Abstract
Proteinase inhibitor-II (PI-II) genes are important defense related genes that play critical regulatory roles in plant growth and development. In the present study, the expression of tomato PI-II gene was investigated under the control of a wound-inducible OsRGLP2 (Oryza sativa root germin like protein 2) promoter in transgenic tobacco plants after wounding, ABA and MeJA applications. Transcript level of target gene in transgenic plants was confirmed by quantitative real time PCR (qPCR). In response to ABA treatment at different concentrations, PI-II gene was strongly induced under OsRGLP2 promoter at higher concentration (100 μM), while considerable level of target gene expression was observed with MeJA application at 50 μM concentration. Upon wounding, relatively high PI-II gene expression was observed after 36-h treatment. Correspondingly, high GUS activity was detected at 36 h with histochemical assay and microscopic analysis in the vascular regions of leaves, stem and roots in wounded transgenic plants. This inducibility of PI-II gene by wounding, ABA and MeJA indirectly indicates its role in plant defense mechanism against biotic and abiotic stresses. Moreover, it was also suggested that ABA and MeJA dependent signaling pathways are involved in stimulation of PI-II gene. To the best of our knowledge, this is the first report describing the induction of PI-II gene under the regulation of OsRGLP2 promoter under stress conditions. The results of present research are useful for potential role of PI-II gene to improve stress tolerance in transgenic crops. Thus, efficacy of this gene can potentially be exploited to test the responses of different plants to various environmental stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Usually plants growing in nature are continuously exposed to biotic and abiotic stresses. Plants secure themselves by triggering several defense mechanisms which control or reduce the effects of such stresses. The plant proteinase inhibitors (PIs) are one of the important defensive proteins that play significant role to resist different kinds of environmental stresses. Most of plant PIs work to act against the herbivores and pathogens by interacting the active sites of their target proteases (Kim et al. 2009; Srinivasan et al. 2009), thereby forming a stable inhibitory complex. Some PIs have also been up-regulated in response to abiotic factors such as salt and drought stresses (Pernas et al. 2000; Gaddour et al. 2001; Huang et al. 2007; Rehman et al. 2017a, b). A variety of PIs are known in plants that have been divided into many families or classes. Among these PIs, serine proteinase inhibitors constitute the largest and well-studied family (Haq and Khan 2003), that have been characterized for their crucial role in various physiological and defense processes in plants. The members of this family are universal throughout the plant kingdom and mostly present as storage proteins in seeds and tubers or also accumulate in vegetative organs of plants.
The wound-inducible proteinase inhibitor-II (PI-II) proteins are one of the important members of serine proteinase inhibitors that have been reported to inhibit trypsin, chymotrypsin, oryzin, subtilisin, elastase and pronase E (Antcheva et al. 1996). They have been identified and characterized from many Solanaceous plant species including tomato, potato, tobacco and pepper, etc. (Bryant et al. 1976; Plunkett et al. 1982; Keil et al. 1986; Pearce et al. 1988, 1993; Tamhane et al. 2009; Mishra et al. 2012). The expression of PI-II genes have been found to be regulated by variety of stresses including wounding and associated signaling molecules. For example, there are many reports on involvement of phytohormones like abscisic acid (ABA) and jasmonic acid (JA) in triggering the local and systemic induction of PI-II genes in many plants including tomato, potato, and tobacco (Farmer et al. 1992; Hildmann et al. 1992; Peña-Cortés et al. 1995; Peña-Cortés and Willmitzer 1995; Wasternack and Parthier 1997). This local and systemic induction of inhibitory genes was apparently to occur via octadecanoid (OD) pathway (Koiwa et al. 1997).
Similarly, it has been established that many defensive traits are expressed in plants distal to the site of injury as a result of wounding (Green and Ryan 1972). Previously, it was reported that wounding of tomato and potato leaves has increased the expression level of PI-II genes (Pena-Cortes et al. 1988). Moreover, endogenous level of ABA and JA was found to elevate in response to mechanical wounding which in turn induces rapid accumulation of PI-II mRNA. In addition, synthesis of PI-II was also reported to be induced by systemin and oligosaccharide elicitors in potato and tomato (Doares et al. 1995). A range of wound-inducible promoters have been identified from many plants, which are involved in regulating the gene expression of many defense related genes under certain stress conditions. OsRGLP2 is a robust promoter that could mediate rapid gene responses by several agents, including wounding, salt, dehydration and pathogenic infection in transgenic plants (Mahmood et al. 2013; Munir et al. 2016; Shah et al. 2017). Based on this information, a recombinant construct was designed by ligating a tomato PI-II gene downstream to OsRGLP2 promoter, which was further investigated for expression analysis in response to wounding, ABA and MeJA treatments after transformation in tobacco plants.
