-Sanchez G, Augur C, Favela-Torres E (2007) Microbial tannases: advances and perspectives. Appl Microbiol Biotechnol 76:47–59
Aithal M, Belur PD (2013) Enhancement of propyl gallate yield in nonaqueous medium using novel cell-associated tannase of Bacillus massiliensis. Prep Biochem Biotechnol 43(5):445–455
Anil KJ (1996) Artificial neural networks: a tutorial. IEEE Xplore, pp 31–44
Ann-Hagerman E, Larry-Butler G (1978) Protein precipitation method for the quantitative determination of tannins. J Agric Food Chem 26(4):809–812
Bali C, Ramesh CK, Mita K, Rammurty A, Summit N (2013) Triphala: a comprehensive ayurvedic review. Int J Res Ayurveda Pharm 4(4):612–617
Beale MH, Martin T, Hagan HB (2018) Deep learning toolbox™, user’s guide. The MathWorks Inc, Natick
Couto SR, Rivela I, Munoz MR, Sanromán A (2000) Ligninolytic enzyme production and the ability of decolourisation of Poly R-478 in packed-bed bioreactors by Phanerochaete chrysosporium. Bioprocess Eng 23(3):287–293
Derakhti S, Shojaosadati SA, Hashemi M et al (2012) Process parameters study of α-amylase production in a packed bed bioreactor under solid state fermentation with possibility of temperature monitoring. Prep Biochem Biotechnol 42(3):203–216
Evans WC, Trease GE (1989) Phamacognosy, 16th edn. Bailere Traiadal, Elsevier, London
Goldberg DE (1989) Genetic algorithms in search, optimization, and machine learning. Addison-Wesley, Reading
Haykin S (1999) Neural networks—a comprehensive foundation, 2nd edn. Prentice Hall, Delhi
Kar B, Banerjee R, Bhattacharyya BC (1999) Microbial production of gallic acid by modified solid state fermentation. J Ind Microbiol Biotechnol 23(3):173–177
Kinnear KE (1994) A perspective on the work in this book. In: Kinnear KE (ed) Advances in genetic programming. MIT Press, Cambridge, pp 3–17
Kumar RA, Gunasekaran P, Lakshmanan M (1999) Biodegradation of tannic acid by Citrobacter freundii isolated from a tannery effluent. J Basic Microbiol 39(3):161–168
Mata-Gómez M, Mussatto SI, Rodríguez R, Teixeira JA, Martinez JL, Hernandez A, Aguilar CN (2015) Gallic acid production with mouldy polyurethane particles obtained from solid state culture of Aspergillus niger GH1. Appl Biochem Biotechnol 176(4):1131–1140
Montgomery DC (2005) Design and analysis of experiments. John Wiley and Sons, New York
Moreira MT, Feijoo G, Palma C, Lema JM (1997) Continuous production of manganese peroxidase by Phanerochaete chrysosporium immobilized on polyurethane foam in a pulsed packed-bed bioreactor. Biotechnol Bioeng 56(2):130–137
Mukesh K, Shiny R, Vikas B, Raj KS (2015) Optimization of tannase production by a novel Klebsiella pneumonia KP715242 using central composite design. Biotechnol Rep 7:128–134
Natarajan K, Rajendran A (2012) Evaluation and optimization of food-grade tannin acyl hydrolase production by a probiotic Lactobacillus plantarum strain in submerged and solid state fermentation. Food Bioprod Process 90(4):780–792
Pandey A, Selvakumar P, Soccol CR, Nigam P (1999) Solid-state fermentation for the production of industrial enzymes. Curr Sci 77:149–152
Pandey A, Soccol CR, Mitchell DA (2000) New developments in solid-state fermentation: I-bioprocesses and products. Process Biochem 35:1153–1169
Pandey A, Soccol CR, Rodriguez-Leon JA, Nigam P (2001) Solid-state fermentation in biotechnology-fundamentals and applications. Asiatech publishers inc., New Delhi
Prasanna DB, Gopal M, Subbalaxmi S (2009) Studies on the extracellular tannase from newly isolated Bacillus thurangiences BN2. In: Kai L (ed) Proceedings of international conference on chemical, biological and environmental engineering. World Scientific, Singapore, pp 379–384
