Abstract
Salinity seriously disrupts the growth and physiology of plants, whereas phytohormones play an important role in regulating plant responses to salinity stress. Biochar is attracting increasing attention in recent years as a potential soil amendment under stress condition. This study addressed the use of biochar to mitigate salt-stressed soil and evaluated the levels of some phytohormones in bean (Phaseolus vulgaris L.) seedlings. A pot experiment was conducted in a climate-controlled greenhouse with three biochar ratios (control, 10, and 20% mass), three salt stress treatments (non-saline, 6, 12 dS m−1 NaCl), and four replications. The results indicated that sodium (Na) concentration, polyamine oxidase (PAO) activity, the contents of polyamines (putrescine, spermidine, and spermine), abscisic acid (ABA), 1-aminocyclopropane-1-carboxylic acid (ACC), jasmonic acid (JA), and salicylic acid (SA) in bean leaf and root increased under salt stress. However, endogenous indole-3-acetic acid (IAA) content was decreased by salinity compared to the non-saline treatment. On the other hand, we observed decreases of Na concentration, PAO activity, polyamines, ABA, ACC, and JA contents in plants treated by biochar. In contrast, biochar enhanced IAA content and the growth of roots and shoots. As a result, the effectiveness of 20% biochar was superior to the 10% treatment in terms of polyamine contents, especially under saline conditions. Interestingly, there were no considerable changes in phytohormone contents by use of biochar under non-saline conditions. Overall, biochar alleviated the negative effects of salt stress on bean seedlings by reduction of Na concentration, endogenous stress hormones, and improvement of growth hormones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salt stress is the one of the most important environmental problems in arid and semi-arid regions where poor groundwater quality and agricultural practices gradually allow the buildup of salts in the soil, posing serious limitations to plant growth and productivity (Hasanuzzaman and others 2013; Xu and others 2016). The response of plants to the adverse effects of salinity is regulated by different external and internal factors. Among the internal plant factors, some phytohormones have important roles in salt stress tolerance and adaptation (Cao and others 2007). It has been proposed that abscisic acid (ABA) acts as a mediator and the major internal signal in the plant response to abiotic stresses (Javid and others 2011). ABA content raised correspondingly with salt stress related to leaf water potential, suggesting that increased ABA content is due to water deficit produced by salts rather than a salt-specific effect (Ghanem and others 2008). Out of all the plant hormones that indicate environmental stress, special attention should be given to ethylene, which is known to be a stress hormone involved in various physiological and molecular plant reactions. Ethylene also regulates several growth and cellular defense mechanisms in response to stress (Van de Poel and others 2015). This implies a synergistic effect between the biosynthesis of reactive oxygen species (ROS) and ethylene (Djanaguiraman and others 2009).
Moreover, the production of ethylene in plants is directly related to endogenous ACC (1-aminocyclopropane-1-carboxylic acid) levels (Shaharoona and others 2006). The phytohormone indole-3-acetic acid (IAA) may also be involved in stress signaling and defense responses (George and others 2010). Recent research has shown that IAA makes some morphogenic responses (Monteiro and others 2012), which may alleviate adverse effects of environmental stresses (Tognetti and others 2011). Polyamines (PA) are naturally active compounds involved in different physiological processes, and it has been suggested that changes in polyamine metabolism under salt stress may be a part of an integrated plant defense mechanism (Flores 1991). The most abundant PAs in plants are putrescine (Put), spermidine (Spd), and spermine (Spm). Because polyamine levels increase significantly upon exposure to saline stress, it has been suggested that this component could be a defensive mechanism of plants against saline stress, thus conferring tolerance against stress (Jimenez-Bremont and Ruiz 2007). PA oxidase (PAO) is one of the main enzymes that adjust the PA metabolic pathway in different model plant systems (Groppa and Benavides 2008).
