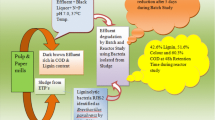
Abstract
This study reports the degradation and decolourization capability of a manganese peroxidase enzyme producing bacterium isolated from pulp and paper mill wastewater. The isolate was identified as Bacillus aryabhattai based on biochemical analysis and 16S rRNA gene sequencing. The strain was designated MG966493. This bacterium was able to reduce 67% and 54% colour and lignin, respectively, from the pulp and paper mill wastewater after 144 h of treatment at 32 °C, pH 7.6 and 120 rpm. Further, FT-IR analysis showed that during the lignin degradation process a number of metabolites were produced comprising different functional groups such as carbonyl (C=C), carboxyl (–COOH), alkene (C=C), amines (–NH2), sulphonic (–SO3) and nitro (–NO2). In addition, the SEM analysis showed that the bacterial cells exposed to pulp and paper mill wastewater have rough surfaces with reduced size as compared to the unexposed cells with smooth surfaces. This study concluded that the isolated bacterium B. aryabhattai has significant potential for the bioremediation of pulp and paper mill wastewater and thus, can be applied for their treatment at an industrial scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulp and paper industry is the third most wastewater producing industry in the world and key contributors to the industrial water pollution (Asghar et al. 2008). According to the Indian Ministry of Environment and Forest (MOEF), pulp and paper industry is categorized as one of the “Red Category” of 17 listed industries, which causes severe environmental pollution. To minimize the environmental pollution, the industry must follow various effluent discharge standards set by the Central Pollution Control Board (CPCB 2010) and other agencies.
Pulp and paper industry discharges a dark brown coloured wastewater produced during the various stages of paper-making process into the environment. This deeply coloured wastewater is reported to have high pollution parameters such as biochemical oxygen demand (BOD), chemical oxygen demand (COD), suspended solids (SS) along with toxic chlorinated compounds, tannins, resin acids, sulfur compounds, lignin and its degradation products (Haq et al. 2016a, b; Pokhrel and Viraraghavan 2004). This dark-coloured wastewater, if discharges into the environment without treatment or partial treatment, can cause undesirable colouration of aquatic resources along with increase in BOD, COD values, reduction in dissolved oxygen content and photosynthetic activity of aquatic plants (Bharagava et al. 2009; Ali and Sreekrishnan 2001; Karrasch et al. 2006). Chlorinated organic compounds of pulp and paper mill wastewater can also lead to genetic mutations and skin disorders in exposed organisms (Easton et al. 1997; Malik et al. 2009). In the terrestrial ecosystem, pulp and paper mill wastewater reduces soil fertility and crop productivity and if the contaminants enter the food chain, these may lead to genotoxic, carcinogenic, and clastogenic effects in human and animals (Savant et al. 2006; Mandal and Bandana 1996).
Due to the above-environmental problems, total chlorine-free (TCF) bleaching process involving the use of oxygen, hydrogen peroxide, ozone, and peracetic acid has been introduced in papermaking process to prevent environmental pollution. This eliminates bleaching with elemental chlorine that releases dioxins and furans into the environment. This also fulfills the market demand of non-chlorine based chemical bleached pulp (Miri et al. 2015; Yaqoob et al. 2010, 2011).
An increased use of TCF bleaching was expected worldwide especially outside of Europe. However, TCF is currently used in few countries only, accounting 5% of the total bleached pulp production worldwide in 2010 because of low market uptake, higher bleaching cost, lower pulp strength, reduction in the removal of hexenuronic acid, and more brightness reversion compared to the elemental chlorine-free bleached pulp (Miri et al. 2015). Thus, it is necessary to adequately treat the generated wastewaters before its final discharge into the environment. Although a number of physico-chemical methods have been reported for the treatment of pulp and paper mill wastewater, they are energy demanding, costly and also generate secondary pollutants (Yang et al. 2008). Intensive research has been carried out on microbial degradation and decolourization of pulp and paper mill wastewater using bacteria or fungi or their enzymes to replace these conventional treatment methods (Saraswathi and Saseetharan 2010).
