Abstract
Experimental investigations were carried out to develop economic production process of cellulase using coconut mesocarp as an inexpensive lignocellulosic inducer while replacing commercial cellulose. Cellulase production was initially investigated from commercial cellulose in different submerged conditions using Trichoderma reesei (MTCC 164). Maximum enzyme production was achieved 6.3 g/l with activity level 37 FPU/ml in the condition where cellulose to water content ratio was maintained at 5:35 (W/V). To achieve similar maximum production of cellulase from coconut mesocarp, response surface methodology was implemented to optimize most influencing parameters. Most influencing nutritional parameters such as coconut mesocarp, glucose and peptone were optimized in the concentration ranges of 35 g/l, 35 g/l and 25 g/l, respectively. Selecting optimized parameter values, fermentations were conducted inside the fermenter with 2 L operating volume to ensure high concentration and activity profiles of enzyme. Enzyme concentration was achieved 7.20 g/l after 96 h of batch fermentation with specific activity levels of 42 FPU/ ml and CMCase 75 U/ml. Enzyme concentration was further improved to 9.58 g/l with activity levels of 54 FPU/ml and CMCase 93 U/ml by adopting sequential feeding of coconut mesocarp in fed-batch fermentation mode. The presence of pure cellulase in the sample was confirmed by FTIR analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To deal with the future energy demand with increasing population and industrialization, concept for conversion of “waste to energy” has been prioritized in most of the developed countries. Among the diversified waste materials, lignocellulosic biomass in the form of plant wastes has been largely explored as a potential biorefinery resource for production of renewable energy. Worldwide annual production of plant dry material in the form of lignocellulosic waste was estimated around 100 billion tones (Sajith et al. 2016) which makes lignocellulosic waste as suitable and abundant feed stock for production of value-added products such as bio-ethanol, xylitol, and carboxylic acids. Among lignocellulosic wastes, coconut mesocarp got very little attention and normally been burned as fuel for different purposes. Air emission from such burning process is not only threat for public health but also related to the wasting of natural resources. Coconut mesocarp is considered as a potential feed stock for production of value-added products including bio-ethanol due to the presence of high content of cellulose (43.4%) and hemicellulose (19.9%) (Miftahul Jannah and Asip 2015). Across the world, 200 countries involved in the cultivation of coconut and global production of coconut was estimated around 55 million tons in 2009 (FAO). Mainly Philippines, Indonesia, India and Brazil are the four largest producer countries of coconut and while producing 11,930,000 metric tonnes of coconut in 2014, India got the third position (FAOSTAT Data 2016).
Bioconversion of valuable chemicals from lignocellulosic biomass requires combined pretreatment processes and catalytic degradation of that substrate. Among the two methods of catalytic degradation: acidic and enzymatic, enzymatic hydrolysis comes up with better saccharification efficiency with maximum sugar extraction (Rana et al. 2014). Hazardous and non-recyclable nature of acids restricts its application from large-scale industrial use. Meanwhile, lower reaction rates and expensive nature of high enzymatic dosages saccharification processes are considered as main bottlenecks for biorefinery (Li et al. 2016). The drawback associated with the expensive nature of enzymatic saccharification process could be overcome through the onsite production of enzymes from inexpensive and abundant carbon sources rather than using commercial cellulose as substrate (Yang 2007; Rana et al. 2014). For more than five decades, Trichoderma reesei has been the prime focus of research for production of such selected cellulase enzymes in the form of cocktail (Hasunuma et al. 2013). The cellulolytic enzymes extracted from T. reesei are most efficient for hydrolysis and conversions of lignocellulosic biomass to fermentable sugars. In addition to cellulases, Trichoderma species are also capable to produce xylanases. Cellulolytic machinery and genome of Trichoderma species specifically T. reesei has been most widely studied. Ten cellulose- and 16 hemicellulose-producing genes were identified from its genome (Martinez et al. 2008). Although from 1960s, the cellulase has been mainly used in food, pulp, paper, textile industries and even pharmaceutical industries (Kataria and Ghosh 2011) but it was less exploited in the hydrolysis process of plant-based lignocellulosic material to produce value-added products (Kataria and Ghosh 2011). For production of such selected cellulase enzymes, ideal interaction between microorganism and lignocellulosic substrate material is the most fundamental criteria during fermentation. To facilitate such condition, suitable pretreatment strategies of such native lignocellulosic material play a vital role. Comparing all advanced pretreatment techniques for breaking down the crystalline structure of lignocellulosic material, liquid hot water (LHW) techniques are considered most economic (Zhuang et al. 2016). At elevated temperature (160–240 °C), liquid hot water shows acid–base catalytic property to cleave ester bonds and it necessities addition of no other chemical (Zhuang et al. 2016) in the same process. Among low-energy-demanding pretreatment process, sodium hydroxide treatment is more susceptible towards production of low toxic compounds during disruption of lignin barrier and can effectively reduce crystallinity of lignocellulosic material (Kataria and Ghosh 2011). Hence, combined pretreatment strategy consisting of LHW treatment followed by sodium hydroxide treatment definitely promotes maximum delignification by breaking down cross-linking ester bonds made by lignin and increases internal surface area of lignocellulosic compounds.
Submerged fermentations (SmF) have been traditionally used for the production of industrially important enzymes due to better control over environmental factors such as temperature, nutrient gradient and pH, more specifically in bioreactor (Howard et al. 2003; Singhania et al. 2010). Controlling such parameters are considered as the biggest drawback of solid-state fermentation (SSF) (Singhania et al. 2010) although SSF comes up with higher yield of enzymes while providing suitable growth environment and conditions in which filamentous fungi can naturally adapt (Cunha et al. 2012). Several submerged fermentation (SmF)-based studies have been successfully conducted for the production of cellulase using T. reesei from substrates such as wheat bran, lactose and kans grass (Sateesh et al. 2012; Rana et al. 2014; Li et al. 2016). For conducting a new research using same genus but different species, it is still necessary to identify in which condition (SmF or SSF) the strain performs optimally well for enzyme production from an unknown substrate. Submerged batch process is the widely used method for cellulase production; however, higher yields of cellulase productions have been claimed through fed-batch techniques (Belghith et al. 2001; Ximenes et al. 2007). Fed-batch technique is also effective to reduce the negative effects of catabolic repression and viscosity (Esterbauer et al. 1991).
