Abstract
Eight yeast isolates identified as Saccharomyces cerevisiae were recovered from molasses-using Cuban distilleries and discriminated by nucleotide sequence analysis of ITS locus. The isolates L/25-7-81 and L/25-7-86 showed the highest ethanol yield from sugarcane juice, while L/25-7-12 and L/25-7-79 showed high ethanol yield from sugarcane molasses. The isolate L/25-7-86 also displayed high fermentation capacity when molasses was diluted with vinasse. In addition, stress tolerance was evaluated on the basis of growth in the presence of inhibitors (acetic acid, lactic acid, 5-hydroxymethylfurfural and sulfuric acid) and the results indicated that L/25-7-77 and L/25-7-79 congregated the highest score for cross-tolerance and fermentation capacity. Hence, these isolates, especially L/25-7-77, could serve as potential biological platform for the arduous task of fermenting complex substrates that contain inhibitors. The use of these yeasts was discussed in the context of second-generation ethanol and the environmental and economic implications of the use of vinasse, saving the use of water for substrate dilution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic ethanol offers the opportunity to revalorize low-cost resources in addition to conventional feedstock used for first-generation fermentations (Naik et al. 2010). Biomass pre-treatment stage represents the biggest challenge for this technology due to, among other issues, the formation of compounds that hampers a key microbial cell factory of the Saccharomyces cerevisiae yeast (Naik et al. 2010).
There are several metabolites that present such inhibitory activity which can be mostly divided into three groups. First, there are the aromatic aldehydes like furfural (2-furaldehyde) and 5-hydroxymethylfurfural (5-hydroxymethyl-2-furaldehyde or HMF) formed by the dehydration of pentoses and hexoses, respectively, during thermal-acid treatment of lignocellulose (Liu and Moon 2009). Their concentrations in the hydrolysates can reach up to 6 g/L depending on the source of the biomass (Larsson et al. 1999; Aguilar et al. 2002). This type of molecules can also be found in industrial substrates like molasses, although at lower concentrations, as a by-product of sugar dehydratation during the milling process (García and Otero 2015). They affect growth and fermentation capacity of the yeast cells by inhibiting key enzymes of the central metabolism, interfering with the rate of protein synthesis and/or increasing the energy demand as ATP and NAD(P)H for repairing damages to cellular structures (Sanchez and Bautista 1988; Modig et al. 2002; Almeida et al. 2008). NADPH-dependent oxo-reductases are very important for the detoxification of these inhibitors (Almeida et al. 2008; Liu and Moon 2009; Sehnem et al. 2013).
The second group of molecules comprises the weak organic acids, as lactic and acetic acids, also produced from the pre-treatment of the biomass and/or are produced by the contaminant yeasts and lactic acid bacteria (Makanjuola and Springham 1984; Bischoff et al. 2009; Beckner et al. 2011; Basso et al. 2014). These molecules in the environment with low pH (below 4.0) are mostly protonated and enter the cells by diffusion. Once inside at neutral pH of the cytoplasm (pH 6.8), the molecules dissociate to H+ and the anions lactate or acetate that lead to the disturbance of cellular homeostasis. Under this condition, yeast cells are required to use energy to drive the efflux pumps to get rid of these inhibitors. Again, both growth and fermentation capacity are compromised.
Lastly, the most common practice of biomass pre-treatment involves the heating the lignocellulose suspended in acid solution (usually diluted sulfuric acid solution). Hence, the produced hydrolysate, despite the already commented inhibitors, also presents the acidic characteristic that imposes acid stress on the yeast cells (De Melo et al. 2010). Though such stressing condition is not so detrimental to the fermentation capacity, it can compromise the cell viability and its recycling during the harvest season. Therefore, the search for strains that deals with this type of stress is also relevant in the context of production of second-generation ethanol.
Cuban distilleries have the characteristics of almost exclusively using molasses as substrate for fermentation, since sugar mills are mostly oriented for the production of sugar. As a consequence, the molasses might be exhausted in terms of sugar content while concentrated in various molecules like those mentioned above. The yeast populations in the processes have been poorly characterized, as well as the physiological profile of the S. cerevisiae population (both the commercial and the native strains). Hence, the yeasts could exhibit distinct traits associated with local fermentation stimuli and should be very adapted to hard conditions as a consequence of the continuous selective process accumulated along the decades of fermentations. Therefore, this kind of process could be considered an interesting source of naturally evolved strains with relevant capacities to produce ethanol even in unfavorable conditions. In the present study, we have surveyed a group of eight local industrial S. cerevisiae strains regarding ethanol yield in four different substrates and their resistance to inhibitors and revealed the relationships between these parameters. The quantification of this cross-analysis allowed us to identify isolates with superior performance for their use to ferment substrates of hard condition.
