Abstract
Alpine treeline ecotones represent ecosystems that are vulnerable to climate change. We investigated the ectomycorrhizal (ECM) community, which has potential to stabilize alpine ecosystems. ECM communities associated with Larix chinensis were studied in four zones along a natural ecotone from a mixed forest stand over pure forest stands, the timberline, and eventually, the treeline (3050–3450 m) in Tabai Mountain, China. Sixty operational taxonomic units (OTUs) of ECM fungi were identified by sequencing the rDNA internal transcribed spacer of ECM tips. The richness of ECM species increased with elevation. The soil C/N ratio was the most important factor explaining ECM species richness. The treeline zone harbored some unique ECM fungi whereas no unique genera were observed in the timberline and pure forest zone. Elevation and topography were equally important factors influencing ECM communities in the alpine region. We suggest that a higher diversity of the ECM fungal community associated with L. chinensis in the treeline zone could result from niche differentiation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The alpine treeline, an ecological transition zone from forest to alpine krummholz, is one of the most important climate-driven ecological boundaries, (Gerhard and Michael 2007) and a potential habitat for many plant species. Research of the alpine treeline ecotone has increased in recent years because this ecosystem is thought to be extremely vulnerable to global climate change (Wieser et al. 2010). However, in contrast to many studies of vegetation cover (Carlson et al. 2014, Rozman et al. 2013, Tomback et al. 2016), belowground biodiversity has received little attention in the alpine treeline ecotone. Nevertheless, it is generally known that belowground organisms play a key role in the ecological functioning of terrestrial ecosystems.
It has been known for a long time that most of the tree species living in the alpine treeline ecotone are mycorrhizal (Horak and Moser 1966). Ectomycorrhizal (ECM) fungi are thought to be particularly important in harsh environments such as the Arctic tundra (Timling et al. 2012). Thus, in the alpine treeline ecotone, ECM associations may be more important than in many other habitats (Johnson et al. 2004). During primary succession, ECM fungi can help seedling establishment through their spores and mycelia, as observed on Fuji Mountain by Ishida et al. (2008). ECM fungi are known to improve soil moisture and nutrient availability and enhance plant vegetative propagation (Reithmeier and Kernaghan 2013, Zhu et al. 2010). However, studies of the ECM community in the alpine treeline ecotone are few in number.
There are several examples where ECM communities associated with different forest stands in mountainous regions have been characterized. On mountain slopes in northern Iran, the ECM fungal richness associated with six host trees decreased with increasing elevation in the range of 400–2700 m above sea level (Bahram et al. 2012). Unlike these Hyrcanian forests of Iran, along Mount Fuji, ECM richness of all host tree species showed a mid-domain effect in the range of 1100–2250 m above the sea level (Miyamoto et al. 2014). This type of change, however, has not been as obvious from previous studies. Both on Cairngorm Mountain (range 300–600 m above the sea level) and in a Mediterranean coastal region (200–800 m), where a single host was studied, there were changes in ECM fungal composition but not in species richness (Jarvis et al. 2015, Scattolin et al. 2014). In these studies, host effects and environmental conditions were defined as the major factors driving change in ECM community composition.
Here, we investigate the ECM fungal community in the alpine treeline ecotone. The Taibai Mountain in China is an ideal site for this study since one tree species, Larix chinensis, is growing along a natural transition from treeline at 3450-m elevation to closed forest stands at 3050-m elevation. We aim to explore the ECM community in the harsh growing conditions of the alpine treeline ecotone. In addition, by analyzing the major environmental factors explaining the changes of ECM community, we identify the major driving factors of the ECM community change.
Materials and methods
Study sites
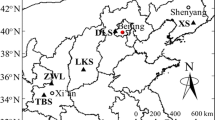
Study sites were selected in the summit region of Taibai Mountain (33° 57′ N, 107° 45′ E), which is the main peak in the Qinling Range. The Qinling Range divides the subtropical and temperate zones in mainland China and also shaped the Yangtze River basin and Yellow River basin in China. Taibai Mountain ranges in elevation from 780 to 3767 m. In the alpine treeline ecotone of Taibai Mountain, annual mean temperature ranges from −2 to −1 °C and precipitation from 800 to 900 mm. Vegetation on Taibai Mountain changes widely and along an elevation gradient. The vegetation changes gradually from deciduous oak forests (780–2300 m) over birch forests (2300–2800 m), to conifer forests (2800–3400 m), and finally, to alpine shrubs and meadows above the treeline (>3400 m). L. chinensis grows in conifer forests at an elevation higher than 3000 m where the ground is covered by snow from October to May (Zhang et al. 2004). Nitrogen deposition for this area is estimated to be 19.2 kg ha−1 year−1 (Liang et al. 2014).