Supplementation of commercial cellulose in production media can definitely be fruitful for production of higher concentration of cellulase but the process will be economically non-favorable. In this article, such commercial cellulose powder was utilized first for maximum production of cellulase while varying water content in submerged fermentations to know specifically the better environmental condition and adaptability of T. reesei (MTCC 164). Such suitable environmental condition was optimized by design expert statistical software for improved production of cellulase from coconut mesocarp-based lignocellulosic bio-waste material. Finally, fed-batch fermentation strategy was developed with response surface-optimized conditions for enhanced production of cellulase. Previously, a significant number of studies were conducted specifically for bio-ethanol production from coconut mesocarp-based substrate (Gonçalves et al. 2014, 2016; Miftahul Jannah and Asip 2015; Avelino et al. 2015; Soares et al. 2016; Cabral et al. 2016; Ebrahimi et al. 2017); however, no cellulase production study was reported from such highly abundant lignocellulosic bio-waste material.
Materials and methods
Mesocarp separation from coconut
Dried coconut fruits without the liquid albumen (coconut water) were collected from different hotels of Karunya Nagar, Coimbatore, where the materials were initially brought as waste fuel material in huge amount. Manual extraction of such fruit mesocarp was carried out with a stainless steel knife and biomass size was further reduced to few mm by a cizer cutter. Mesocarp material is then ground in powder form using ball mill and screened through 20-mesh (0.841 mm) standard sieve prior to pretreatment. The raw material was washed five times with distilled water for the removal of residual compounds and it was dried at 105 °C in a hot air oven to reduce the moisture content. The material was stored in zipper plastic bags and placed in a refrigerator at 4 °C for future use.
Pretreatment processes for delignification
Liquid hot water treatment is considered as most efficient pretreatment method which is capable to cleave the ester and other bonds of lignocellulosic material while having both acid–base catalytic property and can efficiently liberate ferulic acid residue, lignin fragment and carbohydrate. The LCMs (lignocellulosic materials) were mixed with water at a ratio of 1:20 solid/liquid (V/W). The treatment temperature was maintained at 210 °C for 20 min. After such pretreatment, water-insoluble solids (WIS) and prehydrolysates can be separated by filtration. Such WIS was stored at 4 °C for future use. Liquid hot water treatment has been evolved as most promising techniques which can generate complete sugar recovery while utilizing a low capital investment (Menon and Rao 2012; Loaces et al. 2017). To achieve maximum delignification efficiency finally the solid residue was treated with NaOH. In this treatment procedure, 3 gm of liquid hot water-treated material (WIS) was further treated with 0.5% (W/V) NaOH where the samples were maintained at 180 °C for 40 min.
Analysis of delignification
Amount of total cellulose, hemicellulose content and total lignin removal (including acid-soluble lignin and acid-insoluble lignin) ware measured using National renewable Energy Laboratory (NREL) standard protocol (Sluiter et al. 2008). As the total amount of lignin was gradually removed from LCM, the crystallinity of the material was also reduced. To analyze that, surfaces of untreated and pretreated LCM at different conditions were examined by scanning electron microscopy (SEM by NanoSEM 200). During that process, samples were covered by a gold layer and 15 kV voltages were applied.
Microorganism and its maintenance
Trichoderma reesei (MTCC 164) was purchased from MTCC Chandigarh. According to the MTCC procedure, the culture was maintained on potato dextrose agar at 4 °C. The mother culture was prepared and stored in a media composition of malt extract 20 g/l, dextrose 20 g/l, peptone 6 g/l and agar–agar 15 g/l of distilled water with pH 5.5.
Inoculum preparation for fermentation
Fungal spores (4 × 107 spores/ml) from 14- to 20-day-old solid plates were transferred to 50 ml inoculum medium composed of 5 g/l glucose and 10 g/l yeast extract. To develop the adaptability of microorganism in lignocellulosic environment, the medium was supplemented with 1 g/l cellulose powder. The inoculum culture was developed by incubating it at 28 °C and at 150 rpm in 250-ml conical flask for 48 h.
Submerged fermentations using commercial cellulose
The composition of basic nutrient solution used for all the experiments was 0.3 g/l of Urea, 1.4 g/l of (NH4)2SO4, 0.4 g/l of CaCl2, 2H2O, 2 g/l of KH2PO4, 0.3 g/l of MgSO4, 7 H2O, and 1 g/l of peptone, including mineral salt solution with composition of 0.1% of tween-80, 0.005 g/l of FeSO4,7H2O, 0.016 g/l of MnSO4,H2O, 0.014 g/l of ZnSO4, 7H2O, 0.002 g/l of CoCl2, and 6H2O. Now for the variation of different water content in submerged fermentation experiments (semisolid to submerged fermentation), five sets of experiments, namely T1–T5, were conducted with commercial cellulose powder. In experiment T1, 30 g/l of cellulose, 30 g/l of glucose, and 20 g/l of peptone were added for the requirement of 210 ml final media volume in 150 ml of distilled water. The final volume of the solution was maintained at 210 ml while adding 60 ml of basic nutrient solution. In experiment T2, 15 g/l cellulose, 5 g/l lactose, 5 g/l glucose, and 5 g/l peptone were added directly in 90 ml of nutrient solution. In experiments T3–T5, 2 g/l lactose, 2 g/l glucose, and 2 g/l peptone were supplemented and the ratios of commercial cellulose with water content were maintained at 5:24 (W/V), 5:16 (W/V) and 5:8 (W/V). To maintain such water content in all those experiments (T3–T5), 5 gm of cellulose powder was mixed with 24 ml, 16 ml and 8 ml of nutrient solutions, respectively. All the solutions were prepared in different 500-ml conical flasks, pH of the solutions was maintained at 6.0 and finally all the flasks are autoclaved at 121 °C for 15 min prior to inoculation. Inoculum volume was maintained 10% (V/V) of working volume of the reaction. Fermentations were carried out with an agitation rate of 150 rpm at 30 °C for 7 days.
