Abstract
Pinus hartwegii is the only ectomycorrhizal host distributing at 4000 m asl in the Neotropics forming monospecific sky-island forests on the treeline. These ecosystems have unique environmental conditions like high solar radiation, dozens of soil freeze–thaw cycles per year, and high variability in daily temperatures; additionally their soils, as vitric andosols, are poor in nutrients and organic matter. In this extreme environment ectomycorrhizal fungi transform tree fine roots providing the host plant, through the plant-fungal symbiosis, with novel functions (enhanced nutrient acquisition, higher radiation and desiccation tolerance, etc.) improving tree establishment, growth, and survival. We studied the ectomycorrhizal community associated with P. hartwegii from three sites around 3900 m asl in the treeline of Cofre de Perote Volcano, Mexico. We dissected and characterized the mycorrhizal exploration type and morphological traits of mycorrhizae. Fungal identity and distribution were inferred by DNA sequence analysis of the ITS region. Soil conditions were determined by chemistry and macro and micronutrients contents. These fungal communities are characterized by low alpha diversity (less than ten species per site), high beta diversity (only two species shared between sites), and high dominance of Basidiomycota. Dominating genera Tricholoma, Piloderma, Cortinarius and Gautieria ectomycorrhizae belonged to the Medium-Distance exploration type, characterized by abundant external mycelia that often aggregates in mycelial cords and rhizomorphs. These forests constitute Holarctic sky-island refuges in the Neotropic where several potential endemic ectomycorrhizal fungi have evolved. Dominant ectomycorrhizal fungi are specialist soil foragers providing roots with new functions adapted to poor and harsh soil conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The alpine-treeline ecotone is the transition zone from closed-canopy forest to treeless, alpine meadows. It consists of three zones: forest, timberline, and treeline. Treeline is the upper limit of tree growth, where trees form tree islands but are commonly reduced in growth with krummholz morphology (Hasselquist et al. 2005). The alpine-treeline ecotone is one of the most important climate driven ecological boundaries (Gerhard and Michael 2007) and is extremely vulnerable to global climate change (Wieser et al. 2010). However, in contrast to many studies of its vegetation cover, studies on its belowground biodiversity and function are scarce (Han et al. 2017). Ectomycorrhizal (ECM) fungi improve conifer survival and adaptation and are essential elements in treeline ecotone resilience (Reithmeier and Kernaghan 2013). In these ecotones young conifers experience limitations to carbon assimilation and might therefore be more critically affected by mycorrhizal associations than plants in other habitats (Hasselquist et al. 2005).
Alpine and subalpine ECM fungal communities have been studied predominantly in temperate or boreal regions within the Holarctic biological realm. However, tropical subalpine ecosystems are different from temperate or boreal ecosystems since they show high levels of solar radiation, low variability in the annual average temperature, high variability in daily temperatures, and dozens of soil freeze–thaw cycles per year (Cáceres et al. 2014). The combination of such environmental conditions has influenced the evolution of these high-altitude sites (> 4000 m) in tropical latitudes, characterized by native species that play a fundamental role in the structure of a community with a high degree of endemism (Cáceres et al. 2014; Sklenář et al 2014). Few studies focus on Neotropical subalpine ECM fungi diversity (Reverchon et al. 2010; Garibay-Orijel et al. 2013; Baeza-Guzmán et al. 2017), and they point out that these ecosystems have low ECM species richness in comparison to temperate or boreal communities (for e.g. Kjøller and Clemmensen 2009; Gao and Yang 2010; Han et al. 2017).
The mycelia of ectomycorrhizal fungi are the main soil exploration structures for nutrient and water transfer to ECM host trees and lastly are responsible for tree nutrition (Perez-Moreno and Read 2000; Smith and Read 2008). Up to 75% of phosphorus and 80% of nitrogen acquisition is facilitated to symbiont plants by mycorrhizal fungi (Simard et al. 2003; Hobbie and Hobbie 2006; Van der Heijden et al. 2008). Classification into exploration types is a widely used strategy to infer functional traits of ECM fungi and to assess their impact on ecosystem functioning (Agerer 2001; Hobbie and Agerer 2010; Tedersoo et al. 2012; Koide et al. 2014; Rosinger et al. 2018). However, knowledge of these functional traits is restricted to dominant ECM fungal taxa. Moreover, our understanding about functional traits and exploration type patterns on a community-level is still poor and requires further attention (Rosinger et al. 2018).