On the other hand, salicylic acid (SA) is a natural signaling molecule activated by the plant’s defense mechanism in response to environmental stresses such as salinity (Malamy and others 1990). SA mediates the oxidative burst that may lead to cell death in the hypersensitive response and acts as a signal for the development of systemic acquired resistance. In recent years, it has been shown that the endogenous content of SA increased significantly under environmental stresses (Shim and others 2003). Jasmonates are ubiquitously occurring lipid-derived compounds with signal function in plant responses to abiotic and biotic stresses, as well as in plant growth and development (Wasternack 2007). This growth regulator induces a wide-range of physiological and biochemical responses in plants (Kovac and others 2003). In addition, Moons and others (1997) indicated that jasmonic acid (JA) antagonistically adjusts the expression of salt stress-inducible proteins, associated with salt stress in rice. Various tactics such as increasing soil carbon content have been developed to decrease the adverse effects of salinity and other environmental stresses in plants (Lashari and others 2015; Ali and others 2017; Farhangi-Abriz and Torabian 2017; Ramzani and others 2017).
Biochar is made from organic materials through thermal degradation under anoxia conditions. It has been suggested as a soil conditioner to enhance plant growth by supplying and, more importantly, retaining nutrients and by improving soil physical and biological properties (Downie and others 2009). Organic materials such as biochar with pH adjusting and cation exchange capacity of soil, improved the growth of plants under salt and cadmium toxicities (Abbas and others 2017). Moreover, addition of biochar to the soil increased potassium availability in growth medium (Lashari and others 2013). This increment of cation exchange capacity of soil is related to the improvement of soil carbon content and pH, because these factors have a positive correlation with the cation exchange capacity of soil (Lehmann and Joseph 2015). Biochar not only improves crop productivity under normal conditions but also improves crop yield under adverse conditions such as salinity and drought. Application of biochar can decrease adverse effects of salt stress for plant growth (Lashari and others 2013, 2015; Akhtar and others 2015; Kim and others 2016). For instance, biochar mitigated salt-induced mortality in Abutilon theophrasti and extended the survival rate of Prunella vulgaris. The growth rates of A. theophrasti by adding both biochar and salts were similar to plants devoid of salt addition (Thomas and others 2013). Akhtar and others (2015) demonstrated that biochar enhanced crop productivity in salt-affected soils; biochar application ameliorated salt stress by adsorbing Na and increasing xylem K+ content thereby increasing tuber yield in potato. Also, these researchers stated that ABA concentration of plants was decreased by biochar treatments under different levels of salt stress. In fact, biochar has the potential to alleviate salinity-induced reductions in mineral uptake, and may be a novel technique to diminish the effects of salinization in arable and contaminated soils (Thomas and others 2013; Kim and others 2016). Recently, Farhangi-Abriz and Torabian (2017) also reported that biochar could contribute to protect common bean seedlings against NaCl stress by alleviating oxidative stress. However, until now the interaction between biochar and phytohormones involved in salt tolerance has not been addressed in the literature. The objectives of this study were to investigate the effects of biochar on common bean endogenous hormones such as polyamines, ABA, ACC, IAA, JA, and SA under salinity stress.
Materials and Methods
Preparation of Biochar
The biochar was made according to the methods by Qian and Chen (2013). Maple residues (Acer pseudoplatanus L.) were chopped and passed through a 0.5 mm sieve, then heated at 560 °C for 6 h under oxygen limited conditions, with the rate of 5 °C min−1. Elemental content of biochar including carbon (C), hydrogen (H), nitrogen (N), and oxygen (O) were measured with an elemental analyzer (Elementar, Germany). The pH of biochar was measured electrometrically (Ahmed and others 2015) and cation exchange capacity was determined according to the ammonium acetate method (Chapman 1965). The main biochar properties are shown in Table 1.