Most of the research on the degradation of pulp and paper mill wastewater involved fungi as they degrade lignin and other pollutants efficiently. However, fungi require low pH for better performance, while the pH of pulp and paper mill wastewater tends to be neutral or alkaline (between 7 and 9), the requirement to reduce the pH of wastewater prior to the fungal treatment resulting in an additional cost (Raghukumar et al. 2008; Costa et al. 2017). In contrast, bacteria survive well in neutral to alkaline pH (7–9) and are suitable for the treatment of pulp and paper mill wastewater without any need of pH adjustment (Brown and Chang 2014; Rahman et al. 2013). Many bacterial species and their enzymes have been reported for lignin degradation, colour removal and toxicity reduction, but the reduction rate of pollution parameters by bacterial strains have been found lower than that of fungi (Singh et al. 2011; Raj et al. 2014; Costa et al. 2017). Thus, there is a need to search for potential bacterial strains for the effective treatment of pulp and paper mill wastewater. The present study focuses on the isolation, screening and characterization of manganese peroxidase producing bacterial strains and the evaluation of their potential for degradation and decolourization of pulp and paper mill wastewater for environmental safety.
Materials and methods
Chemicals
All the reagents and chemicals used in this study were of analytical grade. The purified synthetic kraft lignin (molecular weight 28,000 daltons) powder was purchased from Sigma Aldrich (USA). Glucose, peptone, agar and salts used in bacterial degradation experiments were purchased from Hi-Media (Mumbai, India).
Sampling site and sample collection
The wastewater samples were collected from the outlet of Century Pulp Paper Mill, Lalkuan, Uttarakhand (India). The wastewater was collected in sterile plastic containers of 5 L capacity, transported to laboratory and stored at 4 °C. The samples were analyzed for various physico-chemical parameters and also used in isolation of potential bacterial strains capable for the degradation and decolourization of pulp and paper mill wastewater.
Physico-chemical analysis of pulp and paper mill effluent
The physico-chemical analysis of pulp and paper mill wastewater was performed in triplicates as per the standard methods (APHA 2012). The collected wastewater sample was analyzed for pH, temperature, COD (open reflux method), BOD (5 days method), total dissolved solids (TDS), total suspended solids (TSS) and total solids (TS) (drying method), total nitrogen (Kjeldhal method), sulfate and phosphate by BaCl2 precipitation method and Vanadomolybdo-phosphoric acid method, respectively (APHA 2012). The colour and lignin in wastewater were measured following the CPPA (1974) and Pearl and Benson (1940) methods, respectively. The pH of wastewater was measured using a digital pH meter (Metrohm, USA). Heavy metals such as copper, zinc and iron were analyzed using atomic absorption spectrophotometry (AAS) (VARIAN AS240FS, Australia) after acid digestion (APHA 2012).
Enrichment of sludge sample and isolation of lignin-degrading bacterial strains
Nutrient enrichment technique was used to isolate potential bacterial strains (Morii et al. 1995). Wastewater sample (20 mL) was added in a Erlenmeyer flask (250 mL) containing 80 mL of sterile mineral salt medium (MSM) of the following composition (in g L−1): Na2HPO4 2.4; KH2PO4 2.0; NH4NO3 0.1; MgSO4 0.01; CaCl2 0.01; D-glucose 10.0; peptone 5.0 (Chandra et al. 2007) and trace elements solution (1 mL L−1) (Pfenning and Lippert 1996). The pH of medium was adjusted to 7.5 ± 0.1. Lignin (100 mg L−1) was added to medium (L-MSM) as carbon source and flasks were incubated at 30 °C under shaking conditions (120 rpm). After 5 days of incubation, 10 mL of sample was transferred to a fresh 90 mL lignin amended mineral salt medium (L-MSM) and incubated for 48 h at 30 °C under shaking conditions (120 rpm) (Hooda et al. 2015).
The culture broth was serially diluted to 10−4 and 10−5 and 50 µL of this culture broth was spread onto the lignin amended MSM agar plates followed by incubation at 32 ± 2 °C for 48 h. The morphologically and phenotypically different colonies developed on the plates were selected, picked up and purified by repeated streak plate method on the same medium. The purified colonies were sub-cultured on MSM agar plates supplemented with the increasing concentration of kraft lignin, i.e., 200–1500 mg L−1. Finally, four bacterial isolates that were able to grow at the highest concentration of 1200 mg L−1 of kraft lignin were selected and streaked on the L-MSM agar plates.
