Abstract
Endophytic fungi are extremely diverse in natural biomes, which display a unique plant-microbe association inside different living host tissues. Among the plants that shelter endophytes, the endophytic fungal assemblages of Cactaceae remain poorly understood. Our study characterized the taxonomy, diversity, and ecology of endophytic fungal assemblages living in different tissues of the cactus Melocactus ernestii present in the Brazilian Caatinga biome. A total of 222 endophytic fungi were obtained from roots, stems, and spines, which were identified in 99 operational taxonomic units (OTUs) of Ascomycota and Basidiomycota phyla. Most of the fungal taxa were recovered from root tissues, followed by stems and spines. The most abundant orders from Ascomycota were Xylariales, Dothideomycetes, and Eurotiomycetes. Basidiomycota is represented by Cantharellales, Agaricales, and Geastrales. Only Nigrospora sp. and Preussia sp. 1 were common among the three plant tissues, and 78.41% of the species were not shared among the populations and tissues. We detected similar richness patterns among the same tissue types using sample-based rarefaction and extrapolation curves. The multiple site dissimilarity across the plants and tissues showed greater disparities in species richness among M. ernestii fungal assemblages. These results highlight the compartmentalization of endophytic fungal species in the root tissue, and the endophytes sharing observed exclusively between spines and stems may reflect interactions of endophytic fungal assemblages with possible dependency on shared resources in cacti. Overall, our findings will an approach to understand changes in the diversity and the key roles of turnover of endophytic fungal assemblages in semi-arid environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Endophytes are microorganisms that form a unique plant-microbe association that is transiently symptomless, unobtrusive, and established entirely inside living host tissues such as bark, flowers, roots, stems, leaves, and seeds (Card et al. 2016). Endophytic communities may vary spatially in different tissues or organ types (Geisen et al. 2017). In addition, endophytic microorganism frequencies are influenced by climatic conditions (Unterseher et al. 2016), plant age (Liu et al. 2017), and other abiotic and biotic environmental factors, such as plant-soil and plant-plant interactions (Shymanovich and Faeth 2019). Among the endophytes, fungi represent one of the most diverse groups studied, which include mainly Ascomycota and Basidiomycota phyla taxa and their anamorphic forms (Rosa et al. 2010); these are represented by cosmopolitan generalist taxa, as well as singlets, which are considered rare and occur as minor taxa (eg. Silva-Hughes et al. 2015).
The Caatinga is a typical dryland ecosystem in Brazil and includes areas where rainfall is <65% of evaporative demand (Delgado-Baquerizo et al. 2013), which is among the most sensitive ecosystems to climate change and land degradation (Berdugo et al. 2020). Caatinga vegetation extends across approximately 735,000 km2 of northeastern Brazil (Leal et al. 2005) and encompass the third center of diversity of the Cactaceae in East Brazil, which in turn, represents 40 different genera and 200 species, of which 80% are endemic cactus (Taylor and Zappi 2004; Goettsch et al. 2015). Cacti exhibit strong ecological specialization and are sensitive to long-term climate change (Martorell et al. 2015; Hughes et al. 2016). Despite the importance of Cactaceae as a hotspot of endophytic fungi in drylands (Hubbard et al. 2014), there are only a handful of published studies on the diversity of these ecosystems. Previous research has reported that endophytic fungi associated with plants from drylands may contribute to the protection of their hosts against drought, salinity, herbivory, climate change, diseases caused by pathogenic microorganisms and improved plant growth by fungal volatile organic compounds (Redman et al. 2002; Hubbard et al. 2014; Camarena-Pozos et al. 2021).
Endophytes associated with Cactaceae have revealed fungal diversity estimates with new taxa associated included the new orders Bezerromycetales (Bezerra et al. 2017). However, only a limited number of studies on endophytic fungi associated with Cactaceae have focused on species from different ecosystems worldwide (Fisher et al. 1994; Suryanarayanan et al. 2005; Silva-Hughes et al. 2015; Fonseca-García et al. 2016; Camarena-Pozos et al. 2021; Gargouri et al. 2021).
Fisher et al. (1994) isolated 617 endophytic fungi (23 taxa, Ascomycota) from the 600-cladode fragments of Opuntia stricta in Australia. Suryanarayanan et al. (2005) screened 1050-cladode fragments 21 cactus species occurring in various localities within Arizona (USA) and isolated 900 endophytic fungi (22 taxa, Ascomycota) while Silva-Hughes et al. (2015) examined 540- cladode fragments from Opuntia humifusa in Missouri (USA) and identified 108 endophytic fungal (17 taxa, Ascomycota and Basidiomycota) by molecular biology techniques.
Fonseca-García et al. (2016) studied the microbial community composition of the two native and sympatric cacti species Myrtillocactus geometrizans and Opuntia robusta and detected 17 fungal classes principally composed for the Ascomycota orders Pleosporales, Chaetothyriales, Capnodiales, Dothideales, and Hypocreales, and of the Basidiomycota orders Agaricales and Hymenochaetales. Camarena-Pozos et al. (2021) detected that some fungal strains associated with agaves and cacti (M. geometrizans and O. robusta) produced volatile organic compounds (VOCs) capable of improving plant growth in Arabidopsis thaliana and host plants (Agave tequilana and Agave salmiana).
Gargouri et al. (2021) identified that fungal of root endosphere and rhizosphere samples of Opuntia ficus-indica from Tunisia belonged to the phylum Ascomycota (classes Eurotiomycetes, Sordariomycetes, Dothiomycetes, Saccharomycetes, and Leotiomycetes), followed by Basidiomycota (class Agaricomycetes), Glomeromycota (genus Glomus), and uncultured taxa. These author observed that increasing aridity correlates with an increase in connectivity of the root endosphere and rhizosphere fungal communities.
In Brazil, there are only four studies of endophytic fungal assemblages associated with cacti Opuntia ficus-indica, Cereus jamacaru, and Tacinga inamoena sampled in the Caatinga biome (Bezerra et al. 2012; Bezerra et al. 2013; Freire et al. 2015; Bezerra et al. 2017); these studies displayed Cactaceae that support a rich and varied endophytic mycobiota. Bezerra et al. (2012) studied 45 cladode fragments Opuntia ficus-indica and isolated 44 endophytic fungi belonging to 13 táxons (Ascomycota). Bezerra et al. (2013) examined 1.215 cladode fragments of Cereus jamacaru and isolated 560 endophytic fungi belonging to 59 táxons distributed in 30 genus: Ascomycota (24), Basidiomycota (4), and traditional Zygomycota (2). Freire et al. (2015) studied the endophytic fungi composition of the hefht cladodes of O. ficus-indica and cladodes infested by an insect (Hemiptera), and observed major frequency of the genus in the healthy cladodes tissue belonging to nine families of seven orders. Bezerra et al. (2017) characterized by morphological and multigene phylogenetic analyses three new strains endophytic fungi associated with the cactus Tacinga inamoena from Caatinga. Those novel taxa were described two new genera Bezerromyces (B. brasiliensis and B. pernambucoensis) and Xiliomyces (X. brasiliensis), which were accommodate in a new family Bezerromycetaceae and a new order Bezerromycetales.
Among the cacti from Brazilian Caatinga, the Melocactus ernestii (Vaupel), known as the “Turk’s cap” or “melon cactus” is a lithophytic cactus endemic to eastern Brazil, and ranks as the species of the genus with the widest geographical range and environmental exclusivity to rocky outcrops (Hughes et al. 2018). Additionally, M. ernestii is used in folk medicine for the treatment of influenza, pneumonia, colic, and bowel disease (Rocha and Agra 2002; Andrade et al. 2006; Taylor and Zappi 2004). According to Hughes et al. (2018), M. ernestii is a species that takes years to reach adulthood (ca. 30 years) and is subjected to longer colonization times and more favorable habitats for fungal establishment. Therefore, understanding whether the endophytic fungi richness is compartmentalized in the different tissues (i.e., compartmental hypothesis), or whether it is characterized by one unique assemblage, is crucial for improving our understanding of the biotic factors that drive species turnover. For the reasons described above, our study aimed to characterize the taxonomy, diversity, and ecology of endophytic fungal assemblages living in different tissues of M. ernestii present in the Brazilian Caatinga biome.