Experimental Conditions
This experiment was conducted in a glass greenhouse with a factorial arrangement on the basis of a randomized complete block design with four replications in 2016. In this experiment, three levels of salinity (non-saline, 6, and 12 dS m−1 of NaCl) and three biochar treatments (non-biochar, 10, and 20% total pot mass) were used for testing bean seedlings (Phaseolus vulgaris L. cv. Derakhshan). Salinity levels were selected according to the range of the salt stress tolerance of bean plants (Grieve and others 2012) and biochar values were chosen for testing the physiological performance of bean seedlings, especially endogenous contents of phytohormones under different values of this organic matter (non-biochar, 10, and 20% total pot mass). Initially, the soil was mixed well with prepared biochar and sieved by passing through a 2-mm mesh, then filled to pots (7 cm radius and 20 cm height), which contained 2.5 kg soil. Physical and chemical properties of the experimental soil are presented in Table 1. In each plastic pot five bean seeds were sown. The plants were kept under a glass greenhouse with 25/20 °C day/night temperature, 65–70% relative humidity, and under natural light intensity and photoperiod. For plant nutrition, 10 g of a fertilizer (Master 20-20-20-Valagro-Italy) was dissolved in a liter of water (with EC 0.8 and pH 7.1) and added to the pots (a week after the sowing). Plants were irrigated daily with tap water during the period of emergence and seedling establishment to keep the soil water content near field capacity. After exposure of the first trifoliate leaf, salt was added to irrigation water for saline treatments.
Sampling Method and Plant Growth
Four weeks after the sowing, five plants from each pot were harvested and these plants were randomly selected for measuring different biochemical traits (each measurement was repeated four times according to the number of replications). The dry weights of roots and shoots were determined after oven drying at 70 °C for 72 h.
Assaying Polyamine Contents
Free polyamine contents of roots and leaves were measured by the method of Aziz and Larher (1995). Plant samples (1 g) were homogenized in 1 ml perchloric acid 6% (v/v) and homogenized samples were centrifuged at 20,000g for 45 min. After that, the supernatant was collected and benzoylated. The prepared samples were analyzed by an HPLC equipped with a C18 reverse-phase column with 5 µm particle size (Model Waters 600E, Waters Inc., USA). From each sample, a 10 µl acetonitrile solution of benzoyl polyamines, was injected into the column with 30 °C temperature. Prepared samples were eluted from the column with 40% acetonitrile at a flow rate of 1 ml min−1. The eluent peaks, with their retention time and area, were recorded using an attached integrator. The standard reagents (putrescine, spermidine, and spermine) were bought from Sigma Chemical Co (Missouri, USA).
Assaying Polyamine Oxidase Activity
For polyamine oxidase (PAO) extraction, potassium phosphate buffer (1.6 ml; 0.1 M) with adjusted pH 6.7 was added to the root and leaf samples. Then the samples were centrifuged at 4 °C for 15 min at 12,000g. The supernatants were collected from surface area and were used for enzyme assays with a UV visible spectrophotometer at 550 nm (100 Conc. UV Visible Spectrophotometer, Varian, California, USA) according to the method by Smith (1972). One unit of enzyme activity is described as 0.001 absorbance units of change in the optical density at 550 nm min−1.
Assaying Endogenous Contents of IAA, ABA, JA, SA, and ACC
For measuring endogenous content of plant hormones, including IAA, ABA, JA, and SA we used the ELISA method (Li and Meng 1996; Wang and others 2002). Fresh samples from roots and leaves with 1.5 g weight were used to measure the contents of the endogenous levels of hormones. Initially powdered tissues were extracted in 10 ml of 80% cold methanol, including butyl-hydroxy-toluene (1 mm) as an antioxidant for 24 h in a dark place at 4 °C. The prepared samples were then centrifuged for 15 min at 10,000 rpm and the supernatant was transferred to a microtube. The residues of samples were extracted with 2 ml of cold methanol for 12 h, and centrifuged again in the same conditions. The supernatants achieved from each step were combined and colored using Sep-Pak C18 cartridges. For exhausting methanol, after filtration the prepared samples were dried with a rotary evaporator at 40 °C. The residue of each sample was dissolved in a buffer with 1 mM Tris, 150 mM NaCl, 1 mM MgCl2, 0.1% gelatin, and 0.1% Tween 20. After that, the hormone content was determined by ELISA according to the guide of testing set (Li and Meng 1996). The antigens and antibodies were obtained from Sigma Chemical Co (Missouri, USA). For the determination of ACC content, the plant tissues were homogenized in 70% ethanol, and after centrifugation at 4000 rpm for 30 min, the ethanol was evaporated under vacuum at 40 °C. The residue was dissolved in 1 ml water, and prepared for assay of ACC according to the method of Concepcion and others (1979) with a gas chromatograph (CP-3800 Varian, California, USA).