We divided L. chinensis habitat into four zones, defined according to timberline and treeline on the southern slope of Taibai Mountain based on previous studies (Duan et al. 2010, Hasselquist et al. 2005, Linjun et al. 2005): treeline zone (zone 1), timberline zone (zone 2), pure forest stand zone (zone 3), and mixed forest stand zone (zone 4). The treeline zone (3375–3450 m) is the upper limit of tree growth, where it usually occupies the upper slope and where trees are sparsely distributed and severely reduced in growth with krummholz morphology (Fig. 1, Supplementary Fig. S1 ). In the timberline zone (3250–3375 m), trees grow into mature stands where they are usually located in the mid-range of the slope. The pure forest zone grows from 3150 to 3250 m, and finally, in the mixed forest zone, L. chinensis and Abies fargesii Franch grow from 3050 to 3150 m, where they are located in the lower portion of the slope.
In the treeline and timberline zones, the main soil type is sandy loam. The shrub layer consists mainly of Rhododendron capitatum, Rhododendron przewalskii and Salix cupularis. The herb layer is composed mostly of Allium pratii, Cardamine macrophylla, and Thalictrum petaloideum. In the pure and mixed stand zones, the soil type is clay loam. The shrub layer consists mostly of Potentilla arbuscula, Berberis amurensis, and Ribes glaciale. The herb layer is composed of Oxalis griffithii, Carex schneideri, and Polygonum sphaerostachyum (Miao et al. 2005).
Sample collection
In August 2014, we selected three sites in each zone, each positioned at least 200 m apart. From each site, we collected samples of fine roots and rhizosphere soil from ten trees, separated by 10 m to ensure independence of the samples (Lilleskov et al. 2004). The geographical coordinates and elevation were recorded on a GPS Garmin (eTrexH-xiaoboshi) for each tree. A total of 120 trees were selected for root and rhizosphere sampling across the L. chinensis habitat. A minimum of three roots (15–30 cm in length) that were connected to their host, were carefully collected both from the east and north of a tree and placed into plastic bags. Each root contained 100–200 root tips. Roots from a single tree were pooled into a single plastic bag (Long et al. 2016). A 20 × 20 × 20 cm soil sample was collected for each tree and placed in a plastic bag. All root and soil samples were stored in a cooler containing several ice bags. Samples were transported to our laboratory within 24 h for subsequent analysis. Mean annual temperature (MAT) and mean annual precipitation (MAP) for each site were obtained from a weather station in Taibai Mountain, 10 km distant from the field plots. Because data for each elevation were not available, we used Tang’s model to calculate MAT and MAP for each site (Tang and Fang 2006).
Root morphotyping and molecular identification
Root samples for each tree were randomly selected and 200 root tips per tree were inspected under a dissecting microscope (PXS9-T, CeWei photoelectric technology Co. Limited) to examine ECM morphotypes. Morphotyping was completed within 3 weeks of collection. Living ECM root tips (n = 5–10) were sampled for each morphotype from each tree sample, placed into 2.0-ml tubes, and freeze-dried using a lyophilizer (FD5-2.5, Sim International Group Co. Ltd., USA). DNA samples were pulverized by using a bead beater containing a stainless steel ball (MM400, Restch, Germany). DNA was extracted by the modified cetyltrimethylammonium bromide (CTAB) method, which was modified according to Long et al. (2016).
The rDNA internal transcribed spacer (ITS) regions were amplified using a Taq MasterMix (Kangwei, Beijing, China). We followed the manufacturer’s instructions and the modified methods described by Long et al. (2016) to make the reaction solution. We used the ITS1F primer (Gardes and Bruns 1993) and the ITS4 primer (White et al. 1990) in the polymerase chain reaction (PCR). The 30-μL reaction system contained 1.5 μL of the templates DNA; 15 μL of 2 × Taq MasterMix (Kangwei, Beijing, China); 12.3 μL deionized water; and 0.6 μL primers.
PCR cycling parameters were as follows: 94 °C for 5 min; 35 cycles at 94 °C for 30 s, 53 °C for 30 s, and 72 °C for 1 min and a final 5 min 72 °C extension. The amplified PCR products were checked on 1.5% agarose gels and inspected under UV light to determine the quality and quantity of amplified bands with the Bio-Rad Gel Doc™ XR+ system (Bio-Rad, CA, USA). Sequencing was conducted on ABI Prism 3730xl genetic analyzer (Applied Biosystems, Foster City, CA) using ITS1F/ITS4.