Materials and methods
Sampling, cell maintenance and molecular identification
Seven industrial must samples were collected directly from fermentation vessels and one from fermenter´s slurry in six bio-ethanol distilleries located along Cuba Island, at different crop harvesting periods. All distilleries produce ethanol from sugarcane molasses, diluted to approximately 120 g of total inverted sugar per liter of substrate. Samples were diluted in sterile saline and plated on Wallerstein nutrient medium (WLN) (Da Silva-Filho et al. 2005a) to produce approximately 10 CFU/plate. Colonies showing prospective Saccharomyces spp. cellular and cultural WLN-morphotype were isolated and named as L/25-7-12, L/25-7-77, L/25-7-79, L/25-7-80, L/25-7-81, L/25-7-82, L/25-7-86 and L/25-7-90. Stock cultures were maintained in glycerol at − 80 °C.
Molecular identification was performed by sequencing the ITS1-5.8S-ITS2 rDNA locus (Da Silva-Filho et al. 2005a; Basilio et al. 2008) using an ABI prisma 3500 device (Applied Biosystems), and previous cells were cultured in yeast extract–peptone–dextrose (YPD) broth. Total DNA was extracted, checked for quality and purity and the concentration evaluated (Basilio et al. 2008). The ITS locus was amplified with the use of the primers ITS4 (5ʹ-TCCTCCGCTTATTGATATGC-3ʹ) and ITS5 (5ʹ-GGAAGTAAAAGTCGTAACAAGG-3ʹ) according to Basilio et al. (2008) and sequenced for both strands (two times each) in ABI prisma 3100 platform (Applied Biosystems, USA). Sequence output files were analyzed by BioEdit v7.0 package and submitted to BLAST analysis in the NCBI nucleotide database (http://www.ncbi.nlm.nih.gov). Yeast identification was assumed if the query sequence showed > 99%identity with DNA sequences from yeast-type strain deposited at NCBI (Basilio et al. 2008). The industrial Saccharomyces cerevisiae strain JP-1 (Da Silva-Filho et al. 2005a, b) was used as reference for molecular characterization. Nucleotide sequences of 140 bp of ITS1 locus were recovered and analyzed in Bioedit™ software and aligned with ClustalW tool (Hall 1999). Clustering analysis was performed in MEGA v7.0 software using the UPGMA method (Kumar et al. 2016). For comparison, nucleotide sequences from S. cerevisiae S288c (internal reference) and Candida glabrata NRRL Y-68 (phylogenetically closed to Saccharomyces group outgroup control) were recovered from NCBI/Genebank and included.
Fermentation trials
Three sugarcane by-products-based fermentation medium (FM) were defined: FM1 with sugarcane juice, FM2 with sugarcane molasses and FM3 consisting of a mixed formulation of sugarcane molasses and distillery vinasse. All substrates were provided by the Sugar Cane Experimental Station of Pernambuco (EECA-PE), Brazil, centrifuged to remove insoluble solids and adjusted to 13(± 1) °Brix with distilled water (FM1 and FM2) or distillery vinasse (FM3) and to pH 4.8 whenever necessary. These industrial substrates were prepared just before the experiments and used without sterilization to mimic the industrial process. A control fermentation medium mimicking industrial C:N ratio was prepared with YNB (1.6 g/L), sucrose (120 g/L) and ammonium sulfate (1.0 g/L), adjusted to pH 4.8 and sterilized by filtration with sterile 0.22 µm filters.
Yeast seed cultures were prepared by cultivating the cells on YPD broth for 48 h at 30 °C, followed by further growth for 24 h after adding fresh YPD sterile medium. Cells were collected by centrifugation and sterile washed twice, and cell cultures were stored at 4°C until use. One gram of cells was transferred to 20 × 180-mm tubes closed with a small off gas tubing containing 15 mL of fermentation media. Fermentation trials were conducted in three biological replicates as previously described (Dutra et al. 2013). Samples were taken at the beginning and the tubes were incubated at 33 °C without agitation. Total suspended solid was monitored with a manual refractometer for °Brix decay for 8 h, after which samples were taken at the final fermentation time.
Samples from the start and end of fermentation were centrifuged to separate cells from the supernatant, which was used for the determination of residual sugar and production of ethanol (E), glycerol (Gly) and acetate (Ace). For this purpose, samples were prepared by filtration through a sterile 0.22 µm filter and freezing at − 20 °C until analysis. Samples were separated by HPLC Agilent device in Aminex HPX-87H Biorad column at 60 °C, using 8 mM H2SO4 solution as mobile phase at a flow rate of 0.6 mL/min. Detection of metabolites was performed by UV and refractive index type detectors. The following parameters were determined:
where ΔE, ΔGly and ΔBrix are the difference between the final and initial concentration of ethanol, glycerol and ºBrix, respectively; Qw is the final volume of the fermentation wort with cell; t is the fermentation time and the constant 14.5 value is the fixed coefficient which correlates reduction of ºBrix and glucose depletion (unpublished results).