Sodium Content
For determination of sodium (Na), 100 mg of dried plant material (leaf and root) was weighed from each pot. Plant material was dry-ashed at 550 °C for 6 h then digested in 5 M HNO3. Flasks were filled up to volume (50 ml) with double-distilled water and analyzed for Na concentration (mg g−1 dry weight) with atomic absorption spectrophotometry (Shimadzu AA-7000, Kyoto, Japan).
Statistical Analysis
Each measurement was repeated four times according to the number of replications and MSTATC 1.0.0 software (MSTATC Inc., East Lansing Michigan, USA) was used for the normality test and variance analyzing of the data. Means were compared with the least significant difference test (LSD test) at p ≤ 0.05 and described as mean ± standard error. The figures were drawn by Microsoft Excel Software.
Results
Shoot and Root Dry Mass
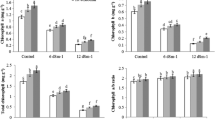
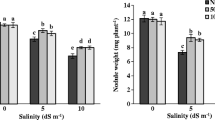
With raising salinity levels to 12 dS m−1 with NaCl, shoot and root dry masses of beans declined; salinity appeared to affect roots more than shoots (Fig. 1). Shoot and root dry mass increased by biochar usage under saline and non-saline conditions. Application of 20% biochar to the soil showed a better effect than 10% biochar for increased shoot and root growth of bean plants under non-saline and salinity conditions (Fig. 1).
Polyamine Contents
The mitigation effect of salt stress and biochar on polyamine contents of common bean seedlings was assessed by leaf and root Put, Spm, and Spd contents (Table 2). In the leaf and root of the NaCl-treated bean plants, there was a significant increase in Put, Spm, and Spd contents. Although, when biochar was added to the soil, polyamine contents were reduced compared to control. Additionally, there was no considerable change of polyamine contents by use of biochar under non-saline conditions. The highest value of Put/Spm + Spd ratio in leaves belonged to the non-biochar treatment under 12 dS m−1; however, there was no remarkable difference among other treatments. Under 12 dS m−1 with 20% biochar, the highest Put/Spm + Spd ratio in roots was observed. The Put/Spm + Spd ratio of leaves and roots under non-saline conditions remained stable when biochar was applied (Table 2).
PAO Activity
The results related to PAO activity of leaves and roots are shown in Table 2. The data indicate that increased salinity led to an increment in PAO activity. Although PAO activity did not vary by biochar application under non-saline conditions, biochar usage increased it at 6 and 12 dS m−1 compared to the non-biochar treatment. Moreover, there was no conspicuous difference between the two biochar doses in the case of PAO activity (Table 2).
ABA Content
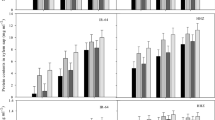
Endogenous leaf and root ABA contents were enhanced significantly in the medium supplemented with NaCl. However, when biochar was applied, ABA content in the two tissues decreased. The highest value of ABA in leaf and root tissues was observed under 12 dS m−1 without biochar (2.93 and 4.8 µg g−1 FW, respectively). As shown in Fig. 2, no appreciable changes in ABA contents were found under non-saline conditions by biochar usage. Furthermore, the effects of the two biochar treatments on ABA content were the same in stressed and non-stressed plants (Fig. 2).