Sequences were edited and manually corrected in BioEdit 7.0.8 and then clustered into species-level operational taxonomic units (OTUs) at 97% sequence similarity for species delimitation using the PlutoF (http://unite.ut.ee) in UNITE (Abarenkov et al. 2010, Kõljalg et al. 2013). If no match was found in the PlutoF system, any resulting OTU assignments were individually checked by BLAST against the UNITE database/NCBI database (http://www.ncbi.nlm.nih.gov). All the sequences were deposited to the DNA Data Bank of Japan (DDBJ) under the accession number LC035136-LC035385.
Soil property analysis
Soil samples were air-dried for 1 week, at which time stones and plant roots were removed. We passed half of the soil sample through a 1-mm mesh screen for pH and electric conductivity measurements. Another quarter of the soil sample was ground to fine powder and passed through 0.15-mm mesh screen for soil elemental analyses. Soil pH and electric conductivity (EC) were measured using FE20 pH meter (Mettler-Toledo Instrument Co., Ltd., Shanghai, China) and DDS-307 conductivity meters (Shanghai REX Instrument Factory, Shanghai, China) after mixing the soil sample with deionized water at 1:2 and 1:5 ratio by volume. The Walkley-Black (WB) method was used to determine soil organic carbon (SOC) by quantifying the amount of oxidizable soil carbon as determined by the reaction with acidic dichromate (Cr2O7 2−). Soil nitrogen content (N) was measured by a semi micro Kjeldahl method (AutoAnalyzer 3, Bran+luebbe, Hamburg, German). Soil phosphorus (P) content was measured with the molybdenum blue method (Hass and Loeppert 2011).
Statistical analyses
Statistical differences among the four zone treatments for soil pH, EC, soil C/N ratio and total P were detected using a one-way ANOVA (Tukey test, P < 0.05), where n = 3 sites. We constructed linear models to investigate the relationship between the soil properties and elevation. ECM richness per tree was the number of OTUs detected for each tree and the mean of the ten trees was used as an observation of a site. ECM richness per site was the number of OTUs detected for all ten trees together. The Simpson’s diversity (1/D) and Shannon–Wiener (H′) indices were calculated from the combined data of the ten trees. Observed species richness was the total number of detected ECM fungal species in each zone. Chao2 richness estimations were calculated for each zone using Estimate S version 9.1 (Colwell et al. 2012).
Linear models were constructed to investigate the relationship between the ECM richness per site and elevation. To determine the soil factors influencing ECM richness, we regressed ECM richness and soil variables (soil pH, EC, C/N ratio, and total P) using stepwise regression analysis (Nlme package, R). The best multivariable linear model was built on the basis of corrected Akaike information criterion (AIC) values.
We calculated the relative abundance of each OTU as the number of tips of a certain OTU/total number of ECM tips at each site and the frequencies of OTUs as the number of trees colonized by each OTU/total number of trees in each site. The relative frequency of OTUs was calculated as the number trees colonized by each OTU in each zone/total number of trees colonized by each OTU in all zones. To analyze the common and unique ECM fungal OTU and genera among the four zone treatments, we draw a Venn diagram using the VennDiagram package in the R programming language (Chen and Boutros 2011).
To test the spatial autocorrelation among sampling sites, we conducted a multivariate spatial autocorrelation test for ECM communities (Smouse and Rod 1999). We analyzed the relationship between geographical distances (calculated from GPS coordinates) and Bray-Curtis distances using a Mantel test (Mantel 1967). Bray-Curtis coefficients were used in the analysis of similarity (ANOSIM) (Clarke 1993) to determine whether the ECM communities differed between the zones. Nonmetric multidimensional scaling (NMDS) was used to visualize the distance between the different sites. The “envfit” function was further used to identify the relative importance of environmental variables for structuring the ECM community (Vegan package, R). All statistical analyses were performed with Estimate S (Colwell et al. 2012) and R 3.2.0 packages (Dixon 2003).
Results
Soil physicochemical variables
Between zones 1 and 4, the mountain slope becomes broad and gentle. In general, the soil in the treeline zone (zone 1) had the highest SOC, C/N ratio, and pH, whereas soil P and N were low. A Tukey’s test showed that MAT, MAP, and EC were not significantly different between zones (Table 1). Only soil pH was positively correlated with elevation in the L. chinensis habitat region (Supplementary Fig. S2).