Here we studied the ECM community associated with Pinus hartwegii fine roots in the upper forest limit from Cofre de Perote Volcano within the Transmexican Volcanic Axis. This pine species inhabits the alpine-treeline ecotone in central Mexico with an altitude range between 3500 and 4200 m, forming monospecific forest sky-islands with adaptation to low temperatures (Perry 1991; Challenger and Soberón 2008). It is an ecologically important species since it grows at the highest elevations in the tropics and has a protective function safeguarding wildlife; it dampens environmental pollution effects and contributes as regulator of water produced by mountain glaciers meltdown (Solís and Musálem 1994). The Trans-Mexican Volcanic Belt (TMVB) is a continental magmatic arch constituted by around 8000 volcanoes (Gómez-Tuena et al. 2005); it divides Mexico in half and constitutes the physical frontier between the Nearctic and Neotropical biological realms. The volcanic activity that conformed the volcanic axis started 19 million years ago in the mid Miocene and ended around 1 million years ago in the Quaternary, however it is still active in some areas (Gómez-Tuena et al. 2005). Cofre de Perote Volcano was built in three phases, the first one 1.3 million years ago, the second 0.4 million years ago, and the third one 0.24 million years ago (Castellón et al. 2008). In consequence, it was fully built by the time of the last glaciation 0.11 million years ago. Given its complex biogeographical history, the TMVB is acknowledged as a biogeographic transition zone and diversification and endemism center (Gámez et al. 2012).
As P. hartwegii tree line develops in poor soils and very harsh environmental conditions, our hypotheses were that: the ECM fungal community associated to P. hartwegii will have low richness and will be dominated by species with abundant external mycelia specialized in nutrient transfer to their host. In consequence, our objectives were: to analyze the diversity, distribution and community structure of ECM fungi associated to P. hartwegii alpine forests and characterize the functions that they provide to P. hartwegii fine roots through its mycorrhizas. We found that these forests constitute Holarctic sky-island refuges in the Neotropic where several potential endemic ECM species have evolved. Dominant ectomycorrhizal fungi, mainly Basidiomycetes, are nutrient foraging specialists equipped with abundant external mycelium with a great capacity to explore the soil, providing roots with functions adapted to poor and harsh soil conditions.
2 Materials and Methods
2.1 Study site
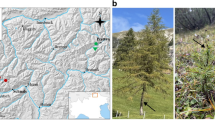
Cofre de Perote is an extinct volcano 4150 m high located in the central region of the Mexican state of Veracruz (19° 29′ 39" N, 97° 08′ 53" W). Above 3000 m vegetation is composed mainly by Pinus ayacahuite, P. hartwegii, P. montezumae, P. pseudostrobus and P. teocote coniferous pine forests. In the highest part of the mountain (3600–4000 m), P. hartwegii establishes monodominant stands in vitric and humic andosol soil. Due to the harsh environmental conditions at high altitude (Vázquez-Ramírez 2014), these forests are characterized by an open canopy (Rzedowski 2006) and low tree density (670 individuals/ha). This species shows low seedling recruitment and fire dependence to regenerate its populations (Sarukhán and Franco 1981). The total dominance of P. hartwegii in the canopy layer is due to its characteristic high tolerance to low temperatures with extremely high solar radiation and many freezing-melting cycles throughout the year (Viveros-Viveros et al. 2007; 2009). In this region, three sampling sites were randomly selected. These sites represent the conifer treeline, where P. hartwegii is the only ECM host. Site 1 is 3860 m high, its slope faces N, it is composed by mature trees with some natural regeneration; the soil has a shallow O horizon. Site 2 has 3890 m high, it has a deep slope facing W and is affected by strong winds and erosion; in consequence, trees have krummholz morphology and soil lacks O horizon. Site 3 is 3620 m with a shallow slope facing E, trees are mature, tall, and soil is well developed with thick O horizon. Site 2 is 1150 m NE away from Site 1, and Site 3 is 1670 m NE away from Site 2, Site 3 is 2800 m NE away from Site 1 (Fig. S1).
2.2 Root collection
To represent 0.6 ha in each sampling site we defined 4 transects 400 m long over the same altitude separated from each other by 50 m. In each transect we established 5 sampling points every 100 m. At each sampling point, 3 soil cores were collected separated 50 cm from each other removing the thin organic layer and collecting the mineral horizon. For this, 15 cm long, sharp-ended PVC tubes with 1.5 cm diameter were used. The 3 soil samples of each point were mixed to obtain approximately 750 g. In total 60 soil samples were collected from 3 sites × 4 transects × 5 points. The samples were cooled and transported to the laboratory. Within one or two days they were sieved through a 2.0 mm mesh; then roots were washed with running water. All fine roots were examined and cleaned under a stereoscopic microscope (Stemi DV4) sorting them into morphotypes according to Baeza-Guzmán et al. (2017). One representative of each OTU by sample was placed in Eppendorf tubes with 2% CTAB at 4 ºC to preserve the DNA.