Submerged fermentation using coconut mesocarp
While considering the suitable condition (T1) for cellulase production from commercial cellulose substrate, media composition for same enzyme production from coconut mesocarp-based substrate was optimized with the help of Design Expert software (version 8.0.4), response surface methodology (RSM). Three most influencing factors which were optimized for submerged fermentative production of cellulase were (a) coconut mesocarp (b) glucose and (c) peptone. The upper limit (+ 1) and lower limit (− 1) of the factors are shown in Table 1. 2n-factorial central composite design (CCD) was developed using Design Expert software (version 8.0.4), which leads to total 20 numbers of experiments with different combinations of the selected variables. All experiments were carried out in 500-ml conical flasks at 150 rpm, 30 °C temperatures for 7 days. For every experiment, inoculum volume was maintained at 10% (V/V). Optimal concentrations of the variables were obtained by the regression and graphical analysis.
Separation of cellulase and determination of its activity
After fermentation, broth was filtered and centrifuged at 10,000 rpm for 5 min to separate enzyme-containing liquid. At that time enzyme concentration was determined. Finally, the broth was lyophilized to concentrate the enzyme solution in powder form and it was stored at − 20 °C for future use. For lyophilization, Labconco lyophilizer Freezone, 2.5 plus, US, was used. Before the analysis of enzyme activity, 4 gm of such crystalline enzyme was dissolved into 50 ml of citrate buffer (0.05 M) at pH 4.8. The process is represented in Fig. 1. For semi-solid culture conditions like T3–T5, 50 ml of 0.05M citrate buffer (pH 4.8) was added directly in the conical flasks and the conical flasks were maintained at 150 rpm for one more hour. After that the solution was centrifuged at 10,000 rpm for 5 min to separate the solid biomass and suspended solids. Finally enzyme concentration and its activity levels were determined for such semisolid cultures. Specific growth rate of biomass (µ) was calculated based on the graphical plotting between logarithmic ratios of individual biomass weight (ln Xf/X0) with different time intervals (t). Here X0 denotes initial total biomass weight and Xf denotes total final biomass weight at time t. As for fungal species, separating fungal biomass from insoluble remaining substrates is difficult; relative increase of total solid mass due to fungal biomass growth was considered for such logarithmic growth analysis. Total protein concentration was determined by Lowry’s method (LOWRY et al. 1951). Enzyme concentration was expressed in terms of total protein concentration and its activity levels were determined by performing filter paper assay and carboxymethyl cellulase (CMC) assay (Ghose 1987). In filter paper assay, 0.5 ml of the enzyme was diluted in 1 ml of 0.05 M Na citrate buffer and Whatman No. 1 filter paper was added in that solution. The condition for the reaction was maintained at 50 °C for 60 min. FPU (Filter paper unit)/ml was determined by measuring the enzyme concentration which can release 2 mg of glucose (Ghose 1987). Similarly, carboxymethyl cellulase assay was conducted by incubating 0.5 ml of diluted enzyme with 0.5 ml of 2% carboxymethyl cellulose substrate at 50 °C for 30 min. Carboxymethyl cellulase (U) unit was determined by analyzing enzyme dilution which can liberate 0.5 mg of glucose (Ghose 1987). Produced glucose concentrations were estimated by dinitrosalicylic acid method (DNS) method (Miller and Miller 1959).
Experimental equipment
Maximum cellulase production with predicted optimized condition was confirmed through batch-mode shake flask studies in triplicates. Finally, the same batch mode cellulase production with optimized condition was conducted in 3-l fermenter (Sartorius, Germany) equipped with pH, DO and temperature probe. The fermenter was fully automated and it was attached with thermostatically controlled water bath to maintain temperature and with a compressor for controlled aeration. For batch-mode enzyme production in fermenter, initially 1.5 L of media with optimized configurations was transferred inside the fermenter and 500 ml of basic nutrient solution was added to make the total operating volume 2 L. The fermenter was autoclaved separately and during 7 days of fermentation, temperature, agitation speed and inoculum volume were maintained at 30 °C, 120 rpm and 10% (V/V), respectively. For fed-batch cultivation in fermenter, fermentation was started with 1 L working volume (500 ml of main essential substrate concentration and 500 ml of basic nutrient solution). The compositions of essential substrates were maintained in 1 L primary volume according to the requirement of 2 L working volume except component-pretreated coconut mesocarp. 40 gm of pretreated mesocarp was added initially in that 1 L primary solution and after every 24 h of consecutive fermentation intervals, 500 ml of sterile solution containing 15 gm of coconut mesocarp was added for two times (at 24 h and 48 h) in that 1 L primary solution to maintain total 35 g/l of main inducer concentration in the media. Schematic representation of the overall experimental setup is presented in Fig. 2. Samples from the fermenter were collected periodically at 24 h of intervals in aseptic condition with the help of sample collector. Every time, 100 ml of sample volumes was collected and they were initially filtered and centrifuged at 10,000 rpm for 5 min to separate enzyme containing solution. At that time enzyme concentration was determined. Finally, enzyme solution was lyophilized to concentrate it in powder form and 4 gm of such powder was dissolved into 50 ml of citrate buffer (0.05 M) at pH 4.8 before analysis of its activity levels.