ECM fungal richness
In total, 20,041 root tips were observed in 120 root samples of L. chinensis. DNA was extracted from 1872 ECM root tips, and we selected one well-amplified product of each morphotype in each root sample for further sequencing. Finally, 607 high-quality sequences were successfully obtained. From these, 60 ECM OTUs belonging to 28 genera and 17 families were delimited at 97% clustering (Table 2). Most OTUs belonged to Basidiomycotina (78.3%) and Ascomycotina (8.3%). Major lineages recorded in the study included Thelephoraceae (16 OTUs), Inocybaceae (10 OTUs), Sebacinaceae (8 OTUs), and Russulaceae (6 OTUs). Total observed richness in the four zones were close to the estimated richness (Chao2 index), indicating that our sampling was adequate to accurately estimate the ECM communities (Table 3).
Richness of ECM fungi per tree and site differed significantly between the four vegetation zones (Table 3). Zone 1 had the most whereas zone 4 had the least ECM OTUs. The lowest mean values of Shannon and Simpson’s diversity indices were also detected in zone 4, but they were not significantly different among the four zones. The ECM richness per site increased with increasing elevation (Fig. 2). ECM richness per site was initially correlated with C/N ratio. The best multivariable linear model constructed, where the AIC value was the lowest, included only the soil C/N ratio as an explanatory variable (r 2 = 0.43, P = 0.012).
Community structure
At the species level, Heyderia sp., Sebacina epigaea, and Tomentella sp. were the dominant species in the study area (Supplementary Table 1). At the genus level, Tomentella (56.6, 25.6%) had the highest frequency and relative abundance, followed by Sebacina (40, 14.8%); Heyderia (33.3, 8.5%); and Inocybe (30, 8.8%) (Fig. 3). Tomentella, Inocybe, and Laccaria occurred in each of the four zones with similar frequency. Sebacina, Unknown 2, Russula, and Cortinarius were frequent in zones 1 and 2, whereas Heyderia, Unknown 7, Pseudotomentella, and Lactarius were frequent in zones 3 and 4 (Supplementary Table 1). At the species level, the Venn diagram showed 16 common OTUs among the four zones (Fig. 4(a)). Zone 1 harbored 18 unique species, while zone 2 and zone 3 had only one unique species. At the genus level, zone 1 had eight unique genera (Humaria, Sistotrema, Lachnum, Helvellosecbacina, Pisolithus, Trichophaea, Unknown 1, and Unknown 4) and no unique genera were found in zones 2 and 3 (Figs. 3, 4b). The number of shared OTUs were 28 (zones 1 vs. 2), 25 (zones 1 vs. 3), and 20 (zones 1 vs. 4). This illustrates that the larger the elevation distance is between two zones, the fewer species they share.
Neither the sites in each zone (P z1 = 0.32, P z2 = 0.31, P z3 = 0.6, and P z4 = 0.7) nor all zones (P = 0.06) were spatially autocorrelated according to the multivariate randomization test, indicating that the sites were distant enough from one another to be considered independent. A Mantel test also showed that Bray-Curtis distances were not correlated with their geographic distance (P = 0.129). According to ANOSIM analysis, ECM communities were significantly different among the four zones (r 2 = 0.41, P = 0.003). NMDS ordination indicated that the ECM fungal communities were most dissimilar at the highest elevation (Fig. 5). Zone, elevation, soil pH, soil C/N ratio, mean annual temperature (MAT) and mean annual precipitation (MAP) were significantly correlated with community structure (Fig. 5, Table 4).
Nonmetric multidimensional scaling (NMDS) plot of ECM communities associated with Larix chinensis (stress of the three-dimensional solution = 0.20). Only significant correlations (elevation, soil pH, soil C/N ratio, MAP, and MAT) between NMDS structure and environmental factors are shown (P < 0.05). C/N, soil carbon/nitrogen ratio; MAT mean annual temperature; MAP mean annual precipitation
Discussion
In several previous studies, ECM community richness has been reported to change with elevation. However, the pattern of change has been inconsistent among studies. Some have indicated decreasing (Bahram et al. 2012), increasing (Pellissier et al. 2014) richness along an increasing elevation, or a peak at mid-elevation (Miyamoto et al. 2014). In addition, some studies report no relationship between ECM fungal richness and elevation (Jarvis et al. 2015, Scattolin et al. 2014, Coince et al. 2014). Our analyses supported the finding that ECM richness associated with a single tree species, L. chinensis, tended to increase along an increasing elevation in the alpine treeline ecotone of Taibai Mountain. This is similar to the results observed in the Alps (Pellissier et al. 2014).