Yeast tolerance to fermentation inhibitors
The synthetic growth medium consisted of YNB (0.67 g/L), glucose (20 g/L) and ammonium sulfate (5 g/L) with initial pH of 5.5, supplemented or not with one of the inhibitors. The inhibitors acetic acid and lactic acid were added to the synthetic medium to concentrations of 5 g/L or 15 g/L, while 5-hydroxymethyl furfural (5-HMF) was added to 5 g/L or 6 g/L (Taherzadeh et al. 2000; Sehnem et al. 2013). In addition, the synthetic medium was adjusted to an initial pH of 2.0 with sulfuric acid to mimic stress by inorganic acid (10 mM dissociated H+) in the industrial processes. Moreover, the protective action of magnesium (Barros de Souza et al. 2015) was tested by adding magnesium sulfate (7 H2O) to 0.5 g/L of the synthetic medium, with or without the inhibitors. That concentration referred to the Mg2+ cation. All media were sterilized by filtration with 0.22 µm sterile Millipore membranes. For all these combinations, the volume of 135 µL of synthetic media was distributed in sterile 96-well microtiter plates.
Seed cultures were prepared by cultivating yeast strains in 5 mL of YPD broth at 30 °C during 16 h at 170 rpm and 15 µL of the cells was used to inoculate the above media combinations for the total volume of 150 µL. These plates were incubated in Sinergy HT Microplate Reader (Biotek, Switzerland) at full speed and 30 °C during 24 h with continuous automated monitoring of absorbance at 660 nm (A660) every hour. All the experiments were carried out in biological replicates (n = 2) with technical triplicate for each condition. Data were recovered as xls files and processed in Excel™ worksheet to prepare the graphics of growth curves. Yeast growth rates were calculated from the slope of exponential growth phase by plotting Ln(A660) versus time. Net increments in biomass (ΔX) were determined as the difference between A660 at the beginning and at the end of cultivations and used to estimate the level of tolerance of the strains to the inhibitors relative to the reference synthetic medium without inhibitors and Mg+ 2.
Statistical analyses
Tukey’s test was used to compare means and differences with a p value < 0.05 considered as significant.
Results and discussion
Molecular yeast identification
Since the fermentation media were not sterilized prior to fermentation, microbial contaminants were continuously introduced to the distillery environment, resulting in a dynamic competition between the desired inoculated strain, native yeast strains and bacteria (Da Silva-Filho et al. 2005a; Basilio et al. 2008; Lucena et al. 2010; Beckner et al. 2011; Basso et al. 2014). The ethanol fermentation industry relies only on a slight portion of yeast diversity (Della-Bianca et al. 2013; Steensels et al. 2014), sometimes overlooking the potential of native strains to overcome harsh environments with high ethanol yields (Da Silva-Filho et al. 2005b; Basso et al. 2008; Lancheros et al. 2015). The huge physiological diversity among the clonal strains, a consequence of an intense process of adaptation to a specific type of substrate, stimulates the search for the most adequate strain for specific substrates. In this context, eight distillery yeast strains recovered from the Cuban ethanol industry were identified as S. cerevisiae using molecular markers and tested for the first time, screening for their profiles of fermentation capacity and tolerance to inhibitors. DNA extracted from eight representative yeast colonies recovered from molasses, using Cuban distillery samples, was submitted to sequencing of the ITS1-5.8S-ITS2 rDNA region. All strains produced an amplicon of 850 bp (data not shown), similar to JP-1 S. cerevisiae strain (Basilio et al. 2008). All DNA sequences obtained from yeast strains presented > 99% identity to S. cerevisiae sequences in the NCBI database (data not shown) and confirmed the identity of the yeast strains (Basilio et al. 2008). Furthermore, nucleotide sequences of the 140 bp region of ITS1 locus were used to verify the intraspecific variability among the isolates by clustering analysis. The results showed that all isolates were separated due to the presence of polymorphic positions in nucleotide sequences (Fig. 1), characterizing their distinct clonal origin. Therefore, the isolates were genetically different and further analysis was performed to test whether these differences were also reflected in the physiological traits.
Clustering analysis using the UPGMA method of the eight industrial isolates of Saccharomyces cerevisiae from a partial sequence of the ITS1 locus. Nucleotide sequences from S. cerevisiae S288c and Candida glabrata NRRL Y-68 were recovered from NCBI/Genebank and used as internal reference and outgroup control, respectively
Fermentation trials
Fermentation experiments performed herein used the synthetic minimal medium as reference for the comparison with three industrial compositions of sugarcane derivatives. In general, the industrial plants work with substrates containing total assimilable sugars in the range of 120–160 g/L, which means that the crude substrates (either the juice or its molasses) are normally diluted with water to reach that range of concentration. However, as the use of water is becoming restrictive and since the constant use of vinasse in the soil has environmental concerns (Christofoletti et al. 2013), some productive areas of the globe decided in recycling vinasse for the dilution of the fermentation substrates.