2.3 DNA Sequencing and analyses
Mycorrhizal DNA extraction, PCR amplification and sequencing followed the procedures detailed by Garibay-Orijel et al. (2013). Briefly, DNA extraction was performed using the kit XNAP (Sigma-Aldrich). PCR was performed with the Ruby Taq PCR Master Mix (Affymetrix) with ITS1F and ITS4 (Gardes and Bruns 1993). Good-quality amplicons were cleaned with ExoSAP-IT (ThermoFisher). Then, they were sequenced in both directions with same PCR primers in an ABI3100 genetic analyzer (Applied Biosystems) in the “Laboratorio de Secuenciación Genómica de la Biodiversidad y la Salud” at the “Instituto de Biología, UNAM”. DNA sample sequences were edited (trimming and base call review) and forward and reverse sequences assembled into contigs; then, contig sequences were grouped into operational taxonomic units (OTUs) at 98% nucleotide similarity in Geneious 10.0.2. Only high quality (HQ > 90%) sequences at least 500 pb were considered in analyses. To assign taxonomic identity to each OTU, we conducted a BLAST search in the UNITE and NCBI databases, this results have been already published in Baeza-Guzmán et al. (2017). Sequences with more than 98% nucleotide similarity were considered conspecific. Metadata of conspecific sequences from published papers were analyzed to determine ECM fungi potential distribution and associated hosts (Bonito et al. 2010; Tse-Laurence and Bidartondo 2011; Avis 2012; Looney et al. 2016). The potential endemism of species is based on the rationale of García-Guzmán et al. (2017) as follows. We used the term "potential endemic" which includes endemic species and species with broad distributions that may not be represented in public databases. For this, we used UNITE and GenBank searches with BLAST; if the higher best match for an OTU was < 98%, we assumed that it is not common and widespread, it is not distributed in the Northern Hemisphere, and in consequence, it is potentially endemic (García-Guzmán et al. 2017).
The most representative sequence of each OTU was deposited in the nucleotide database of GenBank under accession numbers KU871223-KU871244. Once OTUs were assigned with a taxonomic identity they were treated as species.
2.4 Soil data collection
To demonstrate the low nutrient content and soil heterogeneity in the area we conducted physicochemical soil analysis in each sampling site. At each sampling point, a second soil sample was collected using 15 cm long sharp-ended PVC tubes with a 1.5 cm diameter. Samples of each transect were mixed to obtain a total of 1 kg of soil. Samples were transported in Ziploc bags, and dried them at room temperature for three days. The physicochemical properties of the soil samples (pH, soil organic matter (SOM), inorganic N, extractable P, interchangeable K, interchangeable Ca, extractable Mg, interchangeable Fe, and interchangeable Zn) were obtained for each transect in the “Laboratorio de Fertilidad del Suelo y Nutrición Vegetal” at the “Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP)".
2.5 Ecological Analyses
Below ground diversity was assessed by computing species richness and abundance and then calculating Shannon-Wiener diversity index (H') and Simpson diversity index (1/D). Computations were performed using EstimateS version 8.2.0 (Colwell 2019). To demonstrate the total ECM fungal diversity of the study site and to estimate the sufficiency of sample size, a species accumulation curve was calculated. Chao2 and Jacknife2 were used to estimate species richness. To assess ECM community structure associated to P. hartwegii fine roots relative abundance (species number of root tips divided by the total root tips successfully sequenced) and relative frequency (species number of transects present divided by the absolute frequency sum of all species) were computed for each ECM species. The morphological traits of ectomycorrhizae were analyzed according to Agerer (2001) and classified their exploration types as contact, short-distance, medium-distance, and long-distance exploration types and subtypes.
For each site we computed the average N, P, K, Ca, Cu, Fe, Mg, Zn, SOM content and pH using data from the four transects. As data have non-normal distribution we looked for significant differences in soil properties by sampling sites with a non-parametric Kruskal–Wallis test. Pearson correlations were conducted to assess the relationship between edaphic factors and ECM species richness; additionally a Non-Metric Multidimensional Scaling (NMDS) was performed in R to summarize differences in transect soil composition across three sites.
3 Results
3.1 Pinus hartwegii ectomycorrhizal fungi diversity, community structure and distribution
In total, we sequenced successfully 116 mycorrhizal root tips belonging to 22 ectomycorrhizal species associated to P. hartwegii fine roots. These species corresponded to 13 genera and 12 families. The families with the higher number of species were Atheliaceae (5 species) and Cortinariaceae (5 species). Site 2 showed the lowest richness with 7 species, while sites 1 and 3 had 8 and 9 species respectively. Sites 2 (H' 1.92, 4.47 1/D) and 3 (H' 2.2, 5.99 1/D) showed the highest H' and 1/D diversity indexes (Table 1). The species accumulation curve did not reach a plateau when all sampling sites were randomly resampled. Individual site accumulation curves have attenuated slopes depicting the low alpha diversity of each site (Fig. S2). Chao2 estimated a total richness of 64.2 species and Jackknife2 estimated a total richness of 41.2 species (Fig. S3). Site 1 had 7 unique species, while Site 2 had 5 and Site 3 had 8 unique species. We did not find shared species between Sites 1 and 3, and we did not find any EM fungal species present at all three sites (Table 2).