ACC Content
The results indicated that salt stress stimulated the ACC content in leaves and roots of common bean plants. The ACC content rapidly increased after exposure to 12 dS m−1 (Fig. 3). Conversely, ACC content was substantially diminished when biochar was added to the soil, although, ACC content did not change by biochar application under the non-saline treatment. The highest ACC contents were 12.9 and 11.7 nmol g−1 FW in leaf and root, respectively, which belonged to the non-biochar treatment under 12 dS m−1. The results also showed that there was no remarkable discrepancy between the two biochar levels in case of ACC content (Fig. 3).
JA Content
A positive trend between JA contents of leaf and root was observed with rising NaCl concentration in the growth medium. The levels of endogenous JA in response to biochar application significantly decreased as compared to control (Fig. 4). Under 12 dS m−1 without biochar, the highest values of leaf and root JA contents were recorded. JA contents of leaves and roots in the presence of biochar were stable under the non-saline treatment (Fig. 4).
SA Content
The data revealed that NaCl increased the SA content. The effects of salt stress on SA content were also related to the magnitude of the NaCl concentration (Fig. 5). In comparison with the non-saline condition, the extent of increment for SA content in leaves was about 0.7 and 1.7 fold, respectively, under 6 and 12 dS m−1. Additionally, these improvements for root SA content were 0.7 and 2.3 fold, respectively, under 6 and 12 dS m−1 compared to the non-saline condition. On the other hand, the SA data obtained from the experiment showed that biochar had no notable influence on that (Fig. 5).
IAA Content
The presence of NaCl in the culture medium significantly affected the IAA content of bean leaves and roots. A cross over the biochar, leaf IAA content decreased 29 and 39%, and root IAA content declined 26 and 38% under 6 and 12 dS m−1, respectively. Arranged over the salt stress, the IAA content of roots slightly (p < 0.05) increased about 3 and 10% when plants were exposed to 10 and 20% biochar, respectively. However, the increased IAA content in leaves when biochar was added was not significant (Fig. 6).
Na Concentration
The Na concentrations of roots and leaves of bean seedlings were significantly influenced by salinity and biochar application. Enhancing salinity level increased Na content in roots and leaves. In contrast, mixing biochar to soil reduced the Na concentration in comparison with the non-biocar treatment; however, there was no substantial difference between the 10% biochar and the no-biochar treatment in decreasing Na content of roots under 6 dS m−1 salinity. Application of biochar did not show a tangible effect on Na content in roots and leaves (Fig. 7).
Discussion
Salt stress is one of the most communal environmental stress factors in modern agriculture, having harmful effects on crop growth and development. It can unbalance the cellular redox state by generating reactive oxygen species (ROS) such as superoxide radicals (O2 −.), hydroxyl radicals (OH·), and hydrogen peroxide (H2O2) (Munns and Tester 2008). These active radicals exert serious toxic effects on the biochemical, physiological, molecular, and cellular level through lipid peroxidation and damage to proteins and nucleic acids (Nazar and others 2011). Phytohormones are of great significance for the plant’s stress responses and adaptation. To determine the influence of salinity and biochar influence on phytohormones in bean seedlings, the levels of polyamines, ABA, ACC, JA, IAA, and SA were measured. Results showed that salinity induced increased phytohormones in bean plants in terms of increased polyamines, ABA, ACC, JA, and SA. However, the IAA content of bean plants was diminished by salinity compared to the non-saline condition. Phytohormones can participate and interact with redox signaling to control responses to abiotic stresses (Bankaji and others 2014). They regulate salinity tolerance by inducing several protective mechanisms such as the production of proline (Iqbal and others 2014) or enhancing the antioxidant system (Nazar and others 2014) by increasing the accumulation of nutrients. ABA is proposed to serve as a mediator in plant responses to a range of abiotic stresses including salt and drought (Keskin and others 2010). Babu and others (2012) reported that the ABA concentration of tomato increased significantly with increasing NaCl stress as a result of osmotic effects of salt stress. The current study presents evidence that leaf and root ABA levels increase when common bean seedlings are exposed to salinity, especially under 12 dS m−1. Interestingly, the extent of increment in the root was more than the leaf. We think because the root is the first part of the plant exposed to salt stress and the source of ABA production is in the root therefore the level of root ABA is higher than the leaf. Jia and others (2002) suggest that the salt stress-induced ABA accumulation in roots, which may also be triggered by an osmo-sensing mechanism, leads to ABA accumulation in leaves. Furthermore, ABA up-regulates several genes that are responsible for osmotic adjustment (Rhodes and others 2004) and for the mitigation of oxidative stress (Ashraf 2009).