Fermentation experiments were carried out with each one of the eight strains in four different media conditions named as reference medium (synthetic minimal medium—REF) and three industrial compositions of sugarcane juice diluted with water (FM1), sugarcane molasses diluted with water (FM2) or sugarcane molasses diluted with vinasse (FM3). Therefore, eight data were produced for each substrate, while four data were produced for each strain. First, we analyzed the general aspects of fermentation considering each wort by the mean values of the fermentative parameters from all strains (Table 1). Sugar consumption by the yeast cells was significantly higher in sugarcane juice (FM1) and similar between the reference medium (REF) and molasses (FM2). The presence of vinasse lowered sugar uptake from molasses (FM3). This by-product presents a chemical composition that can be detrimental not only to the soils, but also for the microorganisms (Christofoletti et al. 2013). Ethanol production followed the same pattern, with the difference that it was significantly higher in the reference medium than in molasses. Hence, ethanol yield was similarly higher in REF and FM1 and lower in FM2 or FM3 (Table 1). This result agrees with previous report showing better fermentation efficiency in sugarcane juice than in molasses (Kaushal and Phutela 2015). The quality of molasses also influences microbial growth (García and Otero 2015). On the other hand, the presence of vinasse in molasses did not potentiate this lower fermentability of molasses. This was a clear indication that whatever might be present in vinasse composition, its composition made the uptake of sugars difficult, but stimulated its conversion to ethanol by imposing some sort of redox imbalance. This last result was very interesting since there is an increasing concern on the use of vinasse for field fertilization due to environmental concerns in some of the world’s agricultural regions. This means that industries will have to find an efficient means of using this by-product. Some industrial plants located in Valle del Cauca, Colombia, use vinasse to dilute molasses instead of water. In Cuba, vinasse is used to dilute molasses for cultivation of Candida utilis as a single cell protein. Hence, the isolation of vinasse-tolerant strains like those in this work seems a relevant subject for such areas.
Afterward, each strain was analyzed in all four media to identify the most appropriate yeast for a specific substrate. Yeast strains can differ regarding their capacity to assimilate sugars and convert them to ethanol, as well as in their adaptability to specific type of substrate. Da Silva-Filho et al. (2005a) reported the isolation of strain genotypes mostly observed in sugarcane juice than in molasses, and vice versa, from Brazilian industries. In addition, the authors showed that those strains also differed in terms of ethanol yield and glycerol production (Da Silva-Filho et al. 2005b). Later on, other reports also showed the diversity of the Brazilian strains regarding the substrate used for isolation (Basso et al. 2008; Della-Bianca et al. 2013). In the present work, we tested the capacity of the Cuban isolates to assimilate sugars present in industrial substrates (Fig. 2). The strain L/25-7-79 displayed the best performance in FM2, while the strain L/25-7-77 exhibited the highest sugar consumption in both FM1 and FM3 (Fig. 2, data statistically significant at p < 0.05 with HSD test). Calculation of ethanol yield showed that L/25-7-81 and L/25-7-86 more efficiently converted sucrose from FM1 to ethanol, while L/25-7-12 and L/25-7-79 were more efficient when FM2 was the substrate (Table 2). This substrate specificity has been reported, with the strain JP1 adapted to sugarcane juice, while P6 and PE-2 strains were more adapted to molasses (Da Silva-Filho et al. 2005a; Della-Bianca et al. 2013). Interestingly, L/25-7-86 showed the best efficiency when fermenting FM3. It indicates that this isolate might be very tolerant to oxidants and excess of minerals present in the vinasse. Regarding specific ethanol productivity (Qp), L/25-7-81 and L/25-7-79 were the best performing strains in this parameter using FM1 and FM2, respectively (Table 2). Again, L/25-7-86 showed remarkable Qp value when fermenting FM3 (Table 2). Lastly, we calculated the production of the by-product glycerol relative to the main fermentation product ethanol (KGly). Significant differences (p ≤ 0.05) between strains fermenting in all the natural sources were detected despite the synthetic formulation with sucrose. The results highlighted the strains L/25-7-77 and L/25-7-79 with significant reduced glycerol/ethanol ratio in FM1 and FM2, respectively. In this case, L/25-7-79 was more efficient in avoiding glycerol production when fermenting FM3 (Table 2). By scoring all these parameters for all four media, the results showed that the strain L/25-7-81 presented the highest performance for fermentation of FM1, L/25-7-79 for FM2 and L/25-7-86 for FM3.