Screening of isolated bacterial strains for ligninolytic enzyme activity
The screening of purified bacterial isolates was carried out based on the dye decolorization method. The isolated bacterial strains were screened for manganese peroxidase activity using phenol red dye as an indicator. The phenol red dye at the concentration of 0.01% was added to the sterile MSM agar medium under aseptic conditions. All the four bacterial isolates were streaked onto the MSM agar plates amended with phenol red dye and incubated at 32 °C for 48 h. A decolourization zone developed around the bacterial colonies indicates positive result of ligninolytic enzyme activity. Out of the four bacterial isolates, only one bacterium was found capable to produce clear zone of decolorization and was selected for further studies. This bacterium was also grown in liquid medium (MSM broth) containing 0.01% phenol red dye. A loopful of bacterial culture was inoculated into 10 mL nutrient broth followed by incubation at 32 °C under shaking flask condition at 120 rpm. After 24 h, 1 mL of culture was transferred to 99 mL of MSM (pH 7.6) and incubated at 32 °C and 120 rpm for 72 h. At a regular time interval, samples were withdrawn and bacterial growth, dye decolourization and MnP activity measured. The flasks having no bacterial culture were used as control.
Characterization and identification of isolated bacterial strain
Biochemical characterization
The isolated bacterial strain was characterized morphologically and biochemically as per the standard methods of Cowan and Steel’s manual for the identification of medical bacteria (Barrow and Feltham 1993).
Molecular identification
DNA preparation, PCR amplification and 16S rRNA gene sequencing analysis
The genomic DNA from isolated bacterium was isolated following the method described by Atashpaz et al. (2010). The extracted DNA was examined on 0.8% agarose gel, which contains 1 µg mL−1 ethidium bromide and the bands were observed on UV transilluminator.
The 16S rRNA gene was amplified using 5 µL of genomic DNA (as template DNA) and universal eubacterial primers (27F) 5ʹ-AGAGTTTGATCMTGGCTCAG-3ʹ and (1492R) 5ʹ-CGGTTACCTTGTTACGACTT-3ʹ (Narde et al. 2004). The reaction mixture was contained 5 µL DNA template, 200 µM of each dNTP, 1X PCR buffer, 3.0 mM MgCl2, 25 pmol of primer and 2.5 units of Amplitaq DNA polymerase (Perkin Elmer) in a final reaction volume of 50 µL (Bharagava and Mishra 2018). The thermocycling reactions were carried out in a Veriti® 96-well Thermal Cycler (Applied Biosystems, USA). Thirty-five cycles were run to amplify the 16S rRNA gene fragment following the initial denaturation at 95 °C for 2 min, subsequent denaturation at 95 °C for 30 s followed by annealing at 52 °C for 30 s, extension at 72 °C for 2 min and final extension at 72 °C for 15 min.
The PCR products were resolved on 1% agarose gel and purified using gel extraction kit (Merk Biosciences, Bangalore). This gel purified PCR product was sequenced by Aakaar Biotechnologies, Pvt. Ltd. (Lucknow, India) and the obtained partial sequences were subjected to BLAST analysis using the online option available at http://www.ncbi.nlm.nih.gov/BLAST (Altschul et al. 1997) suggesting the closest neighbor of isolated bacterium. The partial sequences obtained were submitted to Gene-Bank under the accession number MG966493.
Biodegradation study of pulp and paper mill effluent by isolated bacterium
The degradation experiments were performed in Erlenmeyer flasks (250 mL) containing 99 mL of autoclaved pulp and paper mill wastewater supplemented with 1% (w/v) glucose and 0.5% (w/v) peptone as carbon and nitrogen source, respectively. Overnight grown culture (1%) with optical density (OD) 2.0 was inoculated followed by incubation at 32 ± 1 °C and 120 rpm for 144 h. The flasks containing autoclaved pulp and paper mill wastewater only were used as control. At a regular interval of 24 h, appropriate sample volume was withdrawn and analyzed for bacterial growth, reduction in colour and lignin content as per the Raj et al. (2014).
Quantification of manganese peroxidase activity
The isolated bacterial strain was grown in 10 mL nutrient broth overnight as pre-culture. One milliliter of this pre-culture was inoculated in Erlenmeyer flask (250 mL) containing 99 mL L-MSM broth amended with lignin (100 mg L−1). The flask was incubated at 32 ± 2 °C, and 120 rpm and pH of broth were maintained at 7.6. Sample (2 mL) was withdrawn from flask and centrifuged at 8000 rpm for 15 min at 4 °C. The culture supernatant was directly used as extracellular crude enzyme to determine the enzyme activity.
Further, the quantitative assay for manganese peroxidase enzyme activity was performed following the method of Orth et al. (1993). The reaction mixture (4 mL) was contained 1 mL of culture supernatant (crude enzyme extract), 1 mL of phosphate buffer (pH 7.0), 500 µL H2O2 (1 mM), 500 µL MnSO4 (1 mM) and 1 mL phenol red (0.1 mM). The reaction was started by adding H2O2 in reaction mixture and enzyme activity was measured against the reagent blank. A sample (1 mL) was taken from reaction mixture and 40 µL NaOH (5 M) was added to this sample to stop the reaction. The samples were withdrawn at every 1 min upto 4 min and absorbance was measured at 610 nm. The enzyme activity was expressed in terms of International Unit (IU) and is defined as the amount of active enzyme required to oxidize 1 µmol substrate per min.