Materials and methods
Selection of plant material and transformation
The tobacco species Nicotiana benthamiana was used for stable transformation with Agrobacterium strain EHA101 carrying p1391Z_OsRGLP2::PI-II vector fused with GUS reporter gene using leaf disc method (Horsch et al. 1985).
Confirmation of transgenic plants
DNA extraction from leaves of transgenic plants was carried out using DNeasy Plant Mini Kit. PCR of transgenic plants was done using PI-II and hygromycin resistant gene primers. The sequences of these primers are PI-II F: 5′TATCCATCATGGCTGTCCAC3′ and PI-IIR: 5′AACACACAACTTGATCCCCACA3′ and Hygro F: 5′ GCTCCATACAAGCCAACCAC 3′ Hygro R: 5′ CGAAAAGTTCGACAGCGTCTC 3′. For amplification, 25 μL of amplification reaction containing 25 pmol of each primer, 2.5 μL of 10× PCR buffer, 1.5 μL of 25 mM MgCl2, 1.5 μL of 2.0 mM dNTPs, 45 ng/μL of genomic DNA, and 1.5 U Taq polymerase was prepared. Gradient Multigene Thermal Cycler (Labnet) was used to run amplification reaction for 35 cycles of denaturing at 94 °C for 40 s, 55 °C for 40 s, 72 °C for 45 s, and a final extension step at 72 °C for 20 min.
In vitro germination and selection of transgenic seeds
T0 seeds obtained from wild type (WT) and transgenic plants (TL1, TL2 and TL3) were surface sterilized according to method described by Srinivasan et al. (2009). The sterilized seeds were selected on hygromycin (50 mg/mL) media and maintained in a growth room at 27 °C with a photoperiod of 16:8 light/dark cycles. T1 progeny was selected on the basis of hygromycin resistance.
ABA and MeJA treatments
For ABA and MeJA stress treatments, ABA and MeJA solutions (10, 50, and 100 μM), were sprayed on 15 days old T1 transgenic lines (TL1, TL2 and TL3) and wild type (WT). Samples were taken after 24 h of spray and immediately frozen in liquid nitrogen and stored at − 80 °C for RNA extraction. Each experiment was repeated at least three times.
Wounding stress
Wounding stress was applied to the young leaves of T1 transgenic plants growing on MS media for 12, 24, and 36 h by injuring the leaves with pre-sterilized forceps. Unstressed transgenic plants were used as control. Wounded leaves were collected, frozen in liquid nitrogen and further processed for RNA isolation and qPCR analysis.
Histochemical assay
GUS histochemical analysis was performed on T1 transgenic seedlings following the procedure of Jefferson (1989). Briefly, plant tissues were incubated at 37 °C in GUS reaction solution containing 50 mM sodium phosphate buffer (pH 7.0) and 2 mM X-Gluc. After incubation, stained tissues were washed with 70% ethanol to remove the chlorophyll and observations were recorded.
RNA isolation and cDNA synthesis
For RT-PCR, ABA and MeJA and wound treated samples were processed for total RNA isolation using Trizol reagent (invitrogen). For complementary DNA (cDNA) synthesis, 1 μg of DNase-treated RNA was reverse transcribed in a 20 μl reaction using M-MuLV reverse transcriptase along with oligo (dT) primers. The total reaction mixture was incubated for 1 h at 42 °C followed by 70 °C for 10 min to stop the reaction. To check the contamination of genomic DNA, a control was run without the reverse transcriptase enzyme. Quantity and quality of cDNA was confirmed through NanoDrop method and PCR using housekeeping gene (actin) primers. Finally cDNA was stored at − 20 °C until further use.
qPCR
For expression analysis, qPCR was performed with Stratagene Mx3005P QPCR System using ten times diluted cDNA, 1× EvaGreen master mix and gene specific primers: 5′-TTCGGGATATGCCCACGTTC-3′ (forward) and 5′-AGGTGCAAGCATTTGGCCTT-3′ (reverse). The N. benthamiana actin primers 5′-GATGAAGATACTCACAGAAAGA-3′ (forward) and 5′-GTGGTTTCATGAATGCCAGCA-3′ (reverse) were used as internal reference. The qPCR programme was carried out in a 40 cycle reaction under the following conditions: 94 °C (30 s), 56 °C (60 s), and 72 °C (10 s). The resultant data were analyzed according to the formula 2−ΔΔCt.