Response of the yeast strains to fermentation inhibitors
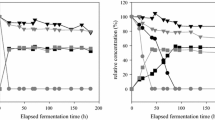
Growth kinetics analyses were carried out only in the REF medium due to difficulty in reading absorbance in the industrial substrates. In general, a huge variation was observed in the growth rates from 0.09 to 0.24 h− 1. Besides, strains could be divided into two groups: the first group composed of strains L/25-7-81 and L/25-7-90 displaying a short exponential growth phase and early entry to stationary phase, and the second group composed of the other six strains that showed slower exponential growth phase (Fig. 2). Afterward, we tested the growth profile of these strains in the presence of inhibitor molecules, such as those found in processes using sugarcane juice (lactic acid produced by lactic acid bacteria) (Beckner et al. 2011; Basso et al. 2014) or lignocellulosic hydrolysates (acetic acid or 5-HMF) (Jönsson and Martín 2016). Strategies are being increasingly sought to increase the tolerance of yeasts to different forms of stress in such a way as to increase ethanol production from non-conventional substrates (Deparis et al. 2017). Molasses also present molecules with oxidant potential, such as 5-HMF, due to thermal-induced transformation of hexoses during sugar milling (García and Otero 2015). Besides, acidic stress can also be trigged when yeast biomass is treated with dilute sulfuric acid during the recycles to control the bacterial population (De Melo et al. 2010). The presence of acetic acid at mild concentrations as 5 g/L did not affect the growth kinetics of all isolates (Fig. 3). Only isolates L/25-7-81 and L/25-7-90 showed abrupt drop in cell density of the cultures after 15 h of cultivation at this concentration. Moreover, 5-HMF at 5 g/L did not influence cell growth as well (Fig. 3). Previous work reported that 5-HMF added up to 4 g/L reduced CO2 evolution rate, but did not impair cell growth unless in the presence of furfural (Taherzadeh et al. 2000).
Comparative analysis of sugar consumption among eight isolates of Saccharomyces cerevisiae isolated from Cuban distilleries in synthetic reference medium (white columns), sugarcane juice (white hatched columns), sugarcane molasses (gray columns) or molasses diluted with vinasse (black columns). All values are presented as the means of biological triplicates (CV < 20%). Asterisks represent significant differences (p < 0.05) for assimilation of sugars in the different substrates for each isolate
Growth profiles of eight S. cerevisiae isolates collected from Cuban ethanol-producing distilleries: a L/25-7-81, b L/25-7-82, c L/25-7-86, d L/25-7-90, e L/25-7-80, f L/25-7-12, g L/25-7-77 and h L/25-7-79. Symbols refer to the following growth conditions: reference medium without inhibitor at pH 4.5 (squares), in the presence of acetic acid at 15 g/L and pH 4.5 (open circles), in the presence of 5-HMF at 6 g/L and pH 4.5 (crosses), in the presence of lactic acid at 15 g/L and pH 4.5 (triangles) and in the absence of inhibitors and pH adjusted to 2.0 with H2SO4 (closed circles). The means of biological triplicates (n = 3) with CV < 20% were only represented
Acidic stress was analyzed using inorganic (sulfuric) or organic (lactic and acetic) acids. The effect of inorganic acid was tested by adjusting the pH of the reference medium to 2.0 with sulfuric acid, which represented 10 mM of dissociated H+ in the medium. In this condition, the cell growth of all isolates did not differ from those displayed by the cells in unadjusted medium (pH 4.5) (Fig. 3). Besides, isolates L/25-7-82, L/25-7-12 and L/25-7-79 stood out as isolates with the best performance in this harsh condition. This result showed that Cuban isolates were perfectly adapted to acidic environment. This is a quite interesting result since yeast isolates, even industrial ones like JP1 (De Melo et al. 2010), are very difficult to grow in synthetic medium adjusted to such low pH. However, this acidic environment seemed to hardly affect the fermentation efficiency of the cells (De Melo et al. 2010). Only when the cells were submitted to an adaptive selection approach, it was possible to select a derivative mutant strain JP1M that grew in synthetic reference medium at pH 2.0 adjusted with sulfuric acid (De Melo et al. 2010). Again, the results showed that Cuban isolates herein are the product of an adaptive selection process along the years of permanence in those distilleries that lead to metabolic re-organization to stress tolerance.
In the presence of lactic acid at 15 g/L, the isolates L/25-7-82, L/25-7-80 and L/25-7-77 showed a growth kinetic profile very close to the reference medium, while L/25-7-81 was highly sensitive to this inhibitor (Fig. 2). Moreover, the isolates L/25-7-86, L/25-7-12 and L/25-7-79 behaved like L/25-7-80 in the presence of this inhibitor. Moreover, lactic acid seemed to be used as carbon co-substrate for the isolates L/25-7-82, L/25-7-86, L/25-7-12, L/25-7-77 and L/25-7-79 in the reference medium, with a two times increase in biomass production of L/25-7-79 after 24 h of cultivation. It is plausible to assume that these isolates submitted to permanent exposure to sub-toxic doses of inhibitors (lactic acid plus furfurals) could evolve to a de-repressed metabolic condition that leads to the release of stress tolerance as well as residual lactate co-consumption with glucose, with increased tolerance to lactate as a consequence. Acetic acid at 15 g/L was very toxic for all eight isolates, including those tolerant to 5-HMF and lactic acid. In this study, a high sensitivity to acetic acid at 15.0 g/L was evident for all isolates. Referencing results were obtained by Guo and Olsson (2014) who conducted a physiological study with the S. cerevisiae CEN.PK 113-7D strain. This type of strain coped with concentrations up to 13.0 g/L with prolonged latency phase, but with a reduced maximum specific growth rate.