Results and discussion
Pretreatment studies
High lignin content (about 40%) (Ebrahimi et al. 2017) makes structure of coconut mesocarp-based lignocellulosic substrate comparatively complex for further processing of fermentation. During the pretreatment process with liquid hot water, total 36% lignin (including acid-soluble and -insoluble lignin) was removed at the initial stage. The achieved result was comparatively better than the result (23.12% lignin removal) shown by Lv et al. (2013) during liquid hot water pretreatment of sugarcane bagasse. Finally, maximum amount of lignin and hemicellulose reductions achieved were 62% and 48.23%, respectively, from 0.5% (W/V) NaOH treatment at 180 °C for 40 min. Such combined pretreatment of liquid hot water followed by NaOH was also effective to retain more than 92% cellulose. Such final result in terms of total lignin removal was comparatively superior than the result (46.88%) obtained by Kim and Han (2012) where rice straw type of lignocellulosic material 5% (w/w) was treated with HPCSH (hydrothermal pretreatment catalyzed with sodium hydroxide) at 80 °C for 1 h. Even better achievement in terms of total lignin removal was also achieved in comparison with Rawat et al. (2013) where Populus deltoides-based lignocellulosic material was treated with HPCSH [2.8% (W/W) followed by NaOH treatment at 94 °C for 1 h and total 12.70% of lignin was reduced]. Just like the conclusion made by Ballesteros et al. (2006), our experimental observations similarly suggest that high-temperature treatment is the main root cause for increased hemicellulose degradation. Our achieved result is also comparable with the result obtained by Kataria et al. (2013), where 79.3% of total lignin was removed from kans grass-based biomass when it was treated with 2% NaOH solution for 90 min. In another study, Zhuang et al. (2016) clearly indicates that combined pretreatment of LHW followed by AA (aqueous ammonia) was effective to degrade 75.36% lignin from lignocellulosic material. Such comprehensive and comparative result analysis clearly indicates that total lignin removal is dependent on initial structural compositions of materials, efficiency of single or combined pretreatment methodologies, temperature, concentration and residence time for NaOH treatment. The SEM images of native (Fig. 3a), LHW (Fig. 3b) and LHW–NaOH-pretreated (Fig. 3c) coconut mesocarp-based lignocellulosic substrate clearly indicate the chronological structural changes. SEM images of the LHW-pretreated and LHW–NaOH-pretreated samples clearly indicate that roughnesses of the fiber surfaces are different from untreated or native sample. Figure 3a shows highly ordered and intact surfaces of the fibers of native coconut mesocarp, whereas Fig. 3b, c specifies the changes in surface morphology, and specifically indicates modified structure with increased surface area. Those images clearly suggest that pretreatment was highly effective to increase the accessible surface area of cellulose through disordered fibers, altering particle size, wall porosity, degree of polymerization, lignin linkages and cellulose crystallinity.
Cellulase production using commercial cellulose substrate
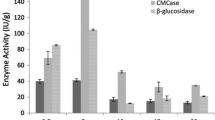
To know the suitable operating conditions for enzyme production using T. reesei (MTCC 164) from commercial cellulose, five different sets of submerged fermentations were conducted with variation of total moisture content. The experiments were termed as T1–T5 with decrease in the cellulose to water content ratios of 5:35 (W/V), 5:30 (W/V) 5:24 (W/V), 5:16 (W/V) and 5:8 (W/V). Surprisingly, the trends of cellulose enzyme productions after seventh day of fermentations were decreasing with decreasing water content ratios. Maximum cellulase production in terms of total soluble protein concentration was measured 6.3 g/l in T1 experimental condition. The corresponding maximum enzyme activity level of 37 FPU/ml was observed in T1 condition where as it was reduced to 2.96 FPU/ml at T5 experimental condition. The variations in total enzymatic concentrations and their activity levels with different experimental conditions are represented in Fig. 4. The variations in total soluble protein concentrations from T3 to T5 were not that much significant. Maximum CMCase activity was measured 72.32 U/ml in T1 operating condition and similarly this activity level of the enzyme decreases in experimental conditions with reducing moisture content. In T1 submerged operating condition, major process parameters such as temperature, agitation and pH (Hansen et al. 2015) are efficiently controlled throughout the process and it finally shows larger effect on microbial growth rate and product formation. Better control of such parameters can successfully overcome heat and mass transfer limitations, whereas effects of such limitations are clearly visible in T4 and T5 operating conditions. Along with better control of process parameters and moisture content, other most important parameters such as concentrations of peptone and glucose influences in cellulase production processes. Peptone acts as a natural source of amino acid and proteins, and its utilization largely affects in microbial growth rate, shorten culturing time, rate of product formation and product yield. On the other side, glucose acts as a basic carbohydrate source and it plays a central role to regulate initial fungal growth and its metabolism. As peptone concentrations and glucose concentrations are also varied along with decreasing water content from 20 to 2 g/l and 30 to 2 g/l, respectively, it exhibits maximum influence in enzyme concentrations and its activity levels. Kataria and Ghosh (2011) clearly defined from their submerged fermentation-based studies that cellulose acts as a most effective substrate among glucose, lactose and xylose for production of cellulase with its enhanced activity levels. In their study, CMCase activity was determined as 0.523 U/ml at pH 6 and temperature 30 °C where as the activity level increased to 1.46 U/ml at pH 5 and temperature 28 °C. At the same temperature and pH, filter paper units (FPU) was calculated as 1.14 FPU/ml. Bendig and Weuster-Botz (2013) clearly reported cellulase production with FPU level of 4.88 FPU/ml from T. reesei RUT-C30 using microcrystalline cellulose. Rana et al. (2014) maintained similar experimental condition during medium formulation while adding 50 ml of nutrient solution with 150 ml of essential nutrient solution and the estimated filter paper activity of the produced enzyme was 4.49 FPU/ml. With respect to enzyme concentration, variations in enzyme activity levels in terms of FPU for all T1–T5 operating conditions are more prominent in Fig. 4. Enzyme activity levels (FPU/ml and CMCase U/ml) achieved in our T1 operating condition were far better than other operating conditions. In T1 submerged condition, enzyme solution was properly separated, concentrated and purified in powder form by lyophilization technique. Some volatile components which can be produced during fungal cultivation can be removed through such lyophilization technique (Ito et al. 1990), whereas the presence of such impurities influences in substrate conversion efficiencies for T3–T5 operating conditions and finally FPU levels for such enzyme solutions decreases.