Statistical analysis
All the data were subjected to analysis of variance (ANOVA) using a PROC GLM procedure of SAS 9.4. The mean within each treatment were compared using the least significant difference (LSD) test with a threshold probability of P < 0.01.
Results
Effect of wounding
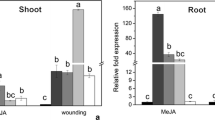
To investigate the induction of PI-II gene under OsRGLP2 promoter in response to wounding, the transgenic plants were wounded at three experimental time periods (12, 24 and 36 h). Overall, transcript level of PI-II gene was found to increase after 24 h reaching to maximum level (6.5-fold) at 36 h in wounded transgenic plants (Fig. 1). The present results were also supported by histochemical assay of GUS reporter gene under OsRGLP2 promoter. The GUS staining revealed that expression pattern of GUS gene was similar to that of PI-II gene (Fig. 2), and was significantly induced at 36 h in transgenic plants after wounding. GUS activity detected by microscopic studies showed strong GUS expression in the vascular bundles of leaf, stem and root after wounding. In leaves, GUS expression was observed in all parts such as leaf epidermis, guard cells, mesophyll cells, and midrib (Fig. 3). After 12 h of injury, diffused expression was noted in vascular tissues and mesophyll cells of leaf which become intense with the passage of time. In stem, high level of GUS activity was detected in vascular bundles and relative low GUS expression was observed in outer cells after 36 h of injury (Fig. 4). In roots, microscopic analysis revealed that GUS activity was mainly associated with vascular bundles, root epidermal layer, and root hairs. Roots showed prominent GUS expression in vascular region than cortex after 24 h. However, GUS activity increased after 36 h and become more uniform in cortex as well as in vascular bundles, while low expression was also detected in outer root epidermal layer (Fig. 5).
Induction of PI-II in response to ABA
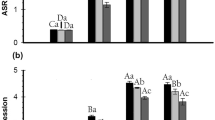
The relative quantification data showed that PI-II gene expression was triggered significantly at higher level under OsRGLP2 promoter in three independent transgenic lines (TL1, TL2 and TL3) than WT under three different ABA concentrations (10, 50, and 100 μM) after 24 h (Fig. 6a). After 10 μM ABA treatment, PI-II gene was initially induced at very low level in all treated plants. The transcript level was found to increase and differ significantly (P < 0.01) between transgenic lines versus WT plants with an increase in ABA concentrations (Table 1). However, transgenic lines displayed comparatively higher expression than WT (Fig. 6a). When treated with 100 μM ABA, TL1, TL2, TL3 showed higher fold change of 9.5, 8.4 and 5.2, respectively, when compared with WT. Moreover, the transcript level of TL1 line was highest among all the other transgenic lines and control plants at all concentrations. The present data indicated that PI-II gene is responsive to ABA application which suggested that ABA might play an important role in the induction of PI-II gene under abiotic stress conditions.
a Expression profile of PI-II gene in transgenic lines and WT in response to ABA treatment with different concentrations. The data are the mean ± SE of three replicates (n = 3). The letters on each bar within each treatment indicate the significant differences at P < 0.01, and bars sharing a common letter are not significantly different. b Expression analysis of PI-II gene in transgenic lines and WT in response to MeJA treatment with different concentrations. The data are the mean ± SE of three replicates (n = 3). The letters on each bar within each treatment indicate the significant differences at P < 0.01, and bars sharing a common letter are not significantly different
Induction of PI-II in response to MeJA
To verify the role of MeJA in the induction of PI-II gene, the relative expression of target gene was analyzed in selected transgenic lines (TL1, TL2 and TL3) and WT by qPCR. The results showed significant difference in expression level of PI-II gene in transgenics and WT following the exogenous MeJA treatment at certain particular concentrations (Fig. 6b; Table 1). Overall, the transcript levels of transgene were transiently induced by MeJA and vary significantly with an increase in concentrations of MeJA in transgenic plants and WT (P < 0.01). After increasing the concentration from 10 to 50 μM MeJA, the expression levels of all lines gradually increased that reached to the maximum level at 50 μM MeJA and then declines at 100 μM MeJA. Compared to WT, a significant higher expression level was noted at 50 μM MeJA in all transgenic lines that was about 8.2-fold for TL1, 7.8-fold for TL2 and 4.5 fold for TL3, while 10 μM MeJA and 100 μM MeJA treatments resulted in decreased PI-II activity (Fig. 6b). However, transcript levels of all transgenic lines were comparatively higher at 100 μM MeJA than at 10 μM MeJA. Moreover, among all transgenic lines tested, TL1 was highly responsive to MeJA treatment at all concentrations. This transcriptional activation of PI-II gene in response to MeJA treatment clearly indicates the positive role of MeJA in the regulation of stress responsive genes.