Fourier transform infrared spectrophotometric (FT-IR) analysis
To identify the functional groups, present in untreated and bacteria treated pulp and paper mill wastewater, the FT-IR analysis was performed using FT-IR Spectrophotometer (Nicolet™ 6700, Thermo Scientific, USA). The untreated and treated pulp and paper mill wastewater samples were centrifuged at 8000 rpm at 4 °C for 10 min and dried in air at 50 °C (Kurade et al. 2012). About 1 mg of air-dried sample was mixed with the 400 mg of potassium bromide in ratio of 5:95. The mixture was taken over translucent disk and given manual hydraulic pressure of 100 kg cm−2 for 10 min and finally the sample disk was fixed in FT-IR Spectrophotometer (Nicolet™ 6700, Thermo Scientific, USA) to carry out analysis. The FT-IR spectrum of samples was recorded in the mid IR region of 400–4000 cm−1 (Bharagava and Mishra 2018).
Scanning electron microscopy (SEM) analysis
Scanning electron microscopy was carried out to observe the surface morphology of bacterium exposed to pulp and paper mill wastewater. The pre-culture of the isolated bacterium was inoculated into pulp and paper mill wastewater, while bacterial cells grown in nutrient broth were used as control. Both the samples were incubated at 32 °C, 120 rpm for 24 h. After 24 h, the samples were centrifuged at 8000 rpm for 10 min. The obtained bacterial pellets were washed three times with phosphate buffer (pH 7.2) and pre-fixed by 2.5% glutaraldehyde for 4–6 h at 4 °C. The cells were again washed twice with phosphate buffer and post-fixed by 1% osmium tetraoxide and left for 1 h to get a clear image. The post-fixed cells were washed thrice with phosphate buffer and dehydrated with increasing acetone concentration 30, 50, 70, 90, 95 and 100% (v/v). These dehydrated cells were dried in a critical point dryer (CPD) and coated with platinum using ion sputter coater (JEOL, Japan JFC 1600 Auto Fine Coater), and observed under the SEM (JEOL JSM-6490LV) for analysis.
Statistical analysis
All the experiments were performed in triplicates (n = 3) to reduce the experimental errors, and confirm the variability and validity of the results. The results obtained from each set of experiment were subjected to the mean, standard deviation analysis.
Results and discussion
Physico-chemical characteristics of pulp and paper mill wastewater
The physico-chemical analysis of untreated pulp and paper mill wastewater showed that it was dark brown in colour and alkaline in nature (pH 8.1) with high values for BOD, COD, phosphate, nitrate and total phenol, as shown in Table 1. Some parameters such as TDS and metals content were below the permissible limit except for Ni in untreated wastewater. The quality of the wastewater improved significantly after the bacterial treatment process. Pulping and bleaching are the main stages of papermaking process, which contribute pollutants to wastewaters. In pulping process, sodium hydroxide and sodium sulfite are used to dissolve lignin and hemicellulosic content that contribute high pH and sulfate content, while bleaching process releases lignin and its derivatives and contribute high BOD and COD to wastewaters. The nitrates present in wastewater mainly associated with lignin, while chlorine used to whiten the pulp reacts with other compounds and form chlorophenols and chlorides, which are released during the bleaching process (Singhal and Thakur 2009; Pokhrel and Viraraghavan 2004).
The dark brown colour of wastewater reduces the photosynthetic activity of aquatic plants and affects the food chain, whereas high BOD, COD values decreases the dissolved oxygen level in aquatic resources creating anoxic conditions to the aquatic organisms. Total solids include organic, inorganic and many dissolved substances, which create toxic environment by changing the ion composition, increase in salinity (USEPA 1986) and poses threats to the aquatic organisms. The presence of metals in pulp and paper mill wastewater might be due to the bioaccumulation in plants, which are used as raw materials as well as also from different types of chemicals used in paper manufacturing process (Hakeem and Bhatnagar 2010).