One explanation for the inconsistency observed among studies may be related to host tree species that also change with elevation. The host tree effect has been generally recognized as one of the main drivers of ECM diversity (Tedersoo et al. 2011). It is also known that there is a positive relationship between tree species diversity and ECM fungal diversity and no significant relationship has been found between ECM diversity and soil factors (Kernaghan et al. 2003). In our study, excluding the effects of multiple host tree species, ECM fungal diversity was significantly different among the four zones. In addition, ECM fungal richness has been suggested to be controlled by both climatic (temperature and precipitation; Bahram et al. 2012) and soil factors, (structure, pH, moisture and nutrient content; Jarvis et al. 2015; Kernaghan and Harper 2001). Soil physicochemical and biological factors, which are largely regulated by climatic factors, share a complicated interdependence. Therefore, it is difficult to definitively separate their controlling factors. N nutrient is a primary determinant controlling ECM fungal species diversity (Cox et al. 2010). ECM genera, such as Cortinarius, Inocybe, and Suillus, have been shown to respond negatively to increasing N (Lilleskov et al. 2001). In our study, they appeared more abundant in the treeline and timberline. In contrast, ECM genera, such as Hygrophorus, Lactarius, and Tomentella (Lilleskov et al. 2001, Cox et al. 2010), have been shown to respond positively to increasing N. They were more abundant in pure forest stands in this study. This might be due to differences in the distribution of N deposits caused by slope position and gradient. Lower sites may retain more N content in the soil than upper sites, because lower sites have shallow slopes and clay soils compared to upper sites with steep slopes and sandy soils. These results are supported by Liu et al. (2007) that found the N content of soil increases in a downslope direction. Most of the N-sensitive ECM species were substantially decreased or were even eliminated once the soil nitrogen increased.
Similar to our results for ECM community richness, and as suggested by Koide et al. (2005), the structure of the ECM community could be influenced by a number of biotic and abiotic factors, such as climate, litter quality, soil type, and soil nutrient status. In addition, Zhang et al. (2013) found a change in soil properties (soil N and organic C), due to slope position and gradient, may affect the growth and distribution of ECM fungi. Increasing soil N content had a negative effect on fungal diversity (Lilleskov et al. 2011, Cox et al. 2010). In our study, ECM fungal diversity indices decreased gradually with increasing soil N content which was related to low slope position and a gradual slope gradient (Table 3). Thus, the topographic factor might be an important variable influencing ECM communities in alpine regions. Additionally, a positive correlation of soil pH with elevation could be another factor influencing ECM communities. Variation in pH values in the soil could also alter the distribution and growth of ECM fungi (Kjøller and Clemmensen 2009, Rousk et al. 2009). In our study, Tuber species increased in abundance with increasing pH while some Lactarius species decreased in abundance when pH became more basic. Previously conducted studies support these findings (Chambers 1999, Becerra et al. 2005). Finally, we were unable to tease apart the suite of environmental variables that are potential candidates for structuring the community.
Previous studies reported that Larix does not associate with a rich ECM fungal community. Only 30 OTUs were found with Larix decidua and 23 OTUs were found with Larix kaempferi (Leski and Rudawska 2012, Nara 2006). In our study, a total of 60 ECM fungal species were observed on L. chinensis roots. The dominant ECM fungal genera (Tomentella, Sebacina, Inocybe, and Russula) are representative of dominant genera found in studies previously conducted in arctic and alpine environments (Deslippe et al. 2011, Gao and Yang 2016, Geml et al. 2012, Welc et al. 2014). Tomentella was the most common genus in our study, which has been reported to be distributed throughout the world (Kõljalg et al. 2000).
ECM species composition was obviously different between the four elevation and vegetation zones. The dominant ECM genera in the treeline and timberline zones (zone 1 and 2) were Sebacina and Unknown 2. Previous studies have shown that some species belonging to Sebacina could help their host plant overcome biotic and abiotic stresses by supplying it with water and nutrients (Gao and Yang 2016, Ghimire and Craven 2011, Illyés et al. 2009, Singh et al. 2013). Sistotrema and Lachnum have been suggested to enhance plant root growth (Bizabani and Dames 2015, Münzenberger et al. 2012). Heyderia and Cortinarius were the dominating genera both in the pure and mixed stand zones (zones 3 and 4). It is worth noting that Heyderia is an ECM genus that is known to form symbiosis only with few plant species, including Larix (Leski and Rudawska 2012). We speculate that higher diversity could as well be the result of niche differentiation, such as nutrient patchiness, exposure or vegetation shelter among others.