Prasanna DB, Mugeraya G, Nirmala KR, Basavaraj N (2010) Production of novel cell-associated tannase from newly isolated Serratia ficaria DTC. J Microbiol Biotechnol 20(4):32–736
Prasanna DB, Rakesh VG, Dinesh CG (2012) Optimization of culture medium for novel cell-associated tannase production from Bacillus massiliensis using response surface methodology. J Microbiol Biotechnol 22(2):199–206
Qureshi N, Annous BA, Ezeji TC et al (2005) Biofilm reactors for industrial bioconversion processes: employing potential of enhanced reaction rates. Microb Cell Fact 21:1–21
Rodriguez-Duran V, Contreras-Esquivel JC, Rodríguez R, Prado-Barragán LA, Aguilar CN (2011) Optimization of tannase production by Aspergillus niger in solid-state packed-bed bioreactor. J Microbiol Biotechnol 21(9):960–967
Rout S, Banerjee R (2006) Production of tannase under mSSF and its application in fruit juice debittering. Indian J Biotechnol 5:346–350
Sabu A, Augur C, Swati C, Pandey A (2006) Tannase production by Lactobacillus sp. ASR-S1 under solid-state fermentation. Proc Biochem 41(3):575–580
Selwal MK, Yadav A, Selwal KK, Aggarwal NK, Gupta R, Gautam SK (2010) Optimization of cultural conditions for tannase production by Pseudomonas aeruginosa IIIB 8914 under submerged fermentation. World J Microbiol Biotechnol 26:599–605
Shaligram NS, Singh SK, Singhal RS, Szakacs G, Pandey A (2008) Compactin production in solid-state fermentation using orthogonal array method by P. brevicompactum. Biochem Eng J 41:295–300
Sharma S, Bhat TK, Dawra RK (2000) A spectrophotometric method for assay of tannase using rhodanine. Anal Biochem 279:85–89
Subbalaxmi S, Vytla RM (2016) Process optimization for tannase production by Bacillus gottheilii M2S2 on inert polyurethane foam support. Biocatal Agric Biotechnol 7:48–55
Subbalaxmi S, Vytla RM (2017a) Evaluation of kinetic parameters for growth, tannic acid utilization and tannase production in Bacillus gottheilii M2S2 using polyurethane foam blocks as support. 3 Biotech 7(5):275
Subbalaxmi S, Vytla RM (2017b) Semi-solid state fermentation: a promising method for production and optimization of tannase from Bacillus gottheilii M2S2. Res J Biotechnol 12(4):39
Subbalaxmi S, Vytla RM (2018) Solid state fermentation of Bacillus gottheilii M2S2 in laboratory-scale packed bed reactor for tannase production. Prep Biochem Biotechnol. https://doi.org/10.1080/10826068.2018.1509086
Van de Lagemaat J, Pyle DL (2001) Solid-state fermentation and bioremediation: development of a continuous process for the production of fungal tannase. Chem Eng J 84(2):115–123
Van de Lagemaat J, Pyle DL (2004) Solid-state fermentation: a continuous process for fungal tannase production. Biotechnol Bioeng 87(7):924–929
Vikas B, Anil K, Gunjan G, Vinod C (2013) A novel low molecular weight acido-thermophilic tannase from Enterobacter cloacae MTCC 9125. Biocatal Agric Biotechnol 2:132–137
Acknowledgements
The authors are grateful to the Department of Biotechnology, Manipal Institute of Technology, Manipal Academy of Higher Education, India, for providing the facilities to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that no actual or potential conflicts of interest in relation to this article exist.
Rights and permissions
About this article
Cite this article
Selvaraj, S., Vytla, R.M., Vijay, G.S. et al. Modeling and optimization of tannase production with Triphala in packed bed reactor by response surface methodology, genetic algorithm, and artificial neural network. 3 Biotech 9, 259 (2019). https://doi.org/10.1007/s13205-019-1763-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-1763-z