Screening and characteristics of isolated bacterium
Initially, eight (PLP 1–PLP 8) morphologically different bacterial strains were isolated from the collected pulp and paper mill wastewater through enrichment technique. Out of these eight bacterial strains, four bacterial strains were capable to grow on the MSM agar plates amended with the highest concentration of lignin (1200 mg mL−1). Out of these four bacterial strains, only one bacterium (PLP 6) produced the clear decolourization zone of phenol red dye around the colonies after 48 h of incubation period, as shown in Fig. 1a, b. During the screening for MnP production in broth medium, the optimum bacterial growth (OD600 1.826) and phenol red dye decolourization (91%) was observed at 48 h of incubation period and later both the bacterial growth and dye decolourization showed a declined trend, as shown in Fig. 2. The activity of extracellular MnP enzyme was also observed maximum (6.1 IU mL−1) at 48 h of incubation period (Fig. 3). However, no dye decolourization was observed in the uninoculated control.
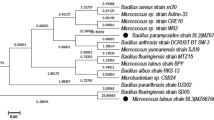
The isolated bacterium PLP 6 appeared as light brown colonies on lignin amended agar plates (L-MSM) and shown to be as Gram positive, rod shaped, endospore forming, motile with circular ends, entire margin and flat elevation. This bacterium showed positive reactions for catalase, gelatinase, amylase, urease and cellulase, while negative reactions for starch hydrolysis, indole and citrate utilization tests. The BLAST analysis of 16S rRNA gene sequence of the isolated bacterium showed the closest relationship (99%) with Bacillus aryabhattai as shown in phylogenetic tree (Fig. 4). Thus, based on the sequence similarity, the isolated bacterium (PLP 6) was identified as B. aryabhattai with accession number MG966493.
Degradation and decolourization of pulp and paper mill wastewater by isolated bacterium
The degradation and decolourization of pulp and paper mill wastewater containing lignin 529 ± 20.10 mg L−1 by the isolated bacterium were performed at pH 7.6 for 144 h and expressed in terms of bacterial growth, lignin degradation and colour reduction. During this study, the bacterial growth was observed optimum at 120 h of incubation period and afterwards a slight decrease in growth was observed (Fig. 5a), whereas maximum reduction in colour (67%) and lignin content (54%) was observer at 144 h of incubation period and after that reduction in colour and lignin is observed in Fig. 5b. Though, the degradation was not observed upto 48 h of incubation period indicating that initially the bacterium utilized glucose and peptone as C and N source and afterwards it started to use lignin and other pollutants as C and N sources resulting in the reduction in lignin and colour of wastewater. Many authors have also reported similar findings (Haq et al. 2016a, b; Singhal and Thakur 2009; Bharagava and Chandra 2010; Bharagava et al. 2009).
In pulp and paper mill wastewater, lignin and chlorinated phenols are the major environmental pollutants and their removal is very essential for environmental and public health safety. The outcome of this study suggests B. aryabhattai might be capable for the effective degradation of pollutants present in pulp and paper mill wastewater. Earlier studies made on the treatment of pulp and paper mill wastewater using Aeromonas formicans showed 70%, 85% and 80% reduction in COD, colour and lignin, respectively, after 8 days of treatment (Gupta et al. 2001). The removal of colour (61%), lignin (53%), BOD (82%), COD (78%) and phenol (77%) from pulp and paper mill wastewater by Bacillus sp. was also reported within 6 days of incubation period (Raj et al. 2007). Chandra et al. (2009) also reported that two bacterial strains Bacillus cereus and Serratia marcescens efficiently reduced colour (45–52%), lignin (30–42%), BOD (40–70%), COD (50–60%) and total phenol (32–40%) after 168 h of incubation period. Raj et al. (2014) found that Paenibacillus sp. effectively reduced colour (68%), phenol (86%), COD (78%), BOD (83%) and lignin (54%) at 34 ± 1 °C and 120 rpm after 144 h of treatment. Haq et al. (2016a, b) reported that a lignin peroxidase producing strain Serratia liquefaciens was capable to effectively reduce the pollution parameters (COD 85%, phenol 95%, colour 72% and lignin 58%) of pulp and paper mill wastewater at 30 °C, pH 7.6 and agitation 120 rpm after 144 h of incubation period.
During the bacterial treatment of pulp and paper mill wastewater, the pH of wastewater changes due to the metabolic activities of bacterium. The initial pH of wastewater was 7.6, but after 48 h, it decreases to pH 5.5 and thereafter gradually increased upto pH 6.9, whereas the pH of the control remained constant. This shift in pH from alkaline to acidic might be due to the acetate efflux along with the intermediates of TCA cycle (Yang et al. 2008).