Conclusions
We demonstrated that ECM community richness increases with increasing elevation in the L. chinensis alpine treeline ecotone. We found that soil pH correlated with elevation and we propose that it may be an important factor influencing ECM fungal communities. Moreover, topography-mediated (slope position and gradient) can also be an important factor in structuring the physical and chemical environment, and hence the ECM fungal community.
References
Abarenkov K, Tedersoo L, Nilsson RH, Vellak K, Saar I, Veldre V (2010) PlutoF— a web based workbench for ecological and taxonomic research, with an online implementation for fungal ITS sequences. Evol Bioinform 6:189–196
Bahram M, Põlme S, Kõljalg U, Zarre S, Tedersoo L (2012) Regional and local patterns of ectomycorrhizal fungal diversity and community structure along an altitudinal gradient in the Hyrcanian forests of northern Iran. New Phytol 193(2):465–473
Becerra A, Zak MR, Horton TR, Micolini J (2005) Ectomycorrhizal and arbuscular mycorrhizal colonization of Alnus acuminata from Calilegua National Park (Argentina). Mycorrhiza 15(7):525–531
Bizabani C, Dames J (2015) Effects of inoculating Lachnum and Cadophora isolates on the growth of Vaccinium corymbosum. Microbiol Res 181:68–74
Carlson BZ, Georges D, Rabatel A, Randin CF, Renaud J, Delestrade A (2014) Accounting for tree line shift, glacier retreat and primary succession in mountain plant distribution models. Divers Distrib 20(12):1379–1391
Chambers SM (1999) Ectomycorrhizal fungi key genera in profile. Springer, Heidelberg
Chen H, Boutros PC (2011) VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics 12(1):35
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18(1):117–143
Coince A, Cordier T, Lengellé J, Defossez E, Vacher C, Robin C, Buée M, Marçais B (2014) Leaf and root-associated fungal assemblages do not follow similar elevational diversity patterns. PLoS One 9(6):e100668
Colwell RK, Chao A, Gotelli NJ, Lin S-Y, Mao CX, Chazdon RL (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol 5(1):3–21
Cox F, Barsoum N, Lilleskov EA, Bidartondo MI (2010) Nitrogen availability is a primary determinant of conifer Mycorrhizas across complex environmental gradients. Ecol Lett 13(9):1103–1113
Deslippe JR, Hartmann M, Mohn WW, Simard SW (2011) Long-term experimental manipulation of climate alters the ectomycorrhizal community of Betula nana in Arctic tundra. Glob Chang Biol 17(4):1625–1636
Dixon P (2003) VEGAN, a package of R functions for community ecology. J Veg Sci 14(6):927–930
Duan R, Wang X, Huang M, Wang Z, Wu G (2010) Ecological characteristics of Larix chinensis population near timberline on Taibai Mountain in China. Acta Ecol Sin 30(2):0519–0526
Gao Q, Yang ZL (2016) Diversity and distribution patterns of root-associated fungi on herbaceous plants in alpine meadows of southwestern China. Mycologia 108(2):281–291
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts. Mol Ecol 2(2):113–118
Geml J, Timling I, Robinson CH, Lennon N, Nusbaum HC, Brochmann C, Noordeloos ME, Taylor DL (2012) An arctic community of symbiotic fungi assembled by long-distance dispersers: phylogenetic diversity of ectomycorrhizal basidiomycetes in Svalbard based on soil and sporocarp DNA. J Biogeogr 39(1):74–88
Gerhard W, Michael T (2007) Trees at their upper limit, vol 5. Springer, Heidelberg
Ghimire SR, Craven KD (2011) Enhancement of Switchgrass (Panicum virgatum L.) biomass production under drought conditions by the ectomycorrhizal fungus Sebacina vermifera. Appl Environ Microb 77(19):7063–7067
Hass A, Loeppert RH (2011) Determination of phosphate in selective extractions for soil iron oxides by the molybdenum blue method in an automated continuance flow injection system. Commun Soil Sci Plan 42(14):1619–1635
Hasselquist N, Germino MJ, McGonigle T, Smith WK (2005) Variability of Cenococcum colonization and its ecophysiological significance for young conifers at alpine–treeline. New Phytol 165(3):867–873