One of the most important consequences generated by salt stress is stimulated ethylene production, as an increased level of ethylene can inhibit root and shoot length and overall plant growth (Nadeem and others 2010). Ethylene, a gaseous plant hormone, plays an important role in tolerance to abiotic stress (Khan and Khan 2014). Production of ethylene in plants is directly correlated with endogenous ACC (1-aminocyclopropane-1-carboxylic acid) levels (Shaharoona and others 2006). As shown in Fig. 3, ACC content in leaves and roots was enhanced with increasing salinity. Wang and others (2009) detected enhanced ethylene synthesis and decreased salt-induced Na content with application of ACC under salinity, and they stated that ethylene might be involved in ion homeostasis under salinity by increasing plasma membrane H+-ATPase activity. Our results indicate that the 6 and 12 dS m−1 salinity treatments conspicuously increased endogenous JA in leaves and roots of bean seedlings relative to the non-saline treatment. There was a similar improvement trend in the JA content of leaves and roots under salt stress. Kramell and others (1995) found a quick rise in endogenous JA content in barley leaf segments exposed to osmotic stress. Moons and others (1997) indicated that jasmonate antagonistically adjusts the expression of salt stress-inducible peptides and proteins, associated with salt stress in rice.
Auxin is one of the famous plant growth regulators that is involved in various plant growth processes and stress responses. Zorb and others (2013) stated that NaCl stress reduced IAA levels in a salt-sensitive maize cultivar. In a study from Shi and others (2014), endogenous IAA negatively modulated H2O2 and O2 − contents, but positively regulated four enzymatic antioxidant enzymes in Arabidopsis under drought stress conditions. Our statistical analysis clearly revealed that salt stress decreased IAA content in leaves and roots of bean. A similar trend was seen in leaves and roots in terms of IAA levels. Cross-talk has been found between auxin and other plant hormones such as SA and ABA (Kazan and Manners 2009). Du and others (2013) stated that the change in auxin content could alter ABA synthesis, and that the balance of auxin and ABA homeostasis played a key role in diverse stress responses.
In the present study, SA contents of leaves and roots in bean were increased by raising salt stress, similarly. SA improves plant tolerance to different environmental stresses by changing the activities of enzymatic antioxidants and decreasing the generation of ROS (Horvath and others 2007). It also regulates various aspects of plant responses to stress through extensive signaling cross-talk with other plant hormones such as ABA and ethylene. SA can increase the concentration of ABA and ABA may promote the biosynthesis of SA in a developing phase- and tissue-specific manner (Seo and Park 2010).
Polyamines are important growth regulators that are essential for plant cell growth and development. They are classified as secondary messengers in plant signaling pathways, and can bind strongly with proteins, phospholipids, and nucleic acids (Alcazar and others 2010), and are involved in stabilizing chromosomes, retarding lipid peroxidation, and preserving membrane integrity. Under stress conditions, plants produce more endogenous PA (Duan and others 2008). In the current study, endogenous Put, Spd, and Spm contents of bean seedlings were enhanced by salinity compared to the non-saline treatment. As an average, the level of PAs in the leaves were higher than those in the root. Among the three major PAs, Put accumulated more than Spd and Spm in both parts of the plant. Polyamine oxidase is a main catabolic enzyme involved in the stress response (Papadakis and Roubelakis-Angelakis 2005). As presented in Table 2, there is a parallel increase in the concentrations of the three PAs and the increase in PAO activity under salt stress. Zhang and Huang (2013) indicated that PAO activity in the roots and leaves of tomato seedlings was generally higher under drought stress than the control. They also found a significant positive correlation between the concentrations of endogenous polyamines and PAO activity.