The activity of manganese peroxidase enzyme (MnP) also increased upto 4.7 IU mL−1 till 72 h, and afterwards it steadily declined, as shown in Fig. 5c. MnPs are the heme-containing glycoproteins that have the capacity to oxidize both phenolic and non-phenolic compounds. MnP catalyzes the peroxide dependent reactions and oxidizes Mn from Mn(II) to Mn(III) and, afterwards, is released from the enzyme surface and complex with oxalate or other chelated compounds. The oxidized or chelated Mn(III) complex acts as a reactive low molecular weight species and diffuses redox mediators such as simple phenols, amines, dyes, phenolic lignin substructures and dimmers. The oxidation potential of Mn(III) chelators is limited only to phenolic compounds, whereas during the oxidation of non-phenolic compounds by Mn(III), the reactive radicals formed in presence of a second mediator (oxalate, malonate etc.). In absence of H2O2, these radicals can be used by MnP as a source of peroxides and increases the pollutants (lignin) degradation efficiency.
FT-IR analysis
The FT-IR analysis of untreated pulp and paper mill wastewater revealed the compounds present in untreated pulp and paper mill wastewater have different functional groups such as carbonyl (C=C), carboxyl (–COOH), alkene (C=C), amines (–NH2), sulfonic (–SO3) and nitro (–NO2), as shown in Fig. 6a. In the bacteria treated pulp and paper mill wastewater, the changes in the appearance of peaks indicated changes in functional groups in the metabolites produced during the bacterial treatment of pulp and paper mill wastewater (Fig. 6b).
The spectrum of lignin compounds has wide range from 3500 to 3100 cm−1. These bands appear due to the presence of phenolic and hydroxyl groups, which make hydrogen bond giving rise to –OH stretching frequencies. The wide absorption peaks in the region of 3400–3300 cm−1 is attributed to O–H stretching, which is related to the aliphatic compounds present in lignin structure. The peaks around 2940–2850 cm−1 showed C–H stretching of –CH2 group, indicating the presence of various amino acids and aliphatic –CH3 groups. Functional groups like NH+, –CH, –SH, –PH, –SiH appeared in the range of 2700–2250 cm−1. The stretching frequencies appearing in the range of 1700–1000 cm−1 allocated aromatic rings, which related to the absorption bands of lignin. The stretching range of aromatic nitro compounds –NO2, secondary C=O and C–N groups was observed at 1540–1515 cm−1. The peaks around 1410–1350 cm−1 region showed O–H stretching bands for phenol or tertiary alcohols. The spectrum in the region of 1250–900 cm−1 referred to the stretching frequencies for cyclic ethers. The stretching of C–S linkage was observed in the region of 700–600 cm−1, whereas brominated compounds was appeared in the range 600–500 cm−1 in infra-red band region (Rajwar et al. 2017; Muruganantham et al. 2009).
SEM analysis
The SEM analysis of unexposed and exposed cells has revealed that the unexposed cells were long, rod shaped with smooth surfaces (Fig. 7a), whereas the cells, which were exposed to pulp and paper mill wastewater become rough, porous, coagulate with wrinkled surfaces and increase in cell number (Fig. 7b). These changes in exposed cells might be due to the stress conditions exerted by various pollutants present in pulp and paper mill wastewater during the treatment process and might be associated with either the precipitation or adsorption of pulp paper mill wastewater pollutants on bacterial cell surface (Kumari et al. 2016).
Conclusion
In present study, a MnP enzyme producing bacterium was isolated from pulp and paper mill wastewater, which was characterized and identified as B. aryabhattai based on the biochemical reactions and 16S rRNA gene sequence analysis. This bacterium was capable to reduce effectively the pollution parameters of pulp and paper mill wastewater. Thus, based on the degradation and detoxification potential of isolated bacterium, it was concluded that the isolated bacterium can be used as a potential agent for the effective degradation and detoxification of pulp and paper mill wastewater and contaminated sites for environmental as well as human health safety.