Cellulase production using coconut mesocarp
From previous experimental observations, cellulose, glucose and peptone are considered as most influencing parameters among variety of nutritional parameters for submerged fermentative production of cellulase by T. reesei (MTCC164). Now the effects of those influencing parameters were optimized for cellulase production from coconut mesocarp-based lignocellulosic waste materials by response surface methodology (RSM), most efficient statistical tool for experimental analysis (Dey and Rangarajan 2017). Experiments were designed based on the central composite design (CCD) which leads to total 20 numbers of experiments with variations of selected parameters and other parameters remained in the same level of T1 experimental condition. In such designed experiments, commercial cellulose was replaced with pretreated and delignified coconut mesocarp-based lignocellulosic material. Experiments were conducted according to the design and results were incorporated in the response parameter position as it is represented in Table 2. Experimental observation in terms of maximum cellulase production was achieved in run 19 condition. To determine the interactive relationships between the variables, transformation none, quadratic order and polynomial model were selected. Model F value 45.18 and value of p (0.0001) being less than 0.05 in fit summery section clearly indicate that the model terms are significant. Empirical relationship between cellulase productions with other three variables is represented by final regression equation, made by analysis of variance (ANOVA). The relationship in terms of coded factors is represented in Eq. 1.
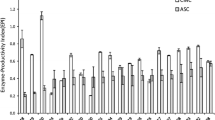
The value of adjusted determination coefficient (adj. R2 = 0.9346) was within reasonable agreement with the predicted R2 of 0.9278 which clearly indicates that the model was significant. The high significance and accuracy of the model was also conferred by the plot of predicted response values versus the actual experimental response values of cellulase production (Fig. 5). As most of the points are clustering around the diagonal line, it signifies the better adaptability of the model to predict experiments. Individual and combined effects of nutrients such as pretreated coconut mesocarp, peptone and glucose on production of cellulase are clear from analysis of variance (ANOVA). Figure 6a demonstrates the combine effect of glucose and peptone in the production of cellulase. At initial peptone concentration of 15 g/l, as glucose concentration was increased from 25 to 35 g/l, there was a slight increase in cellulase production. But at higher glucose concentration of 35 g/l, gradual increase in peptone concentrations during experiments leads to maximum production of cellulase. Similar types of combine effects were observed for the parameters of mesocarp with peptone and mesocarp with glucose on cellulase production. Figure 6b reveals that at both low (25 g/l) and high concentrations (35 g/l) of coconut mesocarp-based lignocellulosic substrate, gradual increase of peptone concentration has very negligible effects on cellulase production. But even at lower concentration of peptone at 15 g/l, cellulase concentration was observed to increase rapidly with increasing concentration of lignocellulosic material. Similarly at lower and higher concentration ranges of lignocellulosic material, slight differences in cellulase production were observed with variations in glucose concentrations (Fig. 6c). At 25 g/l of glucose concentration, maximum cellulase production was achieved little more than 4.65 g/l with increased (35 g/l) utilization of coconut mesocarp. The results clearly indicate that initial pretreatment of coconut mesocarp-based lignocellulosic material with maximum delignification and partial separation of hemicelluloses makes it highly suitable as a substitute for pure cellulose material. Hence, it became justified to replace pure cellulose with pretreated coconut mesocarp-based lignocellulosic material which acts as predominating inducer for enhanced production of cellulase. In design, the criteria were selected for mesocarp substrate as maximize and for both glucose and peptone materials as within range for optimized production of cellulase. The solution selected among suggested optimized results was coconut mesocarp-based substrate: 35 g/l, glucose concentration: 35 g/l, peptone concentration: 25 g/l and the suggested cellulase concentration was 5.80 g/l. The experiments were conducted in triplicates while following the same criteria of variables and the achieved cellulase production was 5.49 ± 0.12 g/l. The corresponding FPU level and CMCase activity levels of the produced enzyme were measured as 35 FPU/ml and 68.92 U/ml, respectively. The optimized concentrations of both peptone and glucose are selected in higher ranges as they largely affects in microbial growth rate, shorten culturing time, rate of product formation and product yield. Utilization of such substances in prescribed concentrations in laboratory scale production process may look little expensive but for industrial-scale production process of pure cellulase which costs around 126.7 US$/50 ml with concentration of 1.20 g/ml (https://www.sigmaaldrich.com/catalog/product/sigma/c2730?lang=en®ion=IN), it will be considered economical.
Enhanced production of cellulase through fermenter
Superior control of environmental factors such as temperature, agitation, nutrient gradient and pH in fermenter plays a most crucial role to achieve enhanced production of cellulase. Moreover, SmF-based fed-batch fermentation was already proved as most useful bioprocess engineering strategy for improved production of cellulase with reduced energy consumption (Hendy et al. 1984). In our case, the same process plays most instrumental role to achieve better cellulase production with enhanced activity level. In batch fermentation mode, specific growth rate of the fungus inside the fermenter was calculated as 0.001 h−1 up to 48 h of time interval and it increased to 0.011 h−1 between 48 and 96 h of cultivation time. Due to filamentous, complex and non-homogeneous growth nature of fungus, the overall growth rate was appeared to be lesser than average bacterial growth rate. Sun et al. (2017) observed similar tenfold lower growth rate of soil fungal species in comparison with soil bacteria, whereas slightly higher fungal growth rate was observed up to 72 h of fed- batch fermentation mode and after that growth rate increases rapidly (Fig. 7). Specific growth rate was observed maximum 0.027 h−1 in between 72 and 96 h of cultivation time. Similarly, cellulase production was improved in batch cultivation mode inside fermenter and enzyme concentration achieved was 7.20 g/l after 96 h of fermentation. The achieved concentration of cellulase was comparatively better than the concentration achieved in shaker flask under RSM-based optimized condition. In this process, specific cellulase activities were developed as 42 FPU/ml and CMCase 75 U/ml, respectively. Such batch-mode submerged cultivation of cellulase is considered comparatively more suitable than solid-state fermentation as longer duration of cultivation (2–3 weeks) under solid-state fermentation consequently limits cellulase production capacity and high probability of microbial contamination hinders its applicability, specifically in biofuel production process through simultaneous saccharification and fermentation process (Li et al. 2016). Although submerged fermentation is considered as energy intensive due to the requirement of high agitation rate for dispersing bubbles and enhanced oxygen mass transfer. But highly shear sensitive nature of mycelial growth which is specifically dependent on agitation rate permits the utilization of impeller speed in optimized level (Ahamed and Vermette 2010). In our case, optimum 120 rpm was maintained using Rushton turbine impeller for both batch and fed-batch mode of cultivation.