Most abundant species were Tricholoma equestre complex (28% of mycorrhizas), Gautieria sp. 1 (18%), Piloderma sp. 2 (13%), and Cortinarius aff. colymbadinus (10%) (Fig. 1). Tricholoma equestre complex was dominant at Sites 1 and 2, while Gautieria sp. 1 was the dominant species in Site 3. Accordingly, the most frequent taxa were Tricholoma equestre complex (present in 9 transects), Piloderma sp. 2 (8 transects), Gautieria sp. 1 and Cortinarius aff. colymbadinus (5 transects each) (Table 2).
Fourteen percent of the ECM fungi found associated with P. hartwegii have never been sequenced elsewhere (Lyophyllum sp. 1, Pseudotomentella sp. 1, and Hygrophorus sp. 1). DNA sequences of fourteen percent of species (Russula aff. betularum, Hydnellum sp. 1, and Sistotrema aff. confluens) are only known from the conifer high mountain forests of the Trans-Mexican Volcanic Belt. Other species (32%) like Clavulina sp. 1, Cortinarius sp. 1, Gautieria sp. 1, Piloderma sp. 1, 2, 3, and Russula sp. 1 have been found previously through North America. In contrast, most species (36%) (Byssocorticium sp. 1, C. mucosus, C. aff. colymbadinus, C. diasemospermus, Pezizomycotina sp. 1, Piloderma olivaceum, Serendipita vermifera, and Tricholoma equestre complex) are widely distributed in the Holarctic and are mainly associated with alpine and boreal forests (Table S1).
3.2 Exploration types of ectomycorrhizae associated with P. hartwegii fine roots
Most ectomycorrhizal fungi (68.2%) in P. hartwegii roots developed Medium-distance exploration type ectomycorrhizas (Fig. 2). The most common exploration subtype was Medium-distance fringe exploration represented by Cortinarius diasemospermus, C. mucossus, Cortinarius sp. 1, 2, 3, Lyophyllum sp. 1, Piloderma olivaceum, Piloderma sp. 1, 2, 3, Byssocorticium sp. 1, and Tricholoma equestre complex. While Gautieria sp. 1 and Hydnellum sp. 1 produced the Medium-distance mat exploration type and Pseudotomentella sp. 1 Medium-distance smooth exploration type. The Contact exploration type was produced by 18.2% of ectomycorrhizal fungi (Clavulina sp. 1, Serendipita vermifera, Russula aff. betularum, and Russula sp. 1). Just 13.6% of species developed Short-distance exploration type (Hygrophorus sp. 1, Sistotrema aff. confluens, and Pezizomycotina sp. 1) (Table 2).
Exploration types of dominant ectomycorrhizal fungi on P. hartwegii fine roots. A Tricholoma equestre complex Medium-distance fringe exploration type. B Gautieria sp. Medium-distance mat exploration type. C Piloderma sp. 2 Medium-distance fringe exploration type. D Cortinarius sp. 3 Medium-distance fringe exploration type. E Tretomyces lutescens Medium-distance fringe exploration type. F Piloderma olivaceum Medium-distance fringe exploration type
3.3 Physicochemical soil properties and mycorrhizal types distribution
The Kruskal–Wallis test showed that all evaluated soil variables were significantly different between the three sites (Table 1). The NMDS analysis demonstrated that sampling sites are separated by pH (r2 = 0.973, P = 0.001) and P extractable (r2 = 0.384, P = 0.05) (Fig. S4). Site 1 showed higher soil concentrations of macronutrients (N, P, K) and micronutrients (Fe, Zn, Cu, Mn). Site 2 showed the lowest concentrations of all the elements evaluated. Site 3 showed the highest values of pH, SOM, Ca, and Mg. Only P was an explanatory variable negatively correlated with EMC fungal richness (r2 = -0.58, P = 0.023). ECM of medium distance exploration type was the most abundant in sites 2 and 3 where inorganic N and extractable P are scarce, in particular it accounted for 95.9% of all mycorrhizas in site 2. In contrast, site 1 with the higher contents of N and P had the lowest proportion of medium distance exploration type (Fig. 3).
4 Discussion
4.1 ECM fungi diversity in alpine P. hartwegii forests in the Neotropic
Tropical alpine forests in the Cofre de Perote are characterized by lower ECM richness than temperate forests but high beta diversity (turnover) and potential endemism. We found just 22 ECM fungi associated with the roots of P. hartwegii. Even while this low richness could be a result of sampling, in fact, P. hartwegii forests have the lowest richness of soil fungi and mycorrhizal fungi among coniferous forests in the Trans-Mexican Volcanic Belt (Argüelles-Moyao and Garibay-Orijel 2018).