Another class of inhibitors includes furfurals and furaldehydes produced from oxidation of sugars and aromatic compounds present in sugarcane juice during sugar milling or from thermal acid treatment of lignocellulosic plant biomass. Among them, 5-hydroxymethyl furfural (5-HMF) is considered a strong inhibitor of yeast growth and fermentation capacity (Taherzadeh et al. 2000). Reduced growth was observed for all isolates in the presence of 5-HMF at 6 g/L, with decreased cell density by 10–15 h of cultivation, followed by restoration of growth thereafter. Growth data showed a remarkable performance of L/25-7-82 and L/25-7-77 isolates that could be assigned as tolerant for this oxidant molecule (Fig. 3b, g). Pereira et al. (2016) reported that S. cerevisiae IMS0351 strain displayed tolerance growing at concentrations up to 1.5 g/L of 5-HMF. Moreover, no growth was observed for any of the eight isolates in the present study when 5-HMF was added to 15 g/L (data not shown), similar to that reported for S. cerevisiae NRRL Y-12632 and ATCC211239 (Liu 2006). Previous work reported that the fuel-ethanol industrial strain JP1 was able to growth in the presence of 5-HMF at 3.5 g/L, while its derivative mutant strain P18R selected from adaptive selection experiments grew at 5 g/L (Sehnem et al. 2013). On the other hand, the fuel-ethanol strain P6 collected from molasses-based industry (Da Silva-Filho et al. 2005a) was already tolerant to this condition. When submitting P6 to the adaptive selection approach, a derivative mutant strain P6H9 was obtained that tolerated 5-HMF even at 6 g/L. These natural strains JP1 and P6 diverge regarding their substrate of selection: JP1 was selected from distillery that uses sugarcane juice, while P6 was selected from molasses-using distillery (Da Silva-Filho et al. 2005a, b). Altogether, these results indicated that cells pre-adapted in molasses environment that contains residual concentration of furfurals, such as P6 or the Cuban isolates selected here (that already tolerate 5-HMF at 5 g/L), are more adequate for the fermentation of plant hydrolysates that contain these inhibitors at higher concentration. The problem is that such metabolic re-organization in the adapted cells that leads to 5-HMF tolerance was accompanied by a decrease in ethanol yield in natural tolerant molasses strain P6 and even more in the adapted derivative P6H9 when fermenting the synthetic reference medium (Sehnem et al. 2013). Indeed, we observed that all the eight Cuban isolates tested here that were naturally tolerant to 5-HMF at 5 g/L also presented lower ethanol yield (Table 2) compared to other industrial isolates from sugarcane juice (Sehnem et al. 2013; Da Silva-Filho et al. 2005a, b).
The protective effect of magnesium ion against copper toxicity has been reported by our previous work (De Barros Souza et al. 2015) and hence we tested whether it could protect the yeast cells against fermentation inhibitors. Its addition to synthetic media at 0.5 g/L did not show significant improvements regarding isolate tolerance for any of the inhibitory conditions assessed. In overall terms, it was observed that L/25-7-82 and L/25-7-77 showed the best performance among the isolates when in the presence of these inhibitors independent of Mg2+, which indicated that these isolates evolved for naturally tolerant yeasts.
With all these physiological data, we attempted to select high-performance isolates that have high fermentative capacity and stress tolerance. For this purpose, each isolate received a score (one to eight) and was ordered for both parameters (Table 3). Regarding fermentative capacity, means of brix consumption (from Fig. 2) and of ethanol yield, ethanol productivity and relative glycerol production (from Table 2) were calculated for each isolate in all four fermentation conditions. Then, the isolates were classified from the highest (score eight) to the lowest (score one) performance for the first three parameters, and highest (score one) to the lowest (score eight) glycerol production. These partial four scores were summed and then the isolates were again classified from eight (higher sum result) to one (lowest sum result) to give the final physiological score (FC parameter in Table 3). A similar approach was used for stress tolerance score (ST parameter in Table 3), in which the isolates were classified from the highest (score eight) to the lowest (score one) tolerance for each of the four stressors. The sum of the four tolerance scores produced the ST parameter. Table 3 summarizes the ranking of the isolates firstly based on the highest fermentation capacity, showing that isolates S. cerevisiae L/25-7-77 and L/25-7-79 were outstanding in their performance of converting sugar for any of the fermentation conditions tested toward ethanol with high yield in a shorter time and high tolerance to fermentation inhibitors. Besides, two contrasting isolates were also revealed from this analysis: L/25-7-81 with high fermentation capacity and stress sensitivity phenotype, and L/25-7-82 with the opposite profile (Table 3). It is well known that strains with higher tolerance to stressing agents are normally less efficient in fermentation, as we reported for tolerance to acid stress (Melo et al. 2010). Therefore, the major challenge in this kind of strategy is now to combine these characteristics (each one depending on the quantitative heritage) in a single strain.