Compared to batch fermentation, fed-batch fermentation enabled improved cellulase titer with better soluble protein concentration and specific activity level. Up to 48 h of time interval in fed-batch cultivation mode, produced cellulase concentration was comparatively lower than the batch cultivation as mesocarp-based most-inducing component was gradually maintained comparatively at lower level up to that time period. But successive increase of such inducing parameter with first two 24 h of intervals through fed-batch feeding and maintaining initial glucose concentration at optimized growth promoting level influenced in improved mycelial growth and conjugated cellulase production after 48 h of fermentation. Cellulase concentration was measured as 9.58 g/l at 96 h (Fig. 7) and associated FPU and CMCase activities were analyzed as 54 FPU/ml and 93 U/ml, respectively. In both batch and fed-batch process, final concentrations of coconut mesocarp were maintained at 35 g/l but improved mass transfer of oxygen with gradual increase of such lignocellulosic inducer concentration in fed-batch fermentation mode helps in achieving the better result. Comparatively higher concentration of such inducing lignocellulosic component at initial phase of batch cultivation comes up with rapid, first and heterogeneous mycelia growth which may leads to non-Newtonian fluid properties and partial oxygen-limited condition. Even uneven clumping of cells leads to reduced oxygen uptake rate and reduced enzyme release (Omojasola et al. 2008). As a result it was observed that after 48 h of batch cultivation, enzyme production stops and its concentration remain almost in the same level, however, biomass growth still continues. For fed-batch fermentation, similar observation was noticed between 72 and 96 h of cultivation. The most possible reason behind such observation may be the presence of high glucose concentrations in media which lead to catabolic repression of cellulase production (Li et al. 2016). Expression of cellulase genes more specifically β-glucosidases genes were inhibited by the presence of high glucose concentration (De Cassia Pereira et al. 2015) or even inhibited by the chances of slight increase in glucose concentration through initial degradation of mesocarp cellulose material by produced enzyme itself. Such limitations were systematically overcome up to 72 h by consecutive feeding of inducing component in fed-batch cultivation mode and it finally confronted with enhanced enzyme production (Hansen et al. 2015). The achieved result in our case is comparable with the similar fed-batch cultivation study of cellulase by Trichoderma reesei RUT C30 (Li et al. 2016). In this study, enhanced cellulase titer of 90.3 FPU/ml was achieved after 144 h of cultivation with continuous feeding of pure lactose as inducer. Utilization of low-cost bio-waste material such as coconut mesocarp-based lignocellulosic inducer for the production of higher titer cellulase production makes the overall process energy and capital friendly (Cen and Xia 1999). Enzyme activity level achieved in our fed-batch fermentation study was better than enzyme activity level achieved by Rana et al. (2014), where lignocellulosic material, peptone and glucose concentrations were maintained even at higher level in batch cultivation mode. Compared to some existing reported studies of cellulase production, our present achievements represent better titers of cellulase and its activity levels (Table 3). The presence of pure cellulase in the fermentation broth, generated during fed-batch fermentation, was confirmed from the FTIR analysis (Fig. 8). The absorption band appeared in the range of 3337.96 cm−1 suggests the changes in the intensities of signals generated by hydrogen bonds arising from amine groups and hydroxyl groups in cellulase (Mishra and Sardar 2015; Zdarta et al. 2017). The broad peak around 1635.71 cm−1 clearly indicates the amide one peak generated due to NH2 scissoring and confirms the presence of cellulase (Mishra and Sardar 2015; Bohara et al. 2016; Zdarta et al. 2017).
Conclusion
The present study has highlighted the design, analysis and optimization of the major nutritional growth parameters in the field of cellulase production from lignocellulosic waste resources such as coconut mesocarp. Coconut mesocarp was explored as natural inducer and suitable alternative of pure cellulose for commercial production of cellulase. For facilitating better microbial conversion of cellulase from such selected substrate, T. reesei (MTCC 164) was identified as most suitable organism. To achieve improved productivity and quality of cellulase, submerged fermentation strategies were implemented due to easier reactor operations with better process control. Production of cellulase under optimized nutritional conditions was arrived through the use of response surface technique in Design Expert Software (version 8.0.4). To further enhance the production of cellulase with maximum activity levels, fed-batch process was adopted by means of successive feeding of coconut mesocarp under established optimized conditions. The study opens an opportunity for cost-effective production of cellulase while ensuring clean environment and proper solid-waste management. The findings are likely to be very useful in the scale-up and design of industrial units representing most simplified but efficient commercial production process of cellulase.