2 Materials and methods
2.1 Study site and plant sampling
Caatinga is characterized by long dry seasons with 240 to 1500 mm annual rainfall and with 5 to 6 months receiving <100 mm, and the potential evapotranspiration is high with 1500 and 2000 mm per year (Pennington et al. 2009; Moro et al. 2016). Tissues from two populations of subsp. Melocactus ernestii subsp. ernestii established on rocky outcrops were collected in 2010/2011 at (i) Pedra Azul (Mee-PA), Minas Gerais state (−15.991457° Lat, −41.308297° Long; 652 m a.s.l.) and (ii) Ipirá (Mee-Ip), Bahia state (−12.184444° Lat, −39.471306° Long; 248 m a.s.l.) in Seasonally Dry Tropical Forest (Fig. 1). Live tissues from 30 adult individuals (characterized by the presence of cephalium) of each population were divided into three classes (hereafter called niches) stems, roots, and spines (Fig. 1). Mee-PA and Mee-Ip populations have, respectively, establishment on kinzigitic geiss and granitoids outcrops, mean annual precipitation levels varying between 1005 and 684 mm, mean annual temperatures of 22.4 and 23 °C, and precipitation seasonality (coefficient of variation) of 70 and 25% according to the Worldclim 2 (~1 km resolution) (Fick and Hijmans 2017). Vouchers were deposited in the Herbarium of the Instituto de Ciências Biológicas of Universidade Federal de Minas Gerais (BHCB-UFMG 141490 and 154,338).
Melocactus ernestii from two sites of Brazilian Caatinga: A Map of northwestern Brazil showing the sites (Ipirá and Pedra Azul) in which the plants were collected; B Ipirá site, Bahia State (BA); C Pedra Azul site, Minas Gerais State (MG); D Adult plants characterized by the presence of cephalium (Ipirá site); E M. ernestii in rocky outcrop; F Screened parts: stems, roots and spines
2.2 Isolation of fungal endophytes
The roots concentrated in the 0–2 cm substratum depth, stem pieces and spines located at one-quarters along stem length from the base of the plants were placed in disinfected individual plastic bags and stored for less than 24 h at 10 °C prior to the isolation of endophytic fungi. Fifteen fragments of each part of the plant were plated, totaling 45 fragments per plant and 2700 fragments in all. The 5 to 10-mm-long fragments were washed, and surface disinfected by immersion 70% ethanol (1 min), 2% sodium hypochlorite (3 min) and washed with sterile distilled water (2 min) (Silva-Hughes et al. 2015). Fragments were plating in potato dextrose agar (PDA, Himedia/India) and malt extract agar Base (MEA, Himedia/India), both media supplemented with chloramphenicol (200 μg/mL) (Sigma/USA). Plates were incubated for up to 60 days at 25 °C and the hyphal tip of each fungus growing out from the plant tissue was excised and transferred to a new PDA plate (Suppl. Fig. 1). To test the effectiveness of the surface disinfection, 100 μL of the last water rinse was plated on PDA and MEA, and incubated at 25 °C. The culture purity was assessed using colony morphology. Samples of the fungal colonies were stored in cryotubes with 20% sterile glycerol at −80 °C and maintained in glass flasks with sterile distilled water (Castellani 1967). The fungi were deposited in the Collection of Microorganisms and Cells of UFMG (UFMGCB).
2.3 Fungal identification
The protocol for DNA extraction followed Rosa et al. (2010). For the filamentous fungi, the internal transcribed spacer (ITS) region was amplified with the universal primers ITS1 and ITS4 (White et al. 1990). Amplification of the ITS region was performed as described by Rosa et al. (2010). The yeasts were grouped and identified according to protocols established by Kurtzman et al. (2011). Yeast molecular identities were confirmed by sequencing the D1-D2 variable domains of the large-subunit rRNA gene using the primers NL1 and NL4 as described by Lachance et al. (1999). Fungal isolates with query coverage and identity ≥99% were considered representing the same taxon. Representative consensus sequences of the fungal taxa were deposited into the GenBank database (Suppl.Table 1). To achieve species-rank identification based on ITS the consensus sequence was aligned with all sequences from related species retrieved from the NCBI GenBank database using BLAST (Altschul et al. 1997). Taxa that displayed query coverage and ≤ 98% identity or an inconclusive taxonomic position were subjected to phylogenetic ITS based analysis for comparison with sequences of ex type species deposited in the GenBank database. The information about fungal classification followed the databases of dictionary Kirk et al. (2008), and websites MycoBank (http://www.mycobank.org) and the Index Fungorum (http://www.indexfungorum.org).
2.4 Analysis of fungal assemblage structure
The recorded abundance of each fungal endophytic across sampling plants (i.e., 30 adult plants of each populations, divided into three tissues or partitions) was used to calculate the three integrated rarefaction/extrapolation curves, based on the first three Hill’s numbers (Hill 1973; Chao et al. 2014): q = 0, an estimation of 0Δ or species richness; q = 1, 1Δ or Shannon index; q = 2, 2Δ or inverse Simpson index. Extrapolations, with values not greater than 2 times the real individual size, were made using the estimation of three Hill’s numbers (q = 0, q = 1, q = 2; Colwell et al. 2012) to testing the sampling sufficiency in each tissue by population. We compared the estimator patterns among partitions using the Hill confidence interval as the mean of 999 replicate bootstrap runs (Chao et al. 2014; Budka et al. 2019). In this case, no overlap of 95% confidence intervals for Hill’s numbers among partitions in each population are differed significantly at p < 0.001 (Colwell et al. 2012). Estimates were obtained using the “iNEXT” R package (Hsieh et al. 2019) in R 4.0 software (R Core Team 2020).
2.5 Abundance-based multiple-site dissimilarities
We explored the differences observed among fungal endophytes communities by additive partitioning of beta diversity among tissues and populations. The presence-absence (i.e., βsor - incidence-based Sørensen index, a beta diversity metric ranges from 0 for no differentiation between assemblages, otherwise it measure to 1; Baselga 2010) and abundance matrices (i.e., β%Diff - abundance-based extension of Sørensen index or percentage difference; Baselga 2013, 2017) were employed to calculate the dissimilarities between tissues and populations. These metrics can be understood with processes driving the species composition of communities. For incidence-based, these metrics were decomposed in two distinct components of dissimilarity: (i) turnover, or replacement of species between sites; (ii) nestedness, or species pool at a site being a strict subset of the species of another richer site (Baselga 2010). For abundance-based dissimilarity, two complementary metrics were obtained: (i) βBC.BAL - balanced variation, or the abundance of a species declines from tissues and populations in the same magnitude than the abundance of other species increases from tissues and populations, and (ii) βBC.GRA - abundance gradient, or the abundance of all species equally declines or increases from one site (tissue or population) to another (Baselga 2013, 2017). Triangular plots were employed to explore similarities among endophytes assemblages present the in different tissues and populations of M. ernestii. Analyses were performed using R package ‘betapart’ in R 4.0 software (R Core Team 2020) with 10,000 permutations [R functions according to Baselga 2010, 2013, 2017].
3 Results
3.1 Taxonomic composition
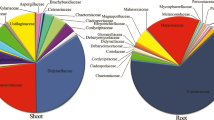
A total of 222 endophytic fungi isolates were obtained from 2700 fragments of roots, stems, and spines of M. ernestii. A total of 136 fungal isolates were recovered from M. ernestii at the Pedra Azul (Mee-PA), as well as 161 from the Ipirá (Mee-Ip) site, which represents 99 operational taxonomic units (OTUs) of Ascomycota and Basidiomycota using molecular biology methods (Suppl. Table 1). Ascomycota was represented by two subphyla, Pezizomycotina and Saccharomycotina. Pezizomycotina was represented by Sordariomycetes (39%), Dothideomycetes (30%), Eurotiomycetes (14%), and Pezizomycetes (1%). Xylariales (Sordariomycetes) was the most abundant, followed by Dothideomycetes (Pleosporales) and Eurotiomycetes (Eurotiales). Basidiomycota was represented by only the Agaricomycetes class, distributed in the following order: Cantharellales, Agaricales, and Geastrales (Fig. 2).