Discussion
Leaves of transgenic plants were mechanically wounded to evaluate the activity of OsRGLP2 promoter in driving the expression of PI-II gene. The present results indicated that mechanical wounding has significantly up-regulated the PI-II gene expression (up to 2–6.5-fold) in wounded transgenic plants as compared to their non-wounded counterparts (control). This is indicative of wound-specific activity of OsRGLP2 promoter which may control the activation of specific defensive genes including PI-II gene. Gulbitti-Onarici et al. (2009) reported similar results by constitutive expression of Cry1Ac (insecticidal crystal proteins) gene under the control of wound-inducible AoPR1 promoter in transgenic tobacco plants after wounding. RT-PCR analysis further showed that PI-II gene is not an early responsive gene as it was induced by wounding over an extended time period (at 36 h) which is in accordance with the observations of Ryan (2000) and Meyer et al. (2016), suggesting the possible role of PI-II gene in late wound signaling pathway and contribution in plant defense against herbivores and pathogens. This notion was also supported by experimental data in transgenic plants over expressing the potato and nightshade PI-II genes that resulted in enhanced resistance against Helicoverpa armigera and Spodoptera litura (Luo et al. 2009; Majeed et al. 2011). Consistent with PI-II quantitative data, the transgenic plants showed strong GUS expression after 36 h in vascular bundles of leaves, stems and roots by microscopic analysis which may suggest that wounding signals are transported through vascular bundles (Keil et al. 1989; Xu et al. 1993). Such microscopic observations with GUS gene were also reported in both wounded leaves and roots of transgenic dicot plants transformed with pin2/Act1 intron/GUS construct (Keil et al. 1989) and in the leaves of transgenic nightshade plants expressing SaPIN2b:GUS construct (Liu et al. 2006) after 24 h of wounding. Collectively, these results indicated that PI-II gene was constitutively expressed under OsRGLP2 promoter and is considered as a part of defense mechanism in plants due to its induction by wound and herbivore predators.
The plant hormone, ABA serves as significant signaling molecule that is critical for growth and development of plants, and provides adaptations to wide range of stresses like drought, salinity and cold (Shinozaki and Yamaguchi-Shinozaki 2000; Sah et al. 2016; Ge et al. 2017). With regard to ABA treatment, transgenic plants showed strong PI-II gene expression under OsRGLP2 promoter than the WT at higher concentration (100 μM) signifying its major role in plants during abiotic stress conditions. This suggests that some critical part of OsRGLP2 promoter region contain ABA responsive elements with possible role in directing the expression of PI-II gene through ABA signaling under abiotic stress condition. These results correlate well with earlier studies in which an increase in PI-II mRNA level was reported in potato leaves and stem after applying 100 μM ABA to leaves (Pena-Cortes et al. 1989, 1995). In another report, Kim et al. (2001) observed elevated CaPI2 expression in pepper after 12 h of ABA treatment (50 μM). Similarly, the transcript levels of cysteine proteinase inhibitors from Panax ginseng (PgCPI) (Jung et al. 2010) and Glycine soja (GsCPI14) (Sun et al. 2014) were strongly up-regulated by ABA treatment. In a related study, Srinivasan et al. (2009) showed higher transcript level in transgenic tobacco plants over expressing the N. benthamiana trypsin inhibitor gene (NtPI) under the regulation of CAM35S promoter in the presence of ABA treatment. In accordance with the previous results, the present data indicated that target gene expression was significantly up-regulated under OsRGLP2 promoter in response to ABA stress treatment.