References
Ahamed A, Vermette P (2010) Effect of mechanical agitation on the production of cellulases by Trichoderma reesei RUT-C30 in a draft-tube airlift bioreactor. Biochem Eng J 49:379–387. https://doi.org/10.1016/J.BEJ.2010.01.014
Avelino F, Alves G, Ruiz HA et al (2015) Bioethanol production from coconuts and cactus pretreated by autohydrolysis. Ind Crops Prod 77:1–12. https://doi.org/10.1016/j.indcrop.2015.06.041
Ballesteros I, Negro MJ, Oliva JM et al (2006) Ethanol production from steam-explosion pretreated wheat straw. Appl Biochem Biotechnol 129–132:496–508
Belghith H, Ellouz-Chaabouni S, Gargouri A (2001) Biostoning of denims by Penicillium occitanis (Pol6) cellulases. J Biotechnol 89:257–262
Bendig C, Weuster-Botz D (2013) Reaction engineering analysis of cellulase production with Trichoderma reesei RUT-C30 with intermittent substrate supply. Bioprocess Biosyst Eng 36:893–900. https://doi.org/10.1007/s00449-012-0822-1
Bohara RA, Thorat ND, Pawar SH (2016) Immobilization of cellulase on functionalized cobalt ferrite nanoparticles. Korean J Chem Eng 33:216–222. https://doi.org/10.1007/s11814-015-0120-0
Cabral MMS, Abud de AKS, Silva de CEF et al (2016) Bioethanol production from coconut husk fiber. Ciência Rural 46:1872–1877. https://doi.org/10.1590/0103-8478cr20151331
Cen P, Xia L (1999) Production of cellulase by solid-state fermentation. Springer, Berlin, pp 69–92
Cunha FM, Esperança MN, Zangirolami TC et al (2012) Sequential solid-state and submerged cultivation of Aspergillus niger on sugarcane bagasse for the production of cellulase. Bioresour Technol 112:270–274. https://doi.org/10.1016/J.BIORTECH.2012.02.082
De Cassia Pereira J, Marques NP, Rodrigues A et al (2015) Thermophilic fungi as new sources for production of cellulases and xylanases with potential use in sugarcane bagasse saccharification. J Appl Microbiol. https://doi.org/10.1111/jam.12757
Dey P, Rangarajan V (2017) Improved fed-batch production of high-purity PHB (poly-3 hydroxy butyrate) by Cupriavidus necator (MTCC 1472) from sucrose-based cheap substrates under response surface-optimized conditions. 3 Biotech. https://doi.org/10.1007/s13205-017-0948-6
Domingues FC, Queiroz JA, Cabral JMS, Fonseca LP (2001) Production of cellulases in batch culture using a mutant strain of Trichoderma reesei growing on soluble carbon source. Biotechnol Lett 23:771–775. https://doi.org/10.1023/A:1010329731010
Ebrahimi M, Caparanga AR, Ordono EE, Villaflores OB (2017) Evaluation of organosolv pretreatment on the enzymatic digestibility of coconut coir fibers and bioethanol production via simultaneous saccharification and fermentation. Renew Energy 109:41–48. https://doi.org/10.1016/j.renene.2017.03.011
Esterbauer H, Steiner W, Labudova I et al (1991) Production of Trichoderma cellulase in laboratory and pilot scale. Bioresour Technol 36:51–65. https://doi.org/10.1016/0960-8524(91)90099-6
FAOSTAT Data (2016) The top 5 coconut producing countries. In: FAOSTAT data, 2016 (last accessed by Top 5 Anything January 2016). https://top5ofanything.com/list/1cb15034/Coconut-Producing-Countries (Accessed 1 Aug 2017). Accessed 23 May 2018
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Gonçalves FA, Ruiz HA, Nogueira da CC, et al (2014) Comparison of delignified coconuts waste and cactus for fuel-ethanol production by the simultaneous and semi-simultaneous saccharification and fermentation strategies. Fuel 131:66–76. https://doi.org/10.1016/j.fuel.2014.04.021
Gonçalves FA, Ruiz HA, Silvino dos Santos E et al (2016) Bioethanol production by Saccharomyces cerevisiae, Pichia stipitis and Zymomonas mobilis from delignified coconut fibre mature and lignin extraction according to biorefinery concept. Renew Energy 94:353–365. https://doi.org/10.1016/j.renene.2016.03.045
Hansen GH, Lübeck M, Frisvad JC et al (2015) Production of cellulolytic enzymes from ascomycetes: comparison of solid state and submerged fermentation. Process Biochem 50:1327–1341. https://doi.org/10.1016/J.PROCBIO.2015.05.017
Hasunuma T, Okazaki F, Okai N et al (2013) A review of enzymes and microbes for lignocellulosic biorefinery and the possibility of their application to consolidated bioprocessing technology. Bioresour Technol 135:513–522. https://doi.org/10.1016/j.biortech.2012.10.047
Hendy NA, Wilke CR, Blanch HW (1984) Enhanced cellulase production in fed-batch culture of Trichoderma reesei C30. Enzyme Microb Technol 6:73–77. https://doi.org/10.1016/0141-0229(84)90038-3
Howard RL, Abotsi E, Jansen van REL, Howard S (2003) Lignocellulose biotechnology: issues of bioconversion and enzyme production. Afr J Biotechnol 2:602–619. https://doi.org/10.5897/AJB2003.000-1115
Ito K, Yoshida K, Ishikawa T, Kobayashi S (1990) Volatile compounds produced by the fungus Aspergillus oryzae in rice Koji and their changes during cultivation. J Ferment Bioeng 70:169–172. https://doi.org/10.1016/0922-338X(90)90178-Y
Juhász T, Szengyel Z, Réczey K et al (2005) Characterization of cellulases and hemicellulases produced by Trichoderma reesei on various carbon sources. Process Biochem 40:3519–3525. https://doi.org/10.1016/J.PROCBIO.2005.03.057
Kataria R, Ghosh S (2011) Saccharification of Kans grass using enzyme mixture from Trichoderma reesei for bioethanol production. Bioresour Technol 102:9970–9975. https://doi.org/10.1016/j.biortech.2011.08.023
Kataria R, Ruhal R, Babu R, Ghosh S (2013) Saccharification of alkali treated biomass of Kans grass contributes higher sugar in contrast to acid treated biomass. Chem Eng J 230:36–47. https://doi.org/10.1016/j.cej.2013.06.045
Kim I, Han J-I (2012) Optimization of alkaline pretreatment conditions for enhancing glucose yield of rice straw by response surface methodology. Biomass Bioenerg 46:210–217. https://doi.org/10.1016/J.BIOMBIOE.2012.08.024