The majority of the fungal taxa were recovered from root tissues, followed by the stem and spine (Fig. 3 and Suppl. Table 2). In general, 78.41% of the species were not shared among the M. ernestii populations and tissues. Only Nigrospora sp. and Preussia sp. 1 were common among the three plant tissues. Preussia sp. 2 and Preussia minimoides occurred in the stem and spine, and Aspergillus calidoustus, Penicillium cf. griseofulvum, and Sphaerobolaceae sp. in the root and spine. Endophytic root and stem assemblages shared Fusarium oxysporum, Hypocreaceae sp. 1, and Phoma sp. Only the yeasts Aureobasidium pullulans and Candida parapsilosis were isolated from M. ernestii stem.
3.2 Fungal assemblage composition
Among the standardized sample size of 30 plants of M. ernestii, the root tissues exhibited the highest values of endophyte diversity: A total of 41 (Ro_BA) and 36 (Ro_MG) endophytes were detected (°Δ), the Shannon index was 29.99 ° and 26.13 ° (1Δ), and the inverse Simpson diversity index was 22.46 and 19.53% (2Δ) (Fig. 4 and Suppl. Table 2). We detected similar richness patterns among the same tissue types using sample-based rarefaction and extrapolation curve; however, the variations among the tissues was mainly due to the low number of species observed in the spines of BA (Sp_BA) and stems of BA and MG (St_BA - St_MG) locations, which did not overlap with St_MG, Ro_BA, and Ro_MG according to rarefaction.
Rarefaction curves indicated that only root tissues showed close to sufficient sampling (Fig. 4). Tissue types revealed different patterns in the rarefaction/extrapolation curves, mainly between roots and spines-stems (Fig. 4), and the confidence intervals did not overlap. In addition, the curve order was similar for every tissue type, with decreasing values of species richness, Shannon diversity, and inverse Simpson index (exception observed in Sp_BA with a small initial sample). These data represent the effects of frequent species numbers. The species richness curve for the tissues continuously increased with increasing sample size, whereas the curves representing the Shannon and inverse Simpson indices grew considerably only in the initial part.
Comparison of sample-size-based rarefaction (solid line) and extrapolation (dashed line, double the size of the reference sample) of tissues fungal endophytes diversity (Ro, roots; Sp, spines; St, stems) on two populations (BA, Bahia; MG, Minas Gerais) of Melocactus ernestii based on Hill’s numbers qΔ with q = 0 (species richness), q = 1 (Shannon index) and q = 2 (inverse Simpson index). The shaded area represents 99% confidence intervals obtained using the bootstrap method on the basis of 999 repetitions. The numbers in parentheses are the sample size and the observed Hill numbers for each reference sample
Comparing tissue types, the highest sample completeness was detected for root tissues, with an initial increase in the °Δ curve and tendency to stationarity. The other tissue types also exhibit an increase after the initial phase, with highlight to St_MG tissue; in this sense, new species are detected with additional survey plants. The extrapolation curves (dotted lines) suggest an increase in species with continued sampling. This evidence was confirmed by extrapolation of the total species number, where the prediction of complete richness in St_MG of 37 species. A total of 21 fungal taxa were expected to be detected by extensive surveying. In contrast, the observed richness of Sp_BA (eight species) requires only an extrapolation of four additional species to be sampled (Suppl. Table 2).
Intersection of root tissue curves and intersection of spines and stems curves was observed (°Δ species richness; Fig. 4), which suggests that the species richness is significantly different among these tissues. In this case, root tissues were the richest in species, and the spines and stem tissues remained the poorest in species. An intermediary pattern of richness was observed in Minas Gerais (St_MG). A similar pattern was observed for diversity metrics related to the number of frequent species (1Δ, Shannon index) and highly frequent species (2Δ, inverse Simpson index) (Fig. 4). In both cases, significant differences were observed between roots and other tissues, except for St_MG, which had an intermediary pattern. Our inferences were made at a significance level of P < 0.001.
The coverage-based sample curves for the tissue types of M. ernestii were variable (Suppl. Fig. 2). The coverage for root tissues was estimated to be ~85% for the reference sample size of 100 (Ro_MG) and 109 (Ro_BA) individuals. For any sample size less than 100, the curve shows that the sample completeness for Ro_MG is estimated to be similar to that of Ro_BA treatment, as evidenced by overlapping confidence intervals. From the sample completeness curve, when the sample size in the Ro_MG treatment was doubled from 100 to 200 individuals, the sample coverage increased from 85% to 96%. A similar coverage pattern was observed for Ro_BA. In general, the other coverage curves for spines and stems show that for any sample size of less than 37 individuals, the estimate is similar. In addition, when the sample size is doubled, the sample coverage increases from St_BA 80% to 86%, from St_MG 60% to 67%, from Sp_BA 60% to 88%, and from Sp_MG 79% to 92%.
We compared the coverage-based diversities of the tissue samples for q = 0 (left panel), q = 1 (middle panel), and q = 2 (right panel), employing our maximum coverage (i.e., 85%, Ro_MG) for the reference sample (Suppl. Fig. 3). Since the confidence envelope overlapped in the root tissues, the richness of endophytes was similar. However, root tissues were significantly higher than in the spine and stem tissues for standardized sample coverage between 10% and 85%. In addition, the richness of St_MG was significantly higher than that of any other, above 55%. For the Shannon diversity (1Δ), the root tissue is more diverse than the spines and stem tissues. The difference between root compositions was observed, but the confidence envelopes overlapped. For Shannon diversity (2Δ) when coverage is less than 60%, Sp_BA and St_MG have almost the same diversity but are more diverse than St_BA and Sp_MG.
4 Abundance-based multiple-site dissimilarities
The multiple site dissimilarity across the total plants and tissues showed higher differences in species richness of endophytic fungal assemblages of M. ernestii (βSor mean ± SD: 0.82 ± 0.08), with similar values to abundance balanced variation (β%Diff 0.85 ± 0.09). Changes in endophyte richness among the six assemblages were mostly due to species replacement through turnover, which accounted for 92.2% (i.e., 0.83 out of 0.90) of the total beta diversity at tissues/population locations. By complementarity, nestedness (species loss or gain) accounted for only 7.8% (0.07) of these changes. In accordance with the aforementioned data, differences in endophyte species were mostly due to individual substitution from tissue to tissue, which accounted for 92.1% (0.84) of total beta diversity. The abundance gradient accounted for the remaining 7.9% (0.07).
Pairwise incidence-based dissimilarities in total beta diversity among tissues ranged from 0.68 (Stem-Spines of Bahia) to 0.91 (BA - MG, Spines; mean of all pairwise comparisons = 0.82; Fig. 5). Turnover values (72.4% ± 0.09) were always higher than the nestedness values (8.3. ± 0.09). Higher turnover values were observed between pairwise spines and populations (0.875, BA and MG), followed by pairwise stems from BA and MG (0.818) and stems and roots from MG (0.813). Abundance-based pairwise dissimilarities among tissues ranged between 0.72 (BA - MG, Stems) and 0.96 (BA - MG, Spines; mean = 0.85 ± 0.09). The balance of variation explained dissimilarities in all pairwise comparisons, in contrast to abundance gradient comparisons.