References
Ali M, Sreekrishnan TR (2001) Aquatic toxicity from pulp and paper mill effluents: a review. Adv Environ Res 5:175–196
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Grapped BLAST and PSIBLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
APHA (2012) Standard methods for the examination of water and wastewaters, 22nd edn. American Public Health Association, American Water Works Association, Water Environmental Federation, Washington, DC, p 981
Asghar MN, Khan S, Mushtaq S (2008) Management of treated pulp and paper effluent to achieve zero discharge. J Environ Manag 88:1285–1299
Atashpaz S, Barzegari AK, Barar J, Vahed SZ, Azarbaijani R, Omidi Y (2010) A robust universal method for the extraction of genomic, DNA Bact. Species Microbiol 79(4):562–566
Barrow GI, Feltham RKA (1993) Cowan and steels manual for the identification medical bacteria, 3rd edn. Cambridge University Press, Cambridge
Bharagava RN, Chandra R (2010) Biodegradation of the major color containing compounds in distillery wastewater by an aerobic bacterial culture and characterization of their metabolites. Biodegrad J 21:703–711
Bharagava RN, Mishra S (2018) Hexavalent chromium reduction potential of Cellulosi microbium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol Environ Saf 147:102–109
Bharagava RN, Chandra R, Rai V (2009) Isolation and characterization of aerobic bacteria capable of the degradation of synthetic and natural melanoidins from distillery wastewater. World J Microbiol Biotechnol 25:737–744
Brown ME, Chang MCW (2014) Exploring bacterial lignin degradation. Curr Opin Chem Biol 19:1–7
Chandra R, Raj A, Purohit HJ, Kapley A (2007) Characterization and optimization of three potential aerobic bacterial strains for kraft lignin degradation from pulp paper waste. Chemos 67:839–846
Chandra R, Raj A, Yadav S, Patel DK (2009) Reduction of pollutants in pulp and paper mill effluent treated by PCP-degrading bacterial strains. Environ Monit Assess 155(1–4):1–11
Costa S, Dedola DG, Pellizzari S, Blo S, Irene RI, Pedrini P, Tamburini E (2017) Lignin biodegradation in pulp and paper mill wastewater by elected white rot fungi. Water 9(12):1–9
CPCB (2010) Central Pollution Control Board, Comprehensive industry document for large pulp and paper industry. COINDS/36/2000-2001
CPPA (1974) Canadian Pulp and Paper Association, Technical Section Standard Method H5P. Montreal, Canada
Easton MDL, Kruzynnski GM, Solar II, Dye HM (1997) Genetic toxicity of pulp mill effluent on juvenile Chinook Salmon (Onchorhychus tshawytscha) using flow cytometry. Water Sci Technol 35:347–355
Gupta VK, Minocha AK, Jain N (2001) Batch and continuous studies on treatment of pulp mill wastewater by Aeromonas formicans. J Chem Technol Biotechnol 76(6):547–552
Hakeem AS, Bhatnagar S (2010) Heavy metal reduction of pulp and paper mill effluent by indigenous microbes. Asian J Exp Biol Sci 1(1):201–203
Haq I, Kumar S, Kumari V, Singh SK, Raj A (2016a) Evaluation of bioremediation potentiality of ligninolytic Serratia liquefaciens for detoxification of pulp and paper mill effluent. J Hazard Mat 305:190–199
Haq I, Kumari V, Kumar S, Raj A, Lohani M, Bhargava RN (2016b) Evaluation of the phytotoxic and genotoxic potential of pulp and paper mill effluent using Vigna radiata and Allium cepa. Adv Biol. https://doi.org/10.1155/2016/8065736
Hooda R, Bhardwaj NK, Singh P (2015) Screening and identification of ligninolytic bacteria for the treatment of pulp and paper mill effluent. Water Air Soil Pollut 226:1–11
Karrasch B, Parra O, Cid H, Mehrens M, Pacheco P, Urrutia R, Valdovinos C, Zaror C (2006) Effect of pulp and paper mill effluents on the microplankton and microbial self-purification capabilities of the Biobio River Chile. Sci Total Environ 359:194–208
Kumari V, Yadav A, Haq I, Kumar S, Bharagava RN, Singh SK, Raj A (2016) Genotoxicity evaluation of tannery effluent treated with newly isolated hexavalent chromium reducing Bacillus cereus. J Environ Manag 183:204–211
Kurade M, Waghmode TR, Kagalkar AN, Govindwar SP (2012) Decolourization of textile industry effluent containing disperse dye Scarlet RR by a newly developed bacterial-yeast consortium BL-GC. Chem Eng J 184:33–41
Malik MK, Kumar P, Sethi R, Rishi S (2009) Genotoxic effect of paper mill effluent on chromosomes of fish Channa punctatus. Curr World Environ 4:353–357