Horak, Moser (1966) Fungi austroamericani VIII. Über neue Gastroboletaceae aus Patagonien: Singeromyces Moser, Paxillogaster Horak und Gymnopaxillus Horak. Nova Hedwig 10:329–338
Illyés Z, Halász K, Rudnóy S, Ouanphanivanh N, Garay T, Bratek Z (2009) Changes in the diversity of the mycorrhizal fungi of orchids as a function of the water supply of the habitat. J Appl Bot Food Qual 83(1):28–36
Ishida TA, Nara K, Tanaka M, Kinoshita A, Hogetsu T (2008) Germination and infectivity of ectomycorrhizal fungal spores in relation to their ecological traits during primary succession. New Phytol 180(2):491–500
Jarvis SG, Woodward S, Taylor AF (2015) Strong altitudinal partitioning in the distributions of ectomycorrhizal fungi along a short (300 m) elevation gradient. New Phytol 206(3):1145–1155
Johnson DM, Germino MJ, Smith WK (2004) Abiotic factors limiting photosynthesis in Abies lasiocarpa and Picea engelmannii seedlings below and above alpine timberline. Tree Physiol 24(4):377–386
Kernaghan G, Harper KA (2001) Community structure of ectomycorrhizal fungi across an alpine/subalpine ecotone. Ecography 24(2):181–188
Kernaghan G, Widden P, Bergeron Y, Légaré S, Paré D (2003) Biotic and abiotic factors affecting ectomycorrhizal diversity in boreal mixed-woods. Oikos 102(3):497–504
Kjøller R, Clemmensen KE (2009) Belowground ectomycorrhizal fungal communities respond to liming in three southern Swedish coniferous forest stands. For Ecol Manag 257(11):2217–2225
Koide RT, Xu B, Sharda J, Lekberg Y, Ostiguy N (2005) Evidence of species interactions within an ectomycorrhizal fungal community. New Phytol 165(1):305–316
Kõljalg U, Dahlberg A, Taylor A, Larsson E, Hallenberg N, Stenlid J, Larsson KH, Fransson PM, Kårén O, Jonsson L (2000) Diversity and abundance of resupinate thelephoroid fungi as ectomycorrhizal symbionts in Swedish boreal forests. Mol Ecol 9(12):1985–1996
Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22(21):5271–5277
Leski T, Rudawska M (2012) Ectomycorrhizal fungal community of naturally regenerated European larch (Larix decidua) seedlings. Symbiosis 56(2):45–53
Liang T, Tong Y, Lin W, Qiao L, Liu X (2014) Spatial-temporal variability of dry and wet deposition of atmospheric nitrogen in different ecological regions of Shaanxi. Acta Ecol Sin 24(3):738–745
Lilleskov EA, Fahey TJ, Lovett GM (2001) Ectomycorrhizal fungal abovegound community change over an atmospheric nitrogen deposition gradient. Ecol Appl 11(2):397–410
Lilleskov EA, Bruns TD, Horton TR, Lee Taylor D, Grogan P (2004) Detection of forest stand-level spatial structure in ectomycorrhizal fungal communities. FEMS Microbiol Ecol 49(2):319–332
Lilleskov EA, Hobbie EA, Horton TR (2011) Conservation of ectomycorrhizal fungi: exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecol 4(2):174–183
Linjun X, Hong P, Cungen C, Hongliang T, Yajuan Y (2005) Quantitative analysis of the Larix chinensis forest's distribution at Qinling Mountains and the character of the alpine timberline at Taibai Mountain. Acta Bot Boreali-Occrident Sin 25(5):968–972
Liu S, Guo XD, Fu BJ, Lian G, Wang J (2007) The effect of environmental variables on soil characteristics at different scales in the transition zone of the Loess Plateau in China. Soil Use Manage 23(1):92–99
Long D, Liu J, Han Q, Wang X, Huang J (2016) Ectomycorrhizal fungal communities associated with Populus simonii and Pinus tabuliformis in the hilly-gully region of the Loess Plateau, China. Sci Rep 6:24336
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Miao LY, Wang XA, Wang ZG (2005) Studies on species diversity of Larix chinensis community ecotone. Front Biol 1(2):163–167
Miyamoto Y, Nakano T, Hattori M, Nara K (2014) The mid-domain effect in ectomycorrhizal fungi: range overlap along an elevation gradient on Mount Fuji, Japan. ISME J 8(8):1739–1746
Münzenberger B, Schneider B, Nilsson RH, Bubner B, Larsson KH, Hüttl RF (2012) Morphology, anatomy, and molecular studies of the ectomycorrhiza formed axenically by the fungus Sistotrema sp. (Basidiomycota). Mycol Prog 11(3):817–826