Triangular plots representing the beta diversity components for pairwise comparisons among tissues and populations. Left panel, incidence-based metrics of species richness (turnover, nestedness); and right panel, abundance-based metrics (balanced variation, abundance gradient). Similarity was estimated as 1-dissimilarity. Two-letter codes identify each tissue as follows: Ro - roots; St - stems; Sp - spines; and two populations: BA = Ipirá (Mee-Ip), Bahia State; MG = Pedra Azul (Mee-PA), Minas Gerais State
5 Discussion
In this present study, we examined the composition, diversity partitioning, and compartmentalization of endophyte fungi in tissues of M. ernestii, a cacti endemic to rock outcrops in hot drylands in eastern Brazil. Most of the fungal genera obtained as endophytes of M. ernestii have been previously described as endophytes. Fewer fungi within the Basidiomycota are isolated as endophytes (Vieira et al. 2012), and only nine species are associated with cacti: Rhodotorula foliorum, R. minuta, R. mucilaginosa, R. pilati, R. sonckii, Sporobolomyces salmonicolor, Sterigmatomyces elviae, Tritirachium dependens recovered from the stem of Cereus jamacaru (Bezerra et al. 2013), Cryptococcus flavescens (Tremellomycetes) recovered from Opuntia humifusa (Silva-Hughes et al. 2015), and eight OTUs which belong to Agaricales, Cantharellales, Corticiales, Filobasidiales, and Tremellales orders from the root (endosphere and rhizosphere) of Opuntia ficus-indica (Gargouri et al. 2021).
In our study, we recovered the Agaricomycetes class, Cantharellales order (Ceratobasidium sp., Ceratobasidiaceae sp. 1, Ceratobasidiaceae sp. 2, and Ceratobasidiaceae sp. 3), Agaricales order (Agaricomycetidae sp. and Tricholomataceae sp.) isolated from roots, and Geastrales order (Sphaerobolaceae sp.) recovered from roots and spines of M. ernestii. These results were similar to previous reports, which obtained Cantharellales and Agaricales orders from roots of Opuntia robusta and O. ficus-indica (Fonseca-García et al. 2016; Gargouri et al. 2021).
Fungi from the Ceratobasidiaceae family play an important ecological role as pathogens, saprotrophs, mycorrhizal symbionts, and endophytes (Veldre et al. 2013). González and Tello (2011) detected two endophytic species of Ceratobasidium from Vitis vinifera, and Decruse et al. (2018) studied the seed germination and seedling growth promoted by a Ceratobasidiaceae sp. in Vanda thwaitesii, an endangered orchid species endemic to South Western Ghats, India, and Sri Lanka. Agaricomycetidae and Tricholomataceae mainly include saprotrophic taxa, but they can be isolated as mycorrhizal and endophytes (Schulz and Boyle 2006; Tejesvi et al. 2010). Fonseca-García et al. (2016) revealed the presence of Agaricales (mainly Henningsomyces species) in the stem endosphere community of the cacti Myrtillocactus geometrizans. Toju et al. (2013) detected endophytic fungi of the genus Cortinarius (Agaricomycetidae) associated with Quercus serrata, whereas Lana et al. (2011) isolated the endophytic Moniliophthora perniciosa (Tricholomataceae) associated with Theobroma cacao. The Sphaerobolaceae family represents mainly hummus species with few lignicolous, termites, and endophytic fungi, as the isolates from its family are associated with Bouteloua gracilis (Herrera et al. 2010).
The Ascomycota genera Alternaria, Aspergillus/Neosartorya, Aureobasidium, Candida, Chaetomium, Cladosporium, Collariella, Curvularia, Diaporthe, Cochliobolus/Curvularia, Epicoccum, Fusarium, Nigrospora, Penicillium, Pestalotiopsis, Phoma, and Xylaria were found to be associated with cacti. However, among the fungi identified as endophytes of M. ernestii, the genera Acrocalymma, Astrocystis, Bartalinia, Ceratobasidium, Colletotrichum, Daldinia, Didymella, Gelasinospora, Neurospora, Lecythophora, Microsphaeropsis, Muyocopron, Neoscytalidium, Preussia, Sclerostagonospora, Setophoma, and Thozetella were reported for the first time as endophytic of cacti. The high number of new registers can be explained by the fact that most fungal endophyte studies focus only on stem or root tissues. In this study, fungi were isolated from root (70.83%) and spine (16.6%) tissues. In addition, other studies on endophytic fungi from cacti may have underestimated species diversity by using only morphological techniques and by not identifying several sterile fungi.
Aureobasidium pullulans and Mycoleptodiscus indicus associated with M. ernestii stems were isolated from dominant species. Suryanarayanan et al. (2005) and Silva-Hughes et al. (2015) showed results similar to those of Aureobasidium pullulans as the dominant species in cacti from USA. However, the Cladosporium genus was the most abundant species in the cladodes of Opuntia stricta, O. ficus-indica, and one of the most abundant species in Cereus jamacaru (Fisher et al. 1994; Bezerra et al. 2012; Bezerra et al. 2013).
Aureobasidium pullulans, a dimorphic species, are often considered transient inhabitants of leaf and fruit surfaces, including the cactus (Ganter et al. 2017). It is one of the most widespread saprophytes and endophytic fungi. Previous studies have reported the ability of A. pullulans to promote plant growth and suppress several fungal plant pathogens, such as Botrytis cinerea, Fusarium culmorum, Penicillium expansum, Rhizopus stolonifer, and Aspergillus niger (Ippolito et al. 2000; Castoria et al. 2001; Wachowska and Głowacka 2014; Sun et al. 2019). However, A. pullulans has been reported as a causal agent of disease in grapes (Morgan and Michailides 2004) and in the stem and fruit of the cactus Pitaya (Hylocereus undatus and H. polyrhizus) in China (Liu et al. 2017).
Mycoleptodiscus indicus is a dematiaceous hyphomycete fungus found as saprotrophs leaflet decomposers of Paubrasilia echinata, which in turn, are endophytes associated with Echinacea purpurea, Borreria verticillata, and Opuntia ficus-indica; phytopathogens that can cause diseases in economically important plants such as Vanilla fragans (Bezerra and Ram 1986; Grandi and Silva 2006; Dewar and Sigler 2010; Bezerra et al. 2012; Rosa et al. 2012; Andrioli et al. 2014; Maboni et al. 2019). Preussia sp. 1 and Sphaerobolaceae sp. were the most abundant species of spines. Members of the Preussia taxa are predominantly coprophilous, but they are isolated from soil, wood, or plant debris and as endophytes (Gonzalez-Menendez et al. 2017). Preussia species were the most frequent genera in plants collected in arid areas, such as the Arizona desert and Sonoran Desert (Massimo et al. 2015; González-Menéndez et al. 2018).
Cochliobolus eragrostidis, Fusarium oxysporum, and F. solani were the dominant species in the roots of M. ernestii. The other OTUs showed low abundance and may represent rare species, included the genera Aspergillus/Neosartorya, Chaetomium and Collariella that were reported as endophytic from the root endosphere and rhizosphere associated with O. ficus-indica in aridity gradient (Gargouri et al. 2021). Species of Cochliobolus/Curvularia are commonly described as endophytes in cacti (Bezerra et al. 2012, 2013; Freire et al. 2015; Gargouri et al. 2021). Bezerra et al. (2013) reported Cochliobolus species as endophytes in the stems of Cereus jamacaru and Opuntia humifusa, and roots of O. ficus-indica cactus (Bezerra et al. 2013; Silva-Hughes et al. 2015; Gargouri et al. 2021). In addition to cacti, species of the genus Cochliobolus cause diseases in banana (Musa sp.), rice (Oryza sativa), sorghum (Sorghum bicolor), wheat (Triticum aestivum), and maize (Zea mays) (Moraes et al. 2006; Völz et al. 2020).
Members of the Fusarium genus have been isolated from plants and soils as pathogens, saprobes, and endophytes, including Cereus jamacaru, Opuntia stricta, and Opuntia ficus-indica cactus (Fisher et al. 1994; Souza et al. 2010; Bezerra et al. 2013; Bonfim et al. 2013; Freire et al. 2015; Gargouri et al. 2021). Usually, Fusarium oxysporum infects the host by the root, obstructing the vascular system and reducing the flow of water from the roots to the top of the plant, consequently producing withering (Leslie and Summerell 2006). However, F. oxysporum has been observed to be asymptomatic in three healthy tissues of E. purpurea and was able to produce extracts with antifungal activity, suggesting a protective effect on the plant (Carvalho et al. 2016).