In alpine areas of North America, Europe, and Asia Cortinarius, Russula, Lactarius, and Tomentella are usually the dominant and richest ECM genera (Gardes and Dahlberg 1996; Mühlmann and Peintner 2008; Gao and Yang 2010; Koizumi et al 2018). In Cofre de Perote, Cortinarius and Piloderma had the highest richness. Cortinarius is a common genus, often a key component in ECM communities of boreal forests (Lindahl et al. 2010); while Piloderma is a mat-forming fungus common in temperate to boreal forests in western North America (Dunham et al. 2007; Trappe et al. 2012). Heinonsalo et al. (2015) and Sun et al. (2015) reported Piloderma as dominant in Finland forests of P. sylvestris, where some species such as P. olivaceum have a great ability to produce protease and enzymes needed to transform organic soil resources. In Neotropical mountains of Mexico, Atheliaceae also has a high abundance in Pinus mycorrhizal roots (Reverchon et al 2010). Piloderma becomes dominant after fire events (Sun et al. 2015) as its resistant propagules have specific adaptations, persistence, and tolerance to high temperatures (Glassman et al. 2016). Pinus hartwegii is also fire tolerant and possesses several adaptations to survive it (Rodríguez-Trejo 2001); indeed, fire is fundamental for the regeneration of its populations (Sarukhán and Franco 1981). As both symbionts are adapted to fire events, the high frequency of the P. hartwegii + Piloderma spp. mycorrhizas is likely to be a key element for the persistence of these ecosystems.
4.2 Distribution and ecology of the ectomycorrhizal fungi associated with P. hartwegii
The complex ECM fungal community of P. hartwegii in the TMVB highlands results from a sky-island dynamic characterized by geographic isolation (0.24 M years) and climate fluctuations (already conformed by the last glaciation 0.11 M years ago). This dynamic in the TMVB allows in situ population persistence, particularly for boreal species, while promoting recent divergence and speciation events (Mastretta-Yanes et al. 2015). Thus, P. hartwegii forests constitute sky-island refuges for Holarctic ECM fungi in the Neotropic as well as a region where allopatric and parapatric speciation, driven by climatic and geological events, favors the potential endemicity of several fungal taxa. The ECM fungi associated with P. hartwegii, whose distribution is only known locally (Cofre de Perote volcano) or regionally (Trans-Mexican Volcanic Belt) (Hydnellum sp. 1, Hygrophorus sp. 1, Lyophyllum sp. 1, Pseudotomentella sp. 1, Russula aff. betularum, and Sistotrema aff. confluens), are only associated with pines. Their fruit bodies and/or mycorrhizae have only been sequenced in Mexican P. montezumae and P. hartwegii forests above 3000 m. Some of the continentally distributed species are particularly associated to Pinaceae, like Clavulina sp. 1, Cortinarius sp. 1, Pilodema sp.1, 3. Widely distributed species are generalists forming mycorrhizas with Pinaceae, Fagaceae, Salicaceae and Betulaceae; some of these like Cortinarius sp. 2, Pezizomycotina sp. 1, and S. vermifera even form arbutoid mycorrhizae. The species Pezizomycotina sp. 1, Piloderma sp. 1 and S. vermifera also establish mycoheterotrophic relationships with understory plants. Therefore available data suggest that specialist species have limited distributions being potentially endemic to the TMVB; while the widely distributed generalist Holarctic species (Koizumi et al. 2018) can establish in the TMBV highlands based on their ability to shift hosts becoming isolated in these refugees during interglacial periods.
4.3 Pinus hartwegii fine roots are dominated by Medium-distance exploration organic N soil forager specialists
The Cofre de Perote volcano soil is highly heterogeneous and nutrient poor. The three evaluated sites were significantly different in all macro and micronutrients (Table 1). In comparison with the normal ranges of soil nutrient content reported by Hossner (2008) Site 2 is extremely poor in all nutrients; while Site 1 and 3 are extremely acid and, even with higher concentrations, are still poor in N, P and Mg. These soils are particularly poor in N inorganic forms, with SOM being the main source of N. In these Neotropical alpine forests the genera with the most abundant ectomycorrhizas were Tricholoma, Cortinarius, Piloderma, and Gautieria; all them have Medium-distance exploration types with hydrophobic mycorrhizas and short rhizomorphs.