The potential role of JA or MeJA in regulating the expression of proteinase inhibitor genes has been the subject of intense research which has been reviewed in many studies (Sivasankar et al. 2000; Sun et al. 2011; Larrieu and Vernoux 2016; Rehman et al. 2017b). Our results obtained by qPCR data revealed that expression of PI-II gene driven by OsRGLP2 promoter was induced at considerable level in transgenic plants at 24 h of post treatment of MeJA. These findings suggest that induction of PI-II gene is dependent on JA-mediated signaling pathway and can be used to increase plant resistance against biotic stresses. Moreover, cis-acting elements present within the OsRGLP2 promoter are involved in mediating the JA-signaling system which might play significant role in up-regulating the PI-II gene. Earlier studies concluded that MeJA or JA application has strongly induced the wound-inducible proteinase inhibitors I and II (PI-I, PI-II) in potato, tobacco and alfalfa (Farmer et al. 1992; Peña-Cortés et al. 1995). In a similar study, a marked increase in Pin2-GUS gene expression was detected in transgenic Solanum brevidens under CaMV35S promoter after 50 μM MeJA treatment (Liu et al. 1996). Moreover, Tian et al. (2014) observed that MeJA has significantly induced the PIN2 gene in def1 (defenseless) mutants and wild type which indicates that MeJA plays an important role in PIN2 induction. Similarly, exogenous application of MeJA (100 μM) to tobacco leaves has increased the transcript level of trypsin inhibitor (NtPI) after 24 h (Srinivasan et al. 2009). All these reports clearly indicate that MeJA has positive role in the regulation of proteinase inhibitor genes.
Conclusion
The present work revealed that expression of PI-II gene under OsRGLP2 promoter was highly up-regulated by mobile wound signals and signaling molecules like ABA and MeJA that are primarily effective for abiotic and biotic responses. These results also provide an evidence for the involvement of signaling cascade-like events in the regulation of inhibitory genes in plants growing under stress conditions. Moreover, fusion of the OsRGLP2 promoter to a defense related genes like PI-II gene is an effective strategy for engineering crops to cope various forms of environmental stresses. However, further studies are still required for elucidating the mechanism and specific physiological function of PI-II gene in response to multiple stresses. In addition, much effort should be focused on multiple signaling molecules to uncover the several features of PI-II gene in plant growth and survival. Conclusively, PI-II gene is a potential candidate gene for developing transgenic crops tolerant to both biotic and abiotic stresses.
References
Antcheva N, Patthy A, Athanasiadis A, Tchorbanov B, Zakhariev S, Pongor S (1996) Primary structure and specificity of a serine proteinase inhibitor from paprika (Capsicum annuum) seeds. Biochim Biophys Acta 1298(1):95–101
Bryant J, Green TR, Gurusaddaiah T, Ryan CA (1976) Proteinase inhibitor II from potatoes: isolation and characterization of its protomer components. Biochemistry 15:3418–3424
Doares SH, Narváez-Vásquez J, Conconi A, Ryan CA (1995) Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol 108:1741–1746
Farmer EE, Johnson RR, Ryan CA (1992) Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol 98(3):995–1002
Gaddour K, Vicente-Carbajosa J, Lara P, Isabel-Lamoneda I, Díaz I, Carbonero P (2001) A constitutive cystatin-encoding gene from barley (Icy) responds differentially to abiotic stimuli. Plant Mol Biol 45:599–608
Ge K, Liu X, Li X, Hu B, Li L (2017) Isolation of an ABA transporter-like 1 gene from Arachis hypogaea that affects ABA import and reduces ABA sensitivity in Arabidopsis. Front Plant Sci 8:1150. https://doi.org/10.3389/fpls.2017.01150
Green TR, Ryan CA (1972) Wound-induced proteinase inhibitor in plant leaves: a possible defense mechanism against insects. Science 175:776–777