Root-associated fungal communities are important components of ecosystem processes that affect plant growth and vigor by influencing the quality, direction, and flow of nutrients and water among plants and fungi (Trivedi et al. 2020). In our study, we also recovered the pigmented fungi Alternaria, Aureobasidium, Aspergillus, Cladosporium, Cochliobolus/Curvularia, Epicoccum, Nigrospora, and Phoma. Pigmented endophytic fungi have been associated with tolerance to UV radiation and desiccation, establish and survive in arid sites, or to confer abiotic and biotic tolerance to their plant hosts (Redman et al. 2002; Mandyam and Junpponem 2005; Sterflinger et al. 2012; Khidir et al. 2010; Ali et al. 2018). For example, melanized dark septate fungi associated with roots could facilitate the uptake of water and nutrients such as N, P, K, and Mg in arid ecosystems (Barrow and Osuna 2002; Barrow 2003; Mandyam and Junpponem 2005; Barrow et al. 2008; Vergara et al. 2018; Gargouri et al. 2021). Morsy et al. (2010) suggested that the endophytic-host relationships might be influenced by the involvement of the melanin pigment and by thermophilic proteins, as observed in cultures of Curvularia protuberata. Melocactus ernestii grows in rock outcrop microhabitats (cavities and crevices) with low substrate amounts, which can make water more ephemeral (Hughes et al. 2018). The joint effect of succulence and crassulacean acid metabolism (CAM) (Lüttge 2004), in association with dark septate fungi, can promote the water storage and water-use efficiency of M. ernestii (Fonseca-García et al. 2016).
We have shown that the species richness of endophytes in M. ernestii varies across different tissue types. Based on a double sample size, the roots showed the highest species richness (°Δ) among the two populations, followed by stems and spines, which were poor in species richness. The same pattern was observed for all diversity metrics (species richness, Shannon (1Δ), and inverse Simpson (2Δ) indices). The multiple comparative approaches employed here (Chao et al. 2014) demonstrated the relationship between sample size and sample completeness in host tissues. More sampling effort is necessary to achieve a stationary level (or completeness) of species richness in spines (>7%) and stems (≈25%). The sterilization protocols and growth conditions, standardized and applied to all samples (minimizing biases in comparative analyses communities), can influence the discrepancy observed in the richness capture in stem and spine tissues. In this case, the rarefaction and extrapolation approach enabled the precise comparison of endophytic diversity in different tissues and improved the precision of diversity estimates (Chao et al. 2014).
The diversity patterns among tissue types in M. ernestii support the hypothesis of compartmentalization into modules (ecological networks), that is, the biotic factors represent the main driver of selection (Toju et al. 2014; Coleman-Derr et al. 2016; Fonseca-García et al. 2016; Trivedi et al. 2020). The difference in the number of isolates from the stem, spine, and root assemblages might be due to variations in the characteristics of each plant tissue sampled, such as the thick and waxy cuticle and the low frequency of stomata in cacti; these adaptations to reduce evaporative water loss may represent additional barriers that inhibit infection by parasitic fungi, as well as the colonization of endophyte species, resulting in less diversity when compared to other plant species (Zimmermann and Granata 2002; Suryanarayanan et al. 2005; Luz et al. 2006; Silva et al. 2006; Fonseca-García et al. 2016).
In addition, there are other characteristics of plant tissue, such as biomass and secondary substances, which may justify the low colonization of stems and spines in relation to the roots (Mauseth 2004; Shade et al. 2017). Finally, below-ground conditions (low UV radiation and atmospheric aridity) are co-determinants of the differences observed among tissues (roots, stems, leaves, and spines; Arnold and Lutzoni 2007; U'Ren et al. 2012).
The endophyte sharing observed exclusively between spines and stems may reflect interactions of endophytic fungal species and/or communication between the two tissues in M. ernestii. According to Schill and Barthlott (1973), chemiluminescence experiments suggest that spines of Discocactus horstii can act as capillaries and provide an increase in water absorption by the plant. Discocactus and Melocactus are the closest in a strongly supported clade (Ritz et al. 2007). In this case, it is not surprising to suppose that the globose genera Melocactus and Discocactus, the closest relatives to each other, present common mechanisms of water supplementation (Schill and Barthlott 1973; Mauseth 2006) and influence the sharing and interactions of endophytic fungal species. In additional, Gargouri et al. (2021) suggested that the interactions among ecological networks could mean that microbes have a higher dependency on shared resources in arid sites.
6 Conclusion
We show that fungal endophytic assemblages across tissues differ with respect to species richness and abundance. The beta diversity among tissues was mainly attributed to the replacement of endophytic species or individuals rather than nestedness. In this context, we suggest that species replacement among tissue compartments is driven by biotic factors. In comparison to complementarities, nestedness and abundance gradient contributed relatively little towards the differences among tissues, suggesting interactions of endophytic fungal species with possible sharing of resources in tissues. Our results highlight the key roles of turnover of endophytic fungal assemblages and summarize the increasing body of evidence. Overall, this work could contribute to estimates of fungal diversity in small scale; to describe novel taxa, which indicates that fungal phylogenetic diversity and function in semi-arid environments.
References
Ali AH, Radwan U, El-Zayat S, El-Sayed MA (2018) Desert plant-fungal endophytic association: the beneficial aspects to their hosts. BFIJ 10:138–145
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. https://doi.org/10.1093/nar/25.17.3389
Andrade CTS, Marques JGW, Zappi DC (2006) Utilização medicinal de cactáceas por sertanejos baianos. Rev Bras Pl Med 8:36–42
Andrioli WJ, Conti R, Araújo MJ, Zanasi R, Cavalcanti BC, Manfrim V, Toledo JS, Tedesco D, Moraes MO, Pessoa C, Cruz AK, Bertucci C, Sabino J, Nanayakkara DNP, Pupo MT, Bastos JK (2014) Mycoleptones A−C and polyketides from the endophyte Mycoleptodiscus indicus. J Nat Prod 77:70–78. https://doi.org/10.1021/np4006822
Arnold AE, Lutzoni F (2007) Diversity and host range of foliar fungal endophytes: are tropical trees biodiversity hot spots? Ecology 88:541–549. https://doi.org/10.1890/05-1459
Barrow JR (2003) Atypical morphology of dark septate fungal root endophytes of Bouteloua in arid southwestern USA rangelands. Mycorrhiza 13:239–247. https://doi.org/10.1007/s00572-003-0222-0