Overall, the ECM fungi with Medium-distance exploration type dominated the ECM community of P. hartwegii. Species with this exploration type accounted for 68.2% of richness, 90.5% of total abundance and 80.9% of total frequency of ectomycorrhizas (Fig. 4). This pattern was particularly evident in site 2, which is the most nutrient depleted (inorganic N = 9 (mg/kg), extractable P = 0.68 (mg/kg). As this site is highly eroded and lacks an O soil Horizon, organic forms of N and P are also scarce. In this site the medium distance exploration type was dominant (Fig. 3) and 71% of the ECM species present produced this specialized exploration type (Table 2). The Medium-distance is the richest exploration type present in the higher number of species across temperate and boreal forests in Europe, accounting for 43%- 51% of the ECM fungi species in Fagus sylvatica, Picea abies and Pinus sylvestris stands; however, in these same forests the numbers of its mycorrhizas are low with an abundance ranging from 4.7% to 5.9% (Rosinger et al. 2018). This exploration type, particularly those of Tricholoma, Cortinarius and Piloderma, is specialized in foraging organic N and becomes dominant in N poor forests (Trudell and Edmonds 2004; Hobbie and Agerer 2010; Lilleskov et al. 2011). Thus, the P. hartwegii fine roots are colonized by a set of specialized fungi that produce abundant extraradical mycelia capable of exploring poor soils and providing trees with the nutrients necessary for their development.
5 Conclusions
The ectomycorrhizal community associated with the fine roots of P. hartwegii Neotropical alpine forest is characterized by low alpha diversity, high beta diversity (turnover), and high dominance of Basidiomycota. The fungi forming this community are a complex mix of Holarctic host generalists, North American Pinaceae specialists and potentially endemic fungi. This community is a result of a sky-island dynamic with geographic isolation and climate fluctuations that allow in situ population persistence of Holarctic species, while promoting recent divergence and speciation events. As a result, even while the ECM fungi richness of these ecosystems is low, there is a considerable unexplored fungal diversity of mushrooms with restricted distribution or potentially endemics.
As these ecosystems are constantly affected by fire and Pinus hartwegii and Piloderma spp. are fire tolerant, the symbiosis P. hartwegii + Piloderma spp. is a key element for the persistence of these ecosystems.
The Cofre de Perote volcano soil is highly heterogeneous and nutrient poor. There, Tricholoma, Cortinarius, Piloderma, and Gautieria formed the most abundant ectomycorrhizas; all them have Medium-distance exploration types. This exploration type is specialized in foraging organic N and becomes dominant in N poor forests.
Data availability
DNA sequences were deposited on GenBank.
Code availability
Software used is cited in methods.
References
Agerer R (2001) Exploration types of ectomycorrhizae. Mycorrhiza 11(2):107–114
Argüelles-Moyao A, Garibay-Orijel R (2018) Ectomycorrhizal fungal communities in high mountain conifer forests in central Mexico and their potential use in the assisted migration of Abies religiosa. Mycorrhiza 28(5–6):509–521
Avis PG (2012) Ectomycorrhizal iconoclasts: the ITS rDNA diversity and nitrophilic tendencies of fetid Russula. Mycologia 104:998–1007
Baeza-Guzmán Y, Medel-Ortiz R, Garibay-Orijel R (2017) Caracterización morfológica y genética de los hongos ectomicorrízicos asociados a bosques de Pinus hartwegii en el Parque Nacional Cofre de Perote, Veracruz. Rev Mex Biodiv 88(1):41–48
Bonito GM, Gryganskyi AP, Trappe JM, Vilgalys R (2010) Global metaanalysis of tuber ITS rDNA sequences: species diversity, host associations and long-distance dispersal. Mol Ecol 19:4994–5008
Cáceres Y, Llambí LD, Rada F (2014) Shrubs as foundation species in a high tropical alpine ecosystem: a multi-scale analysis of plant spatial interactions. Plant Ecol Div 8(2):147–161
Castellón RD, Núñez GC, Aceves AÁM (2008) Mechanical instability quantification of slopes at Cofre de Perote volcano, eastern Mexico. Bol Soc Geol Mex 60(2):187–201
Challenger A, Soberón J (2008) Los ecosistemas terrestres. In: Conabio (ed) Capital Natural de México, vol. I: Conocimiento actual de la biodiversidad. Conabio, Ciudad de Mexico, pp 87–108
Colwell RK, EstimateS: Statistical estimation of species richness and shared species from samples. Version 8.0.0. http://purl.oclc.org/estimates. Accessed 2 Dec 2019
Dunham SM, Larsson KH, Spatafora JW (2007) Species richness and community composition of mat-forming ectomycorrhizal fungi in old- and second-growth Douglas-fir forests of the HJ Andrews Experimental Forest, Oregon, USA. Mycorrhiza 17:633–645