Conclusion
In this study, the fermentation capacity and tolerance to inhibitors of eight S. cerevisiae isolates from Cuban distilleries were studied. Comparing these isolates, S. cerevisiae L/25-7-77 showed considerable tolerance to typical fermentation inhibitors and significantly best fermentation parameters in sugarcane by-products-based media among the isolates tested. Based on the results, we concluded that the relationship between stress tolerance and fermentative performance is a specific trait in S. cerevisiae isolates from molasses-based distilleries. Quantitative physiology assessment must be considered as an important tool in searching local ethanol producer yeasts. The study leads to further investigation toward these industrial S. cerevisiae isolates could be prospect to second ethanol generation approaches.
References
Aguilar R, Ramírez JA, Garrote G, Vázquez M (2002) Kinetic study of the acid hydrolysis of sugar cane bagasse. J Food Eng 55:309–318. https://doi.org/10.1016/S0260-8774(02)00106-1
Almeida J, Modig T, Petersson A, Hähn-Hägerdal B, Lidé G, Gorwa-Grauslund MF (2008) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Tech Biotechnol 82:340–349. https://doi.org/10.1002/jctb.1676
Barros de Souza R, de Menezes JA, Rodrigues de Souza RF, Dutra ED, de Morais MA (2015) Mineral composition of the sugarcane juice and its influence on the ethanol fermentation. Appl Biochem Biotechnol 175: 209–222. https://doi.org/10.1007/s12010-014-1258-7
Basílio ACM, Araújo PRL, Morais JOF, Silva-Filho EA, de Morais MA, Simões DA (2008) Detection and identification of wild yeast contaminants of the industrial fuel ethanol fermentation process. Curr Microbiol 56:322–326. https://doi.org/10.1007/s00284-007-9085-5
Basso LC, de Amorim HV, de Oliveira AJ, Lopes ML (2008) Yeast selection for fuel ethanol production in Brazil. FEMS Yeast Res 8:1155–1163. https://doi.org/10.1111/j.1567-1364.2008.00428.x
Basso TO, Gomes FS, Lopes ML, de Amorim HV, Eggleston G, Basso LC (2014) Homo- and heterofermentative lactobacilli differently affect sugarcane-based fuel ethanol fermentation. Antonie Van Leeuwenhoek 105:169–177. https://doi.org/10.1007/s10482-013-0063-6
Beckner M, Ivey M, Phister T (2011) Microbial contamination of fuel ethanol fermentations. Lett Appl Microbiol 53:387–394. https://doi.org/10.1111/j.1472-765X.2011.03124.x
Bischoff K, Liu S, Leathers TD, Worthington RE, Rich JO (2009) Modeling bacterial contamination of fuel ethanol fermentation. Biotechnol Bioeng 103:117–122. https://doi.org/10.1002/bit.22244
Christofoletti CA, Escher JP, Correia JE, Marinho JF, Fontanetti CS (2013) Sugarcane vinasse: environmental implications of its use. Waste Manag 33:2752–2761. https://doi.org/10.1016/j.wasman.2013.09.005
Da Silva-Filho E, Santos SK, Resende AM, Morais JO, de Morais MA, Simões DA (2005a) Yeast population dynamics of industrial fuel-ethanol fermentation process assessed by PCR fingerprinting. Antonie Van Leeuwenhoek 88:13–23. https://doi.org/10.1007/s10482-004-7283-8
Da Silva-Filho E, Melo HF, Antunes DF, dos Santos SK, Resende MA, Simões DA (2005b) Isolation by genetics and physiological characteristics of a fuel-ethanol fermentative S. cerevisiae strain with potential for genetic manipulation. J Ind Microbiol Biotechnol 32:481–486. https://doi.org/10.1007/s10295-005-0027-6
De Melo H, Bonini BM, Thevelein J, Simões DA, de Morais MA (2010) Physiological and molecular analysis of the stress response of Saccharomyces cerevisiae imposed by strong inorganic acid with implication to industrial fermentations. J Appl Microbiol 109:116–127. https://doi.org/10.1111/j.1365-2672.2009.04633.x
Della-Bianca BE1, Basso TO, Stambuk BU, Basso LC, Gombert AK (2013) What do we know about the yeast strains from the Brazilian fuel ethanol industry? Appl Microbiol Biotechnol 97:979–991. https://doi.org/10.1007/s00253-012-4631-x
Deparis Q, Claes A, Foulquié-Moreno MR, Thevelein JM (2017) Engineering tolerance to industrially relevant stress factors in yeast cell factories. FEMS Yeast Res 17:fox036. https://doi.org/10.1093/femsyr/fox036
Dutra E, Neto A, Barros R, de Morais MA, Tabosa JN, Simões R (2013) Ethanol production from the stem juice of different sweet sorghum cultivars in the state of Pernambuco, northeast of Brazil. Sugar Tech 15:316–321. https://doi.org/10.1007/s12355-013-0240-y
García R, Otero M (2015) Almacenamiento de mieles: reacciones de deterioro y sus consecuencias para el crecimiento microbiano. In: Aprovechamiento de las mieles de la caña de azúcar. Conocimientos y potencial. ICIDCA, Havana, 1–6