Nara K (2006) Pioneer dwarf willow may facilitate tree succession by providing late colonizers with compatible ectomycorrhizal fungi in a primary successional volcanic desert. New Phytol 171(1):187–198
Pellissier L, Niculita-Hirzel H, Dubuis A, Pagni M, Guex N, Ndiribe C, Salamin N, Xenarios I, Goudet J, Sanders I R, Guisan A (2014) Soil fungal communities of grasslands are environmentally structured at a regional scale in the Alps. Mol Ecol 23(17):4274–4290
Reithmeier L, Kernaghan G (2013) Availability of ectomycorrhizal fungi to black spruce above the present treeline in eastern Labrador. PLoS One 8(10):e77527
Rousk J, Brookes P, Baath E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microb 75(6):1589–1596
Rozman A, Diaci J, Batič F (2013) Functional analysis of vegetation on alpine treeline ecotone in the Julian and Kamnik-Savinja alps in Slovenia. Eur J Forest Res 132(4):579–591
Scattolin L, Lancellotti E, Franceschini A, Montecchio L (2014) The ectomycorrhizal community in Mediterranean old-growth Quercus ilex forests along an altitudinal gradient. Plant Biosyst 148(1):74–82
Singh A, Rajpal K, Singh M, Kharkwal AC, Arora M, Varma A (2013) Mass cultivation of Piriformospora indica and Sebacina species. In: Varma A, Kost G, Oelmüller R (eds) Piriformospora indica: Sebacinales and their biotechnological applications. Springer, Heidelberg, pp 377–392
Smouse PE, Rod P (1999) Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity 82(5):561–573
Tang Z, Fang J (2006) Temperature variation along the northern and southern slopes of Mt. Taibai, China. Agric For Meteorol 139(3–4):200–207
Tedersoo L, Bahram M, Jairus T, Bechem E, Chinoya S, Mpumba R, Leal M, Randrianjohany E, Razafimandimbison S, Sadam AV (2011) Spatial structure and the effects of host and soil environments on communities of ectomycorrhizal fungi in wooded savannas and rain forests of continental Africa and Madagascar. Mol Ecol 20(14):3071–3080
Timling I, Dahlberg A, Walker DA, Gardes M, Charcosset JY, Welker JM (2012) Distribution and drivers of ectomycorrhizal fungal communities across the north American Arctic. Ecosphere 3:11
Tomback D, Resler L, Keane R, Pansing E, Andrade A, Wagner A (2016) Community structure, biodiversity, and ecosystem services in treeline whitebark pine communities: potential impacts from a non-native pathogen. Forests 7(1):21
Welc M, Frossard E, Egli S, Bünemann EK, Jansa J (2014) Rhizosphere fungal assemblages and soil enzymatic activities in a 110-years alpine chronosequence. Soil Biol Biochem 74:21–30
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York
Wieser G, Oberhuber W, Walder L, Spieler D, Gruber A (2010) Photosynthetic temperature adaptation of Pinus cembra within the timberline ecotone of the central Austrian alps. Ann Forest Sci 67(2):201
Zhang WH, Wang YP, Kang YX, Liu XJ (2004) Study on the relationship between Larix chinensis population’s structure and environment factors. Acta Ecol Sin 24(1):41–47
Zhang J, Taniguchi T, Tateno R, Xu M, Du S, Liu GB, Yamanaka N (2013) Ectomycorrhizal fungal communities of Quercus liaotungensis along local slopes in the temperate oak forests on the Loess Plateau, China. Ecol Res 28(2):297–305
Zhu LH, Wu XQ, Qu HY, Ji J, Ye JR (2010) Micropropagation of Pinus massoniana and mycorrhiza formation in vitro. Plant Cell Tiss Org 102(1):121–128
Acknowledgements
This research was supported by the Fund for National Science-Technology Support Project in China (2015BAD07B02), National Natural Science Foundation of China (31300525), and the Doctoral Scientific Research Foundation (Z111021309) from Northwest A&F University to Jian Huang.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Han, Q., Huang, J., Long, D. et al. Diversity and community structure of ectomycorrhizal fungi associated with Larix chinensis across the alpine treeline ecotone of Taibai Mountain. Mycorrhiza 27, 487–497 (2017). https://doi.org/10.1007/s00572-017-0766-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-017-0766-z