Gao Q, Yang ZL (2010) Ectomycorrhizal fungi associated with two species of Kobresia in an alpine meadow in the eastern Himalaya. Mycorrhiza 20:281–287
Gámez N, Escalante T, Rodríguez G, Linaje M, Morrone JJ (2012) Caracterización biogeográfica de la Faja Volcánica Transmexicana y análisis de los patrones de distribución de su mastofauna. Rev Mex Biodiv 83(1):258–272
García-Guzmán OM, Garibay-Orijel R, Hernández E, Arellano-Torres E, Oyama K (2017) Word-wide meta-analysis of Quercus forests ectomycorrhizal fungal diversity reveals southwestern Mexico as a hotspot. Mycorrhiza 27(8):811–822
Gardes M, Bruns T (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gardes M, Dahlberg A (1996) Mycorrhizal diversity in arctic and alpine tundra: an open question. New Phytol 133:147–157
Garibay-Orijel R, Morales-Marañón E, Domínguez-Gutiérrez M, Flores-García A (2013) Caracterización morfológica y genética de las ectomicorrizas formadas entre Pinus montezumae y los hongos presentes en los bancos de esporas en la faja volcánica transmexicana. Rev Mex Biodiv 84:153–169
Gerhard W, Michael T (2007) Trees at their upper limit, vol 5. Springer, Heidelberg
Glassman SI, Levine CR, DiRoccI AM, Battles JJ, Bruns T (2016) Ectomycorrhizal fungal spore bank recovery after a severe forest fire: some like it hot. ISME J 10(5):1228–1239
Gómez-Tuena A, Orozco-Esquivel M, Ferrari L (2005) Petrogénesis ígnea de la faja volcánica transmexicana. Bol Soc Geol Mex 57(3):227–283
Han Q, Huang J, Long D, Wang X, Liu J (2017) Diversity and community structure of ectomycorrhizal fungi associated with Larix chinensis across the alpine treeline ecotone of Taibai Mountain. Mycorrhiza 27(5):487–497
Hasselquist N, Germino MJ, McGonigle T, Smith WK (2005) Variability of Cenococcum colonization and its ecophysiological significance for young conifers at alpine–treeline. New Phyt 165(3):867–873
Heinonsalo J, Sun H, Santalahti M, Bäcklund K, Hari P, Pumpanen J (2015) Evidences on the ability of mycorrhizal genus Piloderma to use organic Nitrogen and deliver it to scots pine. PLoS ONE 10(7):e0131561
Hobbie EA, Agerer R (2010) Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Plant Soil 327(1–2):71–83
Hobbie JE, Hobbie EA (2006) 15N in symbiotic fungi and plants estimates nitrogen and carbon flux rates in arctic tundra. Ecology 87:816–822
Hossner LR (2008) Macronutrients. In: Chesworth W (ed) Encyclopedia of Soil Science. Springer, Dordrecht
INEGI (2008) Unidades climáticas de México. Available at: https://idegeo.centrogeo.org.mx/layers/geonode%3Aunidadesclimaticas_gw84
Kjøller R, Clemmensen KE (2009) Belowground ectomycorrhizal fungal communities respond to liming in three southern Swedish coniferous forest stands. For Ecol Manag 257(11):2217–2225
Koide RT, Fernandez C, Malcolm G (2014) Determining place and process: Functional traits of ectomycorrhizal fungi that affect both community structure and ecosystem function. New Phytol 201:433–439
Koizumi T, Hattori M, Nara K (2018) Ectomycorrhizal fungal communities in alpine relict forests of Pinus pumila on Mt. Norikura, Japan. Mycorrhiza 28(2):129–145
Lilleskov EA, Hobbie EA, Horton TR (2011) Conservation of ectomycorrhizal fungi: exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fun Ecol 4(2):174–183
Lindahl BD, de Boer W, Finlay RD (2010) Disruption of root carbon transport into forest humus stimulates fungal opportunists at the expense of mycorrhizal fungi. ISME J 4:872–881
Looney BP, Ryberg M, Hampe F, Sanchez-Garcia M, Matheny PB (2016) Into and out of the tropics: global diversification patterns in a hyperdiverse clade of ectomycorrhizal fungi. Mol Ecol 25:630–647
Mastretta-Yanes A, Moreno-Letelier A, Piñero D, Jorgensen TH, Emerson BC (2015) Biodiversity in the Mexican highlands and the interaction of geology, geography and climate within the Trans-Mexican Volcanic Belt. J Biogeog 42:1586–1600
Mühlmann O, Peintner U (2008) Mycobionts of Salix herbacea on a glacier forefront in the austrian alps. Mycorrhiza 18:171–180
Perez-Moreno J, Read DJ (2000) Mobilization and transfer of nutrients from litter to tree seedlings via the vegetative mycelium of ectomycorrhizal plants. New Phytol 145(2):301–309
Perry J (1991) The pines of México and Central America. Timber Press, Portland
Reithmeier L, Kernaghan G (2013) Availability of ectomycorrhizal fungi to black spruce above the present treeline in Eastern Labrador. PLoS ONE 8(10):e77527
Reverchon F, Ortega-Larrocea MP, Pérez-Moreno J, Peña-Ramírez VM, Siebe C (2010) Changes in community structure of ectomycorrhizal fungi associated to Pinus montezumae across a volcanic soil chronosequence at Sierra Chichinautzin, Mexico. Can J for Res 40:1165–1174