Guo Z, Olsson L (2014) Physiological response of Saccharomyces cerevisiae to weak acids present in lignocellulosic hydrolysate. FEMS Yeast Res 14:1234–1248. https://doi.org/10.1111/1567-1364.12221
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids 41:95–98
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Biores Technol 199:103–112. https://doi.org/10.1016/j.biortech.2015.10.009
Kausal N, Phutela R (2015) Ethanol production from molasses and sugarcane: inoculum effects and costing. J Energy Res Environ Technol (JERET) 2:385–388
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Lancheros S, Morales D, Velásquez M (2015) Increase in second generation ethanol production by different nutritional conditions from sugarcane bagasse hydrolysate using a Saccharomyces cerevisiae native strain. Chem Eng Trans 43:223–228. https://doi.org/10.3303/CET1543038
Larsson S, Palmqvist E, Hahn-Hägerdal B, Tengborg C, Stenberg K, Zacchi G, Nilvebrant N (1999) The generation of inhibitors during dilute acid hydrolysis of softwood. Enz Microb Technol 24:151–159. https://doi.org/10.1016/S0141-0229(98)00101-X
Liu Z (2006) Genomic adaptation of ethanologenic yeast to biomass conversion inhibitors. Appl Microbiol Biotechnol 73:27–36. https://doi.org/10.1007/s00253-006-0567-3
Liu Z, Moon J (2009) A novel NADPH-dependent aldehyde reductase gene from Saccharomyces cerevisiae NRRL Y-12632 involved in the detoxification of aldehyde inhibitors derived from lignocellulosic biomass conversion. Gene 446:1–10. https://doi.org/10.1016/j.gene.2009.06.018
Lucena B, dos Santos BM, Moreira JLS, Moreira APB, Nunes AC, Azevedo V, Miyoshi A, Thompson FL, de Morais MA (2010) Diversity of lactic acid bacteria of the bioethanol process. BMC Microbiol 10:298. https://doi.org/10.1186/1471-2180-10-298
Makanjuola D, Springham D (1984) Identification of lactic acid bacteria isolated from different stages of malt and whisky distillery fermentations. J Inst Brew 90:13–19. https://doi.org/10.1002/j.2050-0416.1984.tb04226.x
Modig T, Liden G, Taherzadeh M (2002) Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem J 363:769–776. https://doi.org/10.1042/bj3630769
Naik S, Goud V, Rout P, Dalai A (2010) Production of first- and second-generation biofuels: A comprehensive review. Renew Sustain Energy Rev 14:578–597. https://doi.org/10.1016/j.rser.2009.10.003
Pereira J, Verheijen P, Straathof A (2016) Growth inhibition of S. cerevisiae. B. subtilis and E. coli by lignocellulosic and fermentation products. Appl Microbiol Biotechnol 100:9069–9080. https://doi.org/10.1007/s00253-016-7642-1
Sanchez B, Bautista J (1988) Effects of furfural and 5-hydroxymethylfurfural on the fermentation of Saccharomyces cerevisiae and biomass production from Candida guilliermondii. Enz Microb Technol 10(5):315–318. https://doi.org/10.1016/0141-0229(88)90135-4
Sehnem N, Machado AS, Leite FC, Pita WB, de Morais MA, Ayub MA (2013) 5-Hydroxymethylfurfural induces ADH7 and ARI1 expression in tolerant industrial Saccharomyces cerevisiae strain P6H9 during bioethanol production. Biores Technol 133:190–196. https://doi.org/10.1016/j.biortech.2013.01.063
Steensels J, Snoek T, Meersman E, Picca Nicolino M, Voordeckers K, Verstrepen KJ (2014) Improving industrial yeasts strain: exploiting natural and artificial diversity. FEMS Microbiol Rev 38:947–995. https://doi.org/10.1111/1574-6976.12073
Taherzadeh M, Gustafsson L, Niklasson C (2000) Physiological effects of 5-hydroxymethylfurfural on Saccharomyces cerevisiae. Appl Microbiol Biotechnol 53(6):701–708. https://doi.org/10.1007/s002530000328
Acknowledgements
K. T. was supported by the United Nations University (UNU-BIOLAC Biotechnology for Latin America and The Caribbean) and by the Pérez-Guerrero Trust Fund for South–South Cooperation of UNDP in the frameworks of projects INT/13/K08 and INT/16/K10. This work was partially sponsored by UNU-BIOLAC program and by the Bioethanol Research Network of the State of Pernambuco (CNPq-FACEPE/PRONEM APQ-1452-2.01/10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Rights and permissions
About this article
Cite this article
Cabañas, K.T., Peña-Moreno, I.C., Parente, D.C. et al. Selection of Saccharomyces cerevisiae isolates for ethanol production in the presence of inhibitors. 3 Biotech 9, 6 (2019). https://doi.org/10.1007/s13205-018-1541-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1541-3