Barrow JR, Osuna P (2002) Phosphorus solubilization and uptake by dark septate fungi in fourwing saltbush, Atriplex canescens (Pursh) Nutt. J Arid Environ 51:449–459. https://doi.org/10.1006/jare.2001.0925
Barrow JR, Lucero ME, Reyes-Vera I, Havstad KM (2008) Do symbiotic microbes have a role in regulating plant performance and response to stress? Commun Integr Biol 1:69–73. https://doi.org/10.4161/cib.1.1.6238
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19:134–143. https://doi.org/10.1111/j.1466-8238.2009.00490.x
Baselga A (2013) Separating the two components of abundance-based dissimilarity: balanced changes in abundance vs. abundance gradients. Methods Ecol Evol 4:552–557. https://doi.org/10.1111/2041-210X.12029
Baselga A (2017) Partitioning abundance-based multiple-site dissimilarity into components: balanced variation in abundance and abundance gradients. Methods Ecol Evol 8:799–808. https://doi.org/10.1111/2041-210X.12693
Berdugo M, Delgado-Baquerizo M, Soliveres S, Hernández-Clemente R, Zhao Y, Gaitán JJ, Gross N, Saiz H, Maire V, Lehman A, Rillig MC, Solé RV, Maestre FT (2020) Global ecosystem thresholds driven by aridity. Science 367:787–790. https://doi.org/10.1126/science.aay5958
Bezerra JL, Ram AA (1986) Crosta negra da baunilha (Vanilla fragans) causada por Mycoleptodiscus indicus (Moniliales, Hiphomycetes). Fitopatol Bras 11(717):724
Bezerra JD, Santos MG, Svedese VM, Lima DM, Fernandes MJ, Paiva LM, Souza-Motta CM (2012) Richness of endophytic fungi isolated from Opuntia ficus-indica Mill. (Cactaceae) and preliminary screening for enzyme production. World J Microbiol Biotechnol 28:1989–1995. https://doi.org/10.1007/s11274-011-1001-2
Bezerra JDP, Santos MGS, Barbosa RN, Svedese VM, Lima DMM, Fernandes MJS, Gomes BS, Paiva LM, Almeida-Cortez JS, Souza-Motta CM (2013) Fungal endophytes from cactus Cereus jamacaru in Brazilian tropical dry forest: a first study. Symbiosis 60:53–63. https://doi.org/10.1007/s13199-013-0243-1
Bezerra JDP, Oliveira RJV, Paiva LM, Silva GA, Groenewald JZ, Crous PW, Souza-Motta CM (2017) Bezerromycetales and Wiesneriomycetales ord. nov. (class Dothideomycetes), with two novel genera to accommodate endophytic fungi from Brazilian cactus. Mycol Prog 16:297–309. https://doi.org/10.1007/s11557-016-1254-0
Bonfim A, Albuquerque GMR, Bezerra JDP, Silva DCV, Svedese VM, Paiva LM, Souza-Motta CM (2013) Fungos fitopatogênicos de Opuntia ficus-indica (L.) Mill. cultivada em área de floresta tropical seca no Brasil. Bol Soc Latin Carib Cact Suc 10:27–33
Budka A, Łacka A, Szoszkiewicz K (2019) The use of rarefaction and extrapolation as methods of estimating the effects of river eutrophication on macrophyte diversity. Biodivers Conserv 28:385–400. https://doi.org/10.1007/s10531-018-1662-3
Camarena-Pozos DA, Flores-Núñez VM, Lopez MG, Partida-Martínez LP (2021) Fungal volatiles emitted by members of the microbiome of desert plants are diverse and capable of promoting plant growth. Environ Microbiol 23:2215–2229. https://doi.org/10.1111/1462-2920.15395
Card S, Johnson L, Teasdale S, Caradus J (2016) Deciphering endophyte behaviour: the link between endophyte biology and efficacious biological control agents. FEMS Microbiol Ecol 92(8). https://doi.org/10.1093/femsec/fiw114
Carvalho CR, Wedge DE, Cantrell CL, Silva-Hughes AF, Pan Z, Moraes RM, Madoxx VL, Rosa LH (2016) Molecular phylogeny, diversity, and bioprospecting of endophytic fungi associated with wild ethnomedicinal north American plant Echinacea purpurea (Asteraceae). Chem Biodivers 13:918–930. https://doi.org/10.1002/cbdv.201500299
Castellani A (1967) A maintenance and cultivation of the common pathogenic fungi of man in sterile distilled water: further researches. J trop Med Hyg 70:181–184
Castoria R, de Curtis F, Lima G, Caputo L, Pacifico S, de Cicco V (2001) Aureobasidium pullulans (LS-30) an antagonist of postharvest pathogens of fruits: study on its modes of action. Postharvest Biol Technol 22:7–17. https://doi.org/10.1016/S0925-5214(00)00186-1
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67
Coleman-Derr D, Desgarennes D, Fonseca-Garcia C, Gross S, Clingenpeel S, Woyke T, North G, Visel A, Partida-Martinez LP, Tringe SG (2016) Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytol 209:798–811. https://doi.org/10.1111/nph.13697
Colwell RK, Chao A, Gotelli NJ, Lin SY, Mao CX, Chazdon RL, Longino JT (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol 5:3–21. https://doi.org/10.1093/jpe/rtr044
Decruse SW, Neethu RS, Pradeep NS (2018) Seed germination and seedling growth promoted by a Ceratobasidiaceae clone in Vanda thwaitesii Hook. f., an endangered orchid species endemic to South Western Ghats, India and Sri Lanka. S Afr J Bot 116:222–229. https://doi.org/10.1016/j.sajb.2018.04.002
Delgado-Baquerizo M, Maestre F, Gallardo A et al (2013) Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 502:672–676. https://doi.org/10.1038/nature12670
Dewar CL, Sigler L (2010) Fungal arthritis of the knee caused by Mycoleptodiscus indicus. Clin Rheumatol 29:1061–1065. https://doi.org/10.1007/s10067-010-1448-9
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. https://doi.org/10.1002/joc.5086
Fisher PJ, Sutton BC, Petrini LE, Petrini O (1994) Fungal endophytes from Opuntia stricta: a first report. Nova Hedwigia 59:195–200
Fonseca-García C, Coleman-Derr D, Garrido E, Visel A, Tringe SG, Partida-Martínez LP (2016) The cacti microbiome: interplay between habitat-filtering and host-specificity. Front Microbiol 12:150. https://doi.org/10.3389/fmicb.2016.00150
Freire KTLS, Araújo GR, Bezerra JDP et al (2015) Fungos endofíticos de Opuntia ficus-indica (L.) Mill. (Cactaceae) sadia e infestada por Dactylopius opuntiae (Cockerell, 1896) (Hemiptera: Dactylopiidae). Revista Gaia Scientia 9:104–110
Ganter PF, Morais PB, Rosa CA (2017) Yeasts in cacti and tropical fruit. In: Buzzini P, Lachance MA, Yurkov A (eds) Yeasts in natural ecosystems: diversity. Springer, Cham. https://doi.org/10.1007/978-3-319-62683-3_8
Gargouri M, Karray F, Chebaane A, Mhiri N, Partida-Martínez LP, Sayadi S, Mliki A (2021) Increasing aridity shapes beta diversity and the network dynamics of the belowground fungal microbiome associated with Opuntia ficus-indica. Sci Total Environ 773:145008. https://doi.org/10.1016/j.scitotenv.2021.145008
Geisen S, Kostenko O, Cnossen MC, Ten Hooven FC, Vreš B, van der Putten WH (2017) Seed and root endophytic fungi in a range expanding and a related plant species. Front Microbiol 8:1645. https://doi.org/10.3389/fmicb.2017.01645