Rodríguez-Trejo DA (2001) Ecología del fuego en el ecosistema de Pinus hartwegii Lindl. Rev Chap Ser Cien for Amb 7:145–151
Rosinger C, Sandén H, Matthews B, Mayer M, Godbold DL (2018) Patterns in ectomycorrhizal diversity, community composition, and exploration types in European beech, pine, and spruce forests. Forests 9(8):445
Rzedowski J (2006) Vegetación de México, 1rst digital ed. CONABIO, Ciudad de Mexico
Sarukhán KJ, Franco BM (1981) Un modelo de simulación de la productividad forestal de un bosque de pino. SARH, Ciudad de Mexico
Simard SW, Jones MD, Durall DM (2003) Carbon and nutrient fluxes within and between mycorrhizal plants. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal Ecology. Springer, Berlin, pp 33–74
Sklenář P, Hedberg I, Cleef AM (2014) Island biogeography of tropical alpine floras. J Biogeograph 41(2):287–297
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic press, New York
Solís PMA, Musálem M (1994) Estado del conocimiento de Pinus hartwegii Lindl. México. In: Arteaga-Martínez B, Musálem MA (eds) IV Reunión Nacional de Plantaciones Forestales. INIFAP, Ciudad de Mexico, pp 139–142
Sun H, Santalahti M, Pumpanen J, Köster K, Berninger F, Raffaello T (2015) Fungal community shifts in structure and function across a boreal forest fire chronosequence. Appl Environ Microbiol 81:7869–7880
Tedersoo L, Naadel T, Bahram M, Pritsch K, Buegger F, Leal M, Kõljalg U, Põldmaa K (2012) Enzymatic activities and stable isotope patterns of ectomycorrhizal fungi in relation to phylogeny and exploration types in an afrotropical rain forest. New Phytol 195:832–843
Trappe MJ, Cromack K, Caldwell BA, Griffiths RP, Trappe JM (2012) Diversity of mat-forming fungi in relation to soil properties, disturbance, and forest ecotype at Crater Lake National Park, Oregon, USA. Diversity 4(2):196–223
Trudell SA, Edmonds RL (2004) Macrofungus communities correlate with moisture and nitrogen abundance in two old-growth conifer forests, Olympic National Park, Washington, USA. Can J Bot 82(6):781–800
Tse-Laurence MA, Bidartondo MI (2011) Mapping fungi from below ground: online genetic resources and ectomycorrhizal geographic distributions. iForest 4:252–255
Van der Heijden MG, Bardgett RD, Van Straalen NM (2008) The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
Vázquez-Ramírez J (2014) Fenología reproductiva de las comunidades vegetales del Parque Nacional Cofre de Perote, Veracruz, México. Masters Dissertation, Universidad Veracruzana
Viveros-Viveros H, Sáenz-Romero C, López-Upton J, Vargas-Hernández JJ (2007) Growth and frost damage variation among Pinus pseudostrobus, P. montezumae and P. hartwegii tested in Michoacán, México. For Ecol Manag 253(1–3):81–88
Viveros-Viveros H, Saenz-Romero C, Vargas-Hernández JJ, López-Upton J, Ramírez-Valverde G, Santacruz-Varela A (2009) Altitudinal genetic variation in Pinus hartwegii Lindl. I: Height growth, shoot phenology, and frost damage in seedlings. For Ecol Manag 257(3):836–842
Wieser G, Oberhuber W, Walder L, Spieler D, Gruber A (2010) Photosynthetic temperature adaptation of Pinus cembra within the timberline ecotone of the central Austrian alps. Ann Forest Sci 67(2):201
Acknowledgements
The first author express her gratitude to "Laboratorio de Organismos Beneficos", Faculty of Agricultural Sciences at Universidad Veracruzana and "Laboratorio de Micología II", Instituto de Ecología (INECOL) for the facilities granted for molecular works. We also thank support of Laura Márquez from the Sequencing Unit of the Biology Institute (UNAM).
Funding
This research was funded by CONACYT Masters scholarship to YBG. CONACYT MEXBOL 251085 funded DNA sequencing.
Author information
Authors and Affiliations
Contributions
RGO and RMO conceived the ideas and designed the study, YBG conducted the field and molecular work, all authors analyzed the data, YBG wrote the first draft of the manuscript; RGO, DTA and RMO commented on data analysis and interpretation. RGO prepared final version and all authors approved it.
Corresponding author
Ethics declarations
Ethics approval
No ethics approval needed.
Consent to participate
All authors consent to participate.
Consent for publication
All authors approved the final version.
Conflicts of interest
Authors declare no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baeza-Guzmán, Y., Medel-Ortiz, R., Trejo Aguilar, D. et al. Medium-distance soil foragers dominate the Pinus hartwegii ectomycorrhizal community at the 3900 m Neotropical treeline. Symbiosis 87, 213–222 (2022). https://doi.org/10.1007/s13199-022-00869-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-022-00869-6