Mandal TN, Bandana TN (1996) Studies on physicochemical and biological characteristics of pulp and paper mill effluents and its impact on human beings. J Freshw Biol 8:191–196
Miri M, Ghasemian A, Resalati H, Zeinaly F (2015) Total chlorine-free bleaching of Populus deltoides kraft pulp by ozone. Int J Carbohydr Chem. https://doi.org/10.1155/2015/381242
Morii H, Nakamiya K, Kinoshita S (1995) Isolation of a lignin-degrading bacterium. J Biosci Bioeng 80:296–299
Muruganantham S, Anbalagan G, Ramamurthy N (2009) FT-IR and SEM-EDS comparative analysis of medicinal plants, Eclipta alba HASSK and Eclipta prostrata LINN. Rom J Biophys 19(4):285–294
Narde G, Kapley A, Purohit HJ (2004) Isolation and characterization of Citrobacter strain HPC 255 for broad range substrate specificity for chlorophenol. Curr Microbiol 48:419–423
Orth AB, Royse DJ, Tien M (1993) Ubiquity of lignin degrading peroxidases among various wood degrading fungi. Appl Environ Microbiol 59:4017–4023
Pearl IA, Benson HK (1940) The determination of lignin in sulphite pulping liquor. Paper Trade J 105–117
Pfenning N, Lippert KD (1966) Uber das vitamin B-12-bedrurbins phototropher schwefelbakterien. Arch Microbiol 55:245–256
Pokhrel D, Viraraghavan T (2004) Treatment of pulp and paper mill wastewater—a review. Sci Total Environ 333:37–58
Raghukumar C, Dsouza-Ticlo D, Verma AK (2008) Treatment of coloured effluent with lignin-degrading enzymes: an emerging role of marine-derived fungi. Crit Rev Microbiol 34(3–4):189–206
Rahman NHA, Rahman NAA, Aziz SA, Hassan MA (2013) Production of ligninolytic enzymes by newly isolated bacteria from palm oil plantation soils. Bioresources 8(4):6136–6150
Raj A, Reddy MMK, Chandra R (2007) Decolourization and treatment of pulp and paper mill effluent by lignin-degrading Bacillus sp. J Chem Technol Biotechnol 82(4):399–406
Raj A, Kumar S, Haq I, Singh SK (2014) Bioremediation and toxicity reduction in pulp and paper mill effluent by newly isolated ligninolytic Paenibacillus sp. Ecol Eng 71:355–362
Rajwar D, Paliwal R, Rai JPN (2017) Biodegradation of pulp and paper mill effluent by co-culturing ascomycetous fungi in repeated batch process. Environ Monit Assess 189(482):1–16
Saraswathi R, Saseetharan MK (2010) Investigation on microorganisms and their degradation efficiency in paper and paper mill effluent. J Water Res Prot 2:660–664
Savant DV, Abdul-Rehman R, Ranade DR (2006) Anaerobic degradation of adsorbable organic halides (AOX) from pulp and paper industry wastewater. Bioresour Technol 97:1092–1104
Singh YP, Dhall P, Mathur RM, Jain RK, Thakur VV, Kumar V, Kumar R, Kumar A (2011) Bioremediation of pulp and paper mill effluent by tannic acid degrading Enterobacter sp. Water Air Soil Pollut 218(1–4):693–701
Singhal A, Thakur IS (2009) Decolourization and detoxification of pulp and paper effluent by Cryptococcus sp. Biochem Eng J 46:21–27
United States Environmental protection Agency, office of Water (1986) Quality criteria for water (gold book). EPA 440/5-86-001. Washington DC
Yang C, Cao G, Li Y, Zhang X, Ren H, Wang X, Feng J, Zhao L, Xu P (2008) A constructed alkaline consortium and its dynamics in treating alkaline black liquor with very high pollution load. PLoS One 3(11):3777
Yaqoob N, Stack K, Nguyen KL (2010) TCF bleaching of eucalypt kraf pulp with Oxone. Appita J 63(5):381–386
Yaqoob N, Rehman I, Chema K, Hameed S, Mateen B (2011) Environmental benign TCF bleaching sequences for AS/AQ wheat straw pulp. United States Patent Patent no: US 8:080, 129 b2
Acknowledgements
Authors are highly thankful to the University Grant Commission (UGC), Government of India (GOI), New Delhi, India for providing the RGNF Fellowship (F1-17.1/2017-18/RGNF-2017-18-SC-UTT-40299) to Ms. Surabhi Zainith for this work. The financial support received from “Science and Engineering Research Board” (SERB), Department of Science & Technology (DST), Government of India (GOI), New Delhi, India, as “Major Research Project” (Grant no: EEQ/2017/000407) is also dully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zainith, S., Purchase, D., Saratale, G.D. et al. Isolation and characterization of lignin-degrading bacterium Bacillus aryabhattai from pulp and paper mill wastewater and evaluation of its lignin-degrading potential. 3 Biotech 9, 92 (2019). https://doi.org/10.1007/s13205-019-1631-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-1631-x