Goettsch B, Hilton-Taylor C, Cruz-Piñón G, Duffy JP, Frances A, Hernández HM, Inger R, Pollock C, Schipper J, Superina M, Taylor NP, Tognelli M, Abba AM, Arias S, Arreola-Nava HJ, Baker MA, Bárcenas RT, Barrios D, Braun P, Butterworth CA, Búrquez A, Caceres F, Chazaro-Basañez M, Corral-Díaz R, del Valle Perea M, Demaio PH, Duarte de Barros WA, Durán R, Yancas LF, Felger RS, Fitz-Maurice B, Fitz-Maurice WA, Gann G, Gómez-Hinostrosa C, Gonzales-Torres LR, Patrick Griffith M, Guerrero PC, Hammel B, Heil KD, Hernández-Oria JG, Hoffmann M, Ishihara MI, Kiesling R, Larocca J, León-de la Luz JL, Loaiza S. CR, Lowry M, Machado MC, Majure LC, Ávalos JGM, Martorell C, Maschinski J, Méndez E, Mittermeier RA, Nassar JM, Negrón-Ortiz V, Oakley LJ, Ortega-Baes P, Ferreira ABP, Pinkava DJ, Porter JM, Puente-Martinez R, Gamarra JR, Pérez PS, Martínez ES, Smith M, Manuel Sotomayor M. del C. J, Stuart SN, Muñoz JLT, Terrazas T, Terry M, Trevisson M, Valverde T, van Devender TR, Véliz-Pérez ME, Walter HE, Wyatt SA, Zappi D, Alejandro Zavala-Hurtado J, Gaston KJ (2015) High proportion of cactus species threatened with extinction. Nat Plants 5:15142. https://doi.org/10.1038/nplants.2015.142
González V, Tello ML (2011) The endophytic mycota associated with Vitis vinifera in central Spain. Fungal Divers 47:29–42. https://doi.org/10.1007/s13225-010-0073-x
Gonzalez-Menendez V, Martin J, Siles JA, Gonzalez-Tejero MR, Reyes F, Platas G, Tormo JR, Genilloud O (2017) Biodiversity and chemotaxonomy of Preussia isolates from the Iberian Peninsula. Mycol Prog 16(7):713–728. https://doi.org/10.1007/s11557-017-1305-1
González-Menéndez V, Crespo G, DE Pedro N et al (2018) Fungal endophytes from arid areas of Andalusia: high potential sources for antifungal and antitumoral agents. Sci Rep 8:9729. https://doi.org/10.1038/s41598-018-28192-5
Grandi RAP, Silva TV (2006) Fungos Anamorfos decompositores do folhedo de Caesalpinia echinata Lam. Rev Bras Bot 29:275–287. https://doi.org/10.1590/S0100-84042006000200009
Herrera J, Khidir HH, Eudy DM, Porras-Alfaro A, Natvig DO, Sinsabaugh RL (2010) Shifting fungal endophyte communities colonize Bouteloua gracilis: effect of host tissue and geographical distribution. Mycologia 102(5):1012–1026. https://doi.org/10.3852/09-264
Hill M (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432
Hsieh TC, Ma KH, Chao A (2019) ‘iNEXT’: iNterpolation and EXTrapolation for species diversity. R package version 2.0.20. https://CRAN.R-project.org/package=iNEXT. Accessed 10 Dec 2020 https://doi.org/10.1890/13-0133.1
Hubbard M, Germida JJ, Vujanovic V (2014) Fungal endophytes enhance wheat heat and drought tolerance in terms of grain yield and second-generation seed viability. J Appl Microbiol 116:109–122. https://doi.org/10.1111/jam.12311
Hughes FM, Jacobi CM, Borba EL (2016) Fate of cohorts in Melocactus (Cactaceae) species is affected by rainfall uncertainty and microrelief structures. Braz J Bot 39:197–205. https://doi.org/10.1007/s40415-014-0116-8
Hughes FM, Figueira JEC, Jacobi CM, Borba EL (2018) Demographic processes and anthropogenic threats of lithophytic cacti in eastern Brazil. Braz J Bot 41:631–640. https://doi.org/10.1007/s40415-018-0483-7
Ippolito A, EL Ghaouth A, Wilson CL, Wisniewski M (2000) Control of postharvest decay of apple fruit by Aureobasidium pullulans and induction of defense responses. Postharvest Biol Technol 19:265–272. https://doi.org/10.1016/S0925-5214(00)00104-6
Khidir HH, Eudy DM, Porras-Alfaro A, Herrera J, Do N, Sinsabaugh RL (2010) A general suite of fungal endophytes dominate the roots of two dominant grasses in a semiarid grassland. J Arid Environ 74:35–42. https://doi.org/10.1016/j.jaridenv.2009.07.014
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Dictionary of the Fungi. CAB International, Wallingford
Kurtzman C, Fell JW, Boekhout T (2011) The yeasts: a taxonomic study. Elsevier
Lachance MA, Bowles JM, Starmer T, Barker JS (1999) Kodamaea kakaduensis and Candida tolerans, two new ascomycetous yeast species from Australian Hibiscus flowers. Can J Microbiol 45:172–177. https://doi.org/10.1139/w98-225
Lana TG, Azevedo JL, Pomella AW, Monteiro RT, Silva CB, Araújo WL (2011) Endophytic and pathogenic isolates of the cacao fungal pathogen Moniliophthora perniciosa (Tricholomataceae) are indistinguishable based on genetic and physiological analysis. Genet Mol Res 10:326–334. https://doi.org/10.4238/vol10-1gmr895
Leal IR, Silva JMC, Tabarelli M, Lacher TE Jr (2005) Changing the course of biodiversity conservation in the caatinga of northeastern Brazil. Conserv Biol 19:701–706. https://doi.org/10.1111/j.1523-1739.2005.00703.x
Leslie J, Summerell BA (2006) The Fusarium laboratory manual. Blackwell, Iowa
Liu QY, Liu MH, Li T, Chen JH, Zhang FG, Hu J (2017) First report of summer patch of Kentucky bluegrass caused by Magnaporthe poae in China. Plant Dis 101(1):250–250
Lüttge U (2004) Ecophysiology of Crassulacean acid metabolism (CAM). Ann Bot 93:629–652. https://doi.org/10.1093/aob/mch087
Luz JS, de Oliveira Silva RL, da Silveira EB, Cavalcante UMT (2006) Atividade enzimática de fungos endofíticos e efeito na promoção do crescimento de mudas de maracujazeiro-amarelo. Rev Caatinga 19:128–134
Maboni G, Krimer P, Baptista R, Lorton A, Anderson C, Sanchez S (2019) Laboratory diagnostics, phylogenetic analysis and clinical outcome of a subcutaneous Mycoleptodiscus indicus infection in an immunocompetent cat. BMC Vet Res 15:354. https://doi.org/10.1186/s12917-019-2132-1
Mandyam K, Junpponem A (2005) Seeking the elusive function of the root-colonising dark sepatele endophyte. Stud Micol 53:173–189. https://doi.org/10.3114/sim.53.1.173
Martorell C, Montañana DM, Ureta C, Mandujano MC (2015) Assessing the importance of multiple threats to an endangered globose cactus in Mexico: cattle grazing, looting and climate change. Biol Conserv 181:73–81. https://doi.org/10.1016/j.biocon.2014.10.035
Massimo NC, Devan MN, Arendt KR, Wilch MH, Riddle JM, Furr SH, Steen C, U'Ren JM, Sandberg DC, Arnold AE (2015) Fungal endophytes in aboveground tissues of desert plants: infrequent in culture, but highly diverse and distinctive symbionts. Microb Ecol 70(1):61–76. https://doi.org/10.1007/s00248-014-0563-6
Mauseth JD (2004) The structure of photosynthetic succulent stems in plants other than cacti. Int J Plant Sci 165:1–9. https://doi.org/10.1086/380978
Mauseth JD (2006) Structure–function relationships in highly modified shoots of Cactaceae. Ann Bot 98:901–926. https://doi.org/10.1093/aob/mcl133
Moraes WS, Zambolim L, Lima JD (2006) Incidence of mushroons in post harvest of banana (Musa spp.) Prata Anã (AAB). Summa Phytopathol 32:67–70
Morgan DP, Michailides TJ (2004) First report of melting decay of “red globe” grapes in California. Plant Dis 88:1047
Moro MF, Nic Lughadha E, de Araújo FS, Martins FR (2016) A phytogeographical metaanalysis of the semiarid caatinga domain in Brazil. Bot Rev 82:91–148. https://doi.org/10.1007/s12229-016-9164-z
Morsy MR, Oswald J, He J, Tang Y, Roossinck MJ (2010) Teasing apart a three-way symbiosis: transcriptome analyses of Curvularia protuberata in response to viral infection and heat stress. Biochem Biophys Res Commun 401:225–230. https://doi.org/10.1016/j.bbrc.2010.09.034
Pennington RT, Lavin M, Oliveira-Filho A (2009) Woody plant diversity, evolution, and ecology in the tropics: perspectives from seasonally dry tropical forests. Annu Rev Ecol Evol Syst 40:37–57. https://doi.org/10.1146/annurev.ecolsys.110308.120327