Kovács K, Szakacs G, Zacchi G (2009) Comparative enzymatic hydrolysis of pretreated spruce by supernatants, whole fermentation broths and washed mycelia of Trichoderma reesei and Trichoderma atroviride. Bioresour Technol 100:1350–1357. https://doi.org/10.1016/j.biortech.2008.08.006
Li Y, Liu C, Bai F, Zhao X (2016) Overproduction of cellulase by Trichoderma reesei RUT C30 through batch-feeding of synthesized low-cost sugar mixture. Bioresour Technol 216:503–510. https://doi.org/10.1016/j.biortech.2016.05.108
Loaces I, Schein S, Noya F (2017) Ethanol production by Escherichia coli from Arundo donax biomass under SSF, SHF or CBP process configurations and in situ production of a multifunctional glucanase and xylanase. Bioresour Technol 224:307–313. https://doi.org/10.1016/j.biortech.2016.10.075
Lowry OH, Rosebrough NJ, Randall FRJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lv S, Yu Q, Zhuang X et al (2013) The influence of hemicellulose and lignin removal on the enzymatic digestibility from sugarcane bagasse. Bio Energy Res 6:1128–1134. https://doi.org/10.1007/s12155-013-9297-4
Ma L, Li C, Yang Z et al (2013) Kinetic studies on batch cultivation of Trichoderma reesei and application to enhance cellulase production by fed-batch fermentation. J Biotechnol 166:192–197. https://doi.org/10.1016/J.JBIOTEC.2013.04.023
Martinez D, Berka RM, Henrissat B et al (2008) Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nat Biotechnol 26:553–560. https://doi.org/10.1038/nbt1403
Menon V, Rao M (2012) Trends in bioconversion of lignocellulose: biofuels, platform chemicals and biorefinery concept. Prog Energy Combust Sci 38:522–550. https://doi.org/10.1016/j.pecs.2012.02.002
Miftahul Jannah A, Asip F (2015) Bioethanol production from coconut fiber using alkaline pretreatment and acid hydrolysis method. Int J Adv Sci Eng Inf Technol 5:320. https://doi.org/10.18517/ijaseit.5.5.570
Miller GL, Miller G (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar no title. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Mishra A, Sardar M (2015) Cellulase assisted synthesis of nano-silver and gold: application as immobilization matrix for biocatalysis. Int J Biol Macromol 77:105–113. https://doi.org/10.1016/j.ijbiomac.2015.03.014
Omojasola PF, Jilani O et al (2008) Cellulase production by some fungi cultured on pineapple waste. Nat Sci 6:64–79
Rana V, Eckard AD, Teller P, Ahring BK (2014) On-site enzymes produced from Trichoderma reesei RUT-C30 and Aspergillus saccharolyticus for hydrolysis of wet exploded corn stover and loblolly pine. Bioresour Technol 154:282–289. https://doi.org/10.1016/J.BIORTECH.2013.12.059
Rawat R, Kumbhar BK, Tewari L (2013) Optimization of alkali pretreatment for bioconversion of poplar (Populus deltoides) biomass into fermentable sugars using response surface methodology. Ind Crops Prod 44:220–226. https://doi.org/10.1016/J.INDCROP.2012.10.029
Sajith S, Priji P, Sreedevi S, Benjamin S (2016) An overview on fungal cellulases with an industrial perspective. J Nutr Food Sci 6:1–13. https://doi.org/10.4172/2155-9600.1000461
Sateesh L, Rodhe AV, Naseeruddin S et al (2012) Simultaneous cellulase production, saccharification and detoxification using dilute acid hydrolysate of S. spontaneum with Trichoderma reesei NCIM 992 and Aspergillus niger. Indian J Microbiol 52:258–262. https://doi.org/10.1007/s12088-011-0184-4
Singhania RR, Sukumaran RK, Patel AK et al (2010) Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb Technol 46:541–549. https://doi.org/10.1016/J.ENZMICTEC.2010.03.010
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D et al (2008) Determination of structural carbohydrates and lignin in biomass. Lab Anal Proced 1617:1–16
Soares J, Demeke MM, Foulquié-Moreno MR et al (2016) Green coconut mesocarp pretreated by an alkaline process as raw material for bioethanol production. Bioresour Technol 216:744–753. https://doi.org/10.1016/j.biortech.2016.05.105
Sun S, Li S, Avera BN et al (2017) Soil bacterial and fungal communities show distinct recovery patterns during forest ecosystem restoration. Appl Environ Microbiol 83:e00966–e00917. https://doi.org/10.1128/AEM.00966-17
Ximenes EA, Dien BS, Ladisch MR et al (2007) Enzyme production by industrially relevant fungi cultured on coproduct from corn dry grind ethanol plants. Appl Biochem Biotechnol 137–140:171–183. https://doi.org/10.1007/s12010-007-9049-z
Yang S-T (2007) Bioprocessing for value-added products from renewable resources: new technologies and applications. Elsevier, New York
Zdarta J, Jędrzak A, Klapiszewski Ł, Jesionowski T (2017) Immobilization of cellulase on a functional inorganic–organic hybrid support: stability and kinetic study. Catalysts 7:374. https://doi.org/10.3390/catal7120374
Zhuang X, Wang W, Yu Q et al (2016) Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products. Bioresour Technol 199:68–75. https://doi.org/10.1016/j.biortech.2015.08.051
Acknowledgements
The research work was financially supported by Short Term research Grant for the faculty members (Sanctioned Letter: KU/AR/KSTG/32/2017, Sr no 9), Karunya Institute of Technology and Sciences (Deemed to be university). Authors are also thankful to each other for their individual support and contribution towards the completion of the research work and writing the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Dey, P., Singh, J., Scaria, J. et al. Improved production of cellulase by Trichoderma reesei (MTCC 164) from coconut mesocarp-based lignocellulosic wastes under response surface-optimized condition. 3 Biotech 8, 402 (2018). https://doi.org/10.1007/s13205-018-1421-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1421-x