Gulbitti-Onarici S, Zaidi MA, Taga I, Ozcan S, Altosaar I (2009) Expression of Cry1Ac in transgenic tobacco plants under the control of a wound-inducible promoter (AoPR1) isolated from Asparagus officinalis to control Heliothis virescens and Manduca sexta. Mol Biotechnol 42:341–349
Haq SK, Khan RH (2003) Characterization of a proteinase inhibitor from Cajanus cajan (L.). J Protein Chem 22:543–554
Hildmann T, Ebneth M, Peña-Cortés H, Sanchez-Serrano J, Willmitzer L, Prat S (1992) General roles of abscisic and jasmonic in gene activation as a result of mechanical wounding. Plant Cell 4:1157–1170
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Huang Y, Xiao B, Xiong L (2007) Characterization of a stress responsive proteinase inhibitor gene with positive effect in improving drought resistance in rice. Planta 226:73–85
Jefferson RA (1989) The GUS reporter gene system. Nature 342:837–838
Jung DY, Lee OR, Kim YJ, Lee JH, Pulla RK, Sathiyaraj G, Shim JS, Yang DC (2010) Molecular characterization of a cysteine proteinase inhibitor, PgCPI, from Panax ginseng CA Meyer. Acta Physiol Plant 5:961–970
Keil M, Sanchez-Serrano J, Schell J, Willmitzer L (1986) Primary structure of a proteinase inhibitor II gene from potato (Solanum tuberosum). Nucleic Acids Res 14:5641–5650
Keil M, Sánchez-Serrano JJ, Willmitzer L (1989) Both wound-inducible and tuber-specific expression are mediated by the promoter of a single member of the potato proteinase inhibitor II gene family. EMBO J 8:1323–1330
Kim JY, Park SC, Hwang I, Cheong H, Nah JW, Hahm KS, Park Y (2009) Protease inhibitors from plants with antimicrobial activity. Int J Mol Sci 10:2860–2872
Kim S, Hong Y, An CS, Lee K (2001) Expression characteristics of serine proteinase inhibitor II under variable environmental stresses in hot pepper (Capsicum annuum L.). Plant Sci 161:27–33
Koiwa H, Bressan RA, Hasegawa PM (1997) Regulation of protease inhibitors and plant defense. Trends Plant Sci 2:379–384
Larrieu A, Vernoux T (2016) Q&A: how does jasmonate signaling enable plants to adapt and survive? BMC Biol 14:79
Liu THA, Stephens LC, Hannapel DJ (1996) Expression of a chimeric proteinase inhibitor II-GUS gene in transgenic Solanum brevidens plants. J Plant Physiol 149:533–538
Liu J, Xia KF, Deng YG, Huang XL, Hu BL, Xu X, Xu ZF (2006) The nightshade proteinase inhibitor IIb gene is constitutively expressed in glandular trichomes. Plant Cell Physiol 47:1274–1284
Luo M, Wang Z, Li H, Xia KF, Cai Y, Xu ZF (2009) Overexpression of a weed (Solanum americanum) proteinase inhibitor in transgenic tobacco results in increased glandular trichome density and enhanced resistance to Helicoverpa armigera and Spodoptera litura. Int JMol Sci 10:1896–1910
Mahmood T, Yasmin T, Haque MI, Naqvi SMS (2013) Characterization of a rice germin-like protein gene promoter. Genet Mol Res 12:360–369
Majeed A, Makhdoom R, Husnain T, Riazuddin S (2011) Assessment of potato proteinase inhibitor-II gene as an antifungal and insecticidal agent. Acta Agric Scand Sect B 61:92–96
Meyer M, Huttenlocher F, Cedzich A, Procopio S, Stroeder J, Pau-Roblot C, Lequart-Pillon M, Pelloux J, Stintzi A, Schaller A (2016) The subtilisin-like protease SBT3 contributes to insect resistance in tomato. J Exp Bot 67(14):4325–4338
Mishra M, Mahajan N, Tamhane VA, Kulkarni MJ, Baldwin IT, Gupta VS, Giri AP (2012) Stress inducible proteinase inhibitor diversity in Capsicum annuum. BMC Plant Biol 12:217
Munir F, Hayashi S, Batley J, Naqvi SM, Mahmood T (2016) Germin-like protein 2 gene promoter from rice is responsive to fungal pathogens in transgenic potato plants. Funct Integr Genom 16:19–27
Pearce G, Ryan CA, Liljegren D (1988) Proteinase inhibitor-I and inhibitor-II in fruit of wild tomato species—transient components of a mechanism for defense and seed dispersal. Planta 175:527–531
Pearce G, Johnson S, Ryan CA (1993) Purification and characterization from tobacco (Nicotina tabacum) leaves of six small, wound inducible, proteinase iso inhibitors of the potato inhibitor II family. Plant Physiol 102:639–644