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at https://www.R-project.org/
Redman RS, Sheehan KB, Stout TG, Rodriguez RJ, Henson JM (2002) Thermotolerance generated by plant/fungal symbiosis. Science 298(5598):1581. https://doi.org/10.1126/science.1072191
Ritz CM, Martins L, Mecklenburg R, Goremykin V, Hellwig FH (2007) The molecular phylogeny of Rebutia (Cactaceae) and its allies demonstrates the influence of paleogeography on the evolution of south American mountain cacti. Am J Bot 94:1321–1332. https://doi.org/10.3732/ajb.94.8.1321
Rocha EA, Agra MF (2002) Flora do Pico do Jabre, Paraíba, Brasil: Cactaceae Juss. Acta Bot Bras 16:15–21. https://doi.org/10.1590/S0102-33062002000100004
Rosa LH, Gonçalves VN, Caligiorne RB, Almeida Alves TM, Rabelo ALT, Sales PA, Romanha AJ, Sobral MEG, Rosa CA, Zani CL (2010) Leishmanicidal, trypanocidal, and cytotóxic activities of endophytic fungi associated with bioactive plants in Brazil. Braz J Microbiol 41:1–11. https://doi.org/10.1590/S1517-83822010000200024
Rosa LH, Tabanca N, Techen N, Wedge DE, Pan Z, Bernier UR, Becnel JJ, Agramonte NM, Walker LA, Moraes RM (2012) Diversity and biological activities of endophytic fungi associated with micropropagated medicinal plant Echinacea purpurea (L.) Moench. Am. J. Plant Sci 3:1105–1114. https://doi.org/10.4236/ajps.2012.38133
Schill R, Barthlott W (1973) Kakteendornen als wasserabsorbierende organe. Die Naturwissenschaften 60:202–203. https://doi.org/10.1007/BF00599438
Schulz BJE, Boyle CJC (2006) What are endophytes? In: Schulz BJE, Boyle CJC, Sieber TN (eds) Microbial root endophytes. Springer-Verlag, Berlin, pp 1–13
Shade A, Jacques MA, Barret M (2017) Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr Opin Microbiol 37:15–22. https://doi.org/10.1016/j.mib.2017.03.010
Shymanovich T, Faeth SH (2019) Environmental factors affect the distribution of two Epichloë fungal endophyte species inhabiting a common host grove bluegrass (Poa alsodes). Ecol Evol 9(11):6624–6642. https://doi.org/10.1002/ece3.5241
Silva RLDO, Luz JS, Silveira EBD, Cavalcante UMT (2006) Fungos endofíticos em Annona spp.: isolamento, caracterização enzimática e promoção do crescimento em mudas de pinha (Annona squamosa L.). Acta Bot Bras 20:649–655. https://doi.org/10.1590/S0102-33062006000300015
Silva-Hughes AF, Wedge DE, Cantrell CL, Carvalho CR, Pan Z, Moraes RM, Madoxx ML, Rosa LH (2015) Diversity and antifungal activity of the endophytic fungi associated with the native medicinal cactus Opuntia humifusa (Cactaceae) from the United States. Microbiol Res 175:67–77. https://doi.org/10.1016/j.micres.2015.03.007
Souza AEF, Nascimento LC, Araújo E, Lopes EB, Souto FM (2010) Occurrence and identification of the etiologic agents of plant diseases in cactus (Opuntia ficus-indica Mill.) in the semi-arid region of Paraiba. Biotemas 23:11–20. https://doi.org/10.5007/2175-7925.2010v23n3p11
Sterflinger K, Tesei D, Zakharova K (2012) Fungi in hot and cold deserts with particular reference to microcolonial fungi. Fungal Ecol 5:453–462. https://doi.org/10.1016/j.funeco.2011.12.007
Sun PF, Chien IA, Xiao HS, Fang WT, Hsu CH, Chou JY (2019) Intra specific variation in plant growth-promoting traits of Aureobasidium pullulans. Chiang Mai J Sci 46(1):15–31
Suryanarayanan T, Wittlinger SK, Faeth SH (2005) Endophytic fungi associated with cacti in Arizona. Mycol Res 109(5):635–639. https://doi.org/10.1017/s0953756205002753
Taylor NP, Zappi DC (2004) Cacti of eastern Brazil. Royal Botanic Gardens, Kew
Tejesvi MV, Ruotsalainen AL, Markkola AM, Pirttilä AM (2010) Root endophytes along a primary succession gradient in northern Finland. Fungal Divers 41(1):125–134. https://doi.org/10.1007/s13225-009-0016-6
Toju H, Yamamoto S, Sato H, Tanabe AS, Gilbert GS, Kadowaki K (2013) Community composition of root-associated fungi in a Quercus-dominated temperate forest: "codominance" of mycorrhizal and root-endophytic fungi. Ecol Evol 3:1281–1293. https://doi.org/10.1002/ece3.546
Toju H, Guimarães P, Olesen J, Thompson J (2014) Assembly of complex plant–fungus networks. Nat Commun 5:5273. https://doi.org/10.1038/ncomms6273
Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK (2020) Plant-microbiome interactions: from community assembly to plant health. Nat Rev Microbiol 18(11):607–621. https://doi.org/10.1038/s41579-020-0412-1
Unterseher M, Siddique AB, Brachmann A, Peršoh D (2016) Diversity and composition of the leaf mycobiome of beech (fagus sylvatica) are affected by local habitat conditions and leaf biochemistry. PloS one 11(4):e0152878. https://doi.org/10.1371/journal.pone.0152878
U'Ren JM, Lutzoni F, Miadlikowska J, Laetsch AD, Arnold AE (2012) Host- and geographic structure of endophytic and endolichenic fungi at a continental scale. Am J Bot 99:898–9143. https://doi.org/10.3732/ajb.1100459
Veldre V, Abarenkov K, Bahram M, Martos F, Selosse MA, Tamm H, Koljalg U, Tedersoo L (2013) Evolution of nutritional modes of Ceratobasidiaceae (Cantharellales, Basidiomycota) as revealed from publicly available ITS sequences. Fungal Ecol 6(4):256–268. https://doi.org/10.1016/j.funeco.2013.03.004
Vergara C, Araujo KEC, Alves LS, Souza SR, Santos LA, Santa-Catarina C, Silva K, Pereira GMD, Xavier GR, Zilli JE (2018) Contribution of dark septate fungi to the nutrient uptake and growth of rice plants. Braz J Microbiol 49:67–78. https://doi.org/10.1016/j.bjm.2017.04.010
Vieira ML, Hughes AF, Gil VB, Vaz AB, Alves TM, Zani CL, Rosa CA, Rosa LH (2012) Diversity and antimicrobial activities of the fungal endophyte community associated with the traditional Brazilian medicinal plant Solanum cernuum Vell. (Solanaceae). Can J Microbiol 58:54–66. https://doi.org/10.1139/w11-105
Völz R, Park JY, Kim S, Park SY, Harris W, Chung H, Lee YH (2020) The rice/maize pathogen Cochliobolus spp. infect and reproduce on Arabidopsis revealing differences in defensive phytohormone function between monocots and dicots. Plant J 103:412–429. https://doi.org/10.1111/tpj.14743
Wachowska U, Głowacka K (2014) Antagonistic interactions between Aureobasidium pullulans and Fusarium culmorum, a fungal pathogen of winter wheat. BioControl 59:635–645. https://doi.org/10.1007/s10526-014-9596-5
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Zimmermann HG, Granata G (2002) Insect pests and diseases. In: NOBEL PS (ed) Cacti: biology and uses. University of California Press, Berkeley, pp 235–254
Acknowledgements
Alice Ferreira-Silva was financially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (grant:141684/2010-0). Frederic M. Hughes acknowledges CNPq grant 302381/2020-1. The study was supported by FAPEMIG, CAPES and CNPq.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferreira-Silva, A., Hughes, F.M., Rosa, C.A. et al. Higher turnover of endophytic fungal assemblages in the tissues of globose cactus Melocactus ernestii from Brazilian semi-arid biome. Symbiosis 85, 79–91 (2021). https://doi.org/10.1007/s13199-021-00795-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-021-00795-z