Peña-Cortés H, Willmitzer L (1995) The role of hormones in gene activation in response to wounding. In: Davis PJ (ed) Plant hormones: physiology, biochemistry and molecular biology. Kluwer Academic Publisher, The Netherlands, pp 395–414
Pena-Cortes H, Sanchez-Serrano J, Rocha-Sosa M, Willmitzer L (1988) Systemic induction of proteinase-inhibitor-II gene expression in potato plants by wounding. Planta 174:84–89
Peña-Cortés H, Fisahn J, Willmitzer L (1995) Signals involved in wound-induced proteinase inhibitor II gene expression in tomato and potato plants. Proc Natl Acad Sci USA 92:4106–4113
Pernas M, Sánchez-Monge R, Salcedo G (2000) Biotic and abiotic stress can induce cystatin expression in chestnut. FEBS Lett 467:206–210
Ryan CA (2000) The systemin signaling pathway: Differential activation of plant defensive genes. Biochim Biophys Acta 1477:112–121
Plunkett G, Senear DF, Zuroske G, Ryan CA (1982) Proteinase inhibitors I and II from leaves of wounded tomato plants: purification and properties. Arch Biochem Biophys 213:463–472
Rehman S, Jørgensen B, Rasmussen SK, Mahmood Tariq (2017a) Characterization of Proteinase inhibitor-II gene under OsRGLP2 promoter for salt stress in transgenic Nicotiana benthamiana. Turk J Biol 41:494–502
Rehman S, Aziz E, Akhtar W, Ilyas M, Mahmood T (2017b) Structural and functional characteristics of plant proteinase inhibitor-II (PI-II) family. Biotechnol Lett. https://doi.org/10.1007/s10529-017-2298-1
Sah SK, Reddy KR, Li J (2016) Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci 7:571
Shah SH, Noureen A, Deeba F, Sultana T, Dukowic-Schulze S, Chen C, Naqvi SMS (2017) Transgenic analysis reveals 5′ abbreviated OsRGLP2 promoter(s) as responsive to abiotic stresses. Mol Biotechnol 59:459–468
Shinozaki K, Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol 3:217–223
Sivasankar S, Sheldrick B, Rothstein SJ (2000) Expression of allene oxide synthase determines defense gene activation in tomato. Plant Physiol 122:1335–1342
Srinivasan T, Kumar KRR, Kirti PB (2009) Constitutive expression of a trypsin protease inhibitor confers multiple stress tolerance in transgenic tobacco. Plant Cell Physiol 50:541–553
Sun JQ, Jiang HL, Li CY (2011) Systemin/Jasmonate-mediated systemic defense signaling in tomato. Mol Plant 4:607–615
Sun X, Yang S, Sun M, Wang S, Ding X, Zhu D, Ji W, Cai H, Zhao C, Wang X, Zhu Y (2014) A novel Glycine soja cysteine proteinase inhibitor GsCPI14, interacting with the calcium/calmodulin-binding receptor-like kinase GsCBRLK, regulated plant tolerance to alkali stress. Plant Mol Biol 85:33–48
Tamhane VA, Giri AP, Kumar P, Gupta VS (2009) Spatial and temporal expression patterns of diverse Pin-II proteinase inhibitor genes in Capsicum annuum Linn. Gene 442:88–98
Tian D, Peiffer M, De Moraes CM, Felton GW (2014) Roles of ethylene and jasmonic acid in systemic induced defense in tomato (Solanum lycopersicum) against Helicoverpa zea. Planta 239:577–589
Wasternack C, Parthier B (1997) Jasmonate-signalled plant gene expression. Trends Plant Sci 2:302–307
Xu D, McElroy D, Thornburg RW, Wu R (1993) Systemic induction of a potato pin2 promoter by wounding, methyl jasmonate, and abscisic acid in transgenic rice plants. Plant Mol Biol 22:573–588
Acknowledgements
This work was financially supported by the Higher Education Commission, Islamabad, Pakistan. We are grateful to the Department of Plant and Environmental Sciences, University of Copenhagen for providing research facilities. The authors are thankful to the Kirsten Jørgensen for her guidance to set up the qPCR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rehman, S., Jørgensen, B., Rasmussen, S.K. et al. Expression analysis of proteinase inhibitor-II under OsRGLP2 promoter in response to wounding and signaling molecules in transgenic Nicotiana benthamiana. 3 Biotech 8, 51 (2018). https://doi.org/10.1007/s13205-017-1070-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-1070-5