When plants were treated with biochar, Na concentration, the contents of polyamines, ABA, ACC, and JA, decreased; however, IAA increased. In other words, using biochar reduced the content of the phytohormones involved in the plant stress responses (PA, ABA, ACC, and JA) and increased the phytohormone that regulates plant growth (IAA). Additionally, the content of JA was stable under biochar treatment. The content of SA was not altered by biochar treatments under saline or non-saline conditions; this response may be related to the gene expression in this plant and can be different in other cultivars and plant species. Studies have also shown that biochar can enhance plant growth either by direct or indirect mechanisms of action. Direct growth promotion under biochar amendment involves supplying mineral nutrients, that is, Ca, Mg, P, K, and S and so on, to the plant, whereas, the indirect mechanism involves improving the soil’s physical, chemical, and biological characteristics (Cheng and others 2012; Enders and others 2012; Peng and others 2012). The increased availability of such minerals in the soil solution may result in reduced Na uptake thereby reducing salinity stress in plants (Grattan and Grieve 1998), which leads to the alleviation of salt stress in plants. In the present experiment, biochar enhanced shoot and root growth and decreased Na concentrations of bean seedlings compared to the non-biochar treatment. In a previous study, we have stated that incorporation of biochar into salt-affected soil could mitigate salinity stress in common bean (Farhangi-Abriz and Torabian 2017) by reducing ROS levels. Biochar can also be used to ameliorate salinity stress by reducing the Na uptake in plants, which is quite stable in soil (Lashari and others 2014). In fact, application of biochar reduces the negative effect of salt stress by adsorbing Na from the soil solution (Akhtar and others 2015), which alleviates salinity impacts. Our results clearly showed that decreasing the Na concentration of plant tissues by adding biochar to the soil increased plant growth promoting hormones in roots and leaves of bean.
Conclusion
In the present study, the relationship between biochar and salt stress with regard to phytohormones has been demonstrated in common bean seedlings. Our results revealed that salinity increased Na concentrations, ACC content, the activity of PAO, polyamines, ABA, JA, and SA contents as stress hormones; whereas it decreased IAA in bean leaves and roots. When biochar treatments at two rates were applied, an antithetical trend was observed in phytohormones compared to salinity stress. In fact, biochar could reduce the effects of salt on bean seedlings, which is obvious in levels of phytohormones. We concluded that biochar alleviated the negative effects of NaCl on common bean seedlings by reducing Na concentrations in the leaves and roots of bean plants, which could change the levels of phytohormones indirectly, under non-optimal conditions.
References
Abbas T, Rizwan M, Ali S, Adrees M, Zia-ur-Rehman M, Qayyum MF, Ok YS, Murtaza G (2017) Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ Sci Pollut Res 1–13
Ahmed AA, Roosens N, Dewaele E, Jacobs M, Angenon G (2015) Overexpression of a novel feedback-desensitized ∆1-pyrroline-5-carboxylate synthetase increases proline accumulation and confers salt tolerance in transgenic Nicotiana plumbaginifolia. Plant Cell Tissue Organ Cult 122:383–393
Akhtar SS, Andersen MN, Liu F (2015) Biochar mitigates salinity stress in potato. J Agron Crop Sci 201:368–378
Alcazar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Ali S, Rizwan M, Qayyum MF, Ok YS, Ibrahim M, Riaz M, Arif MS, Hafeez F, Al-Wabel MI, Shahzad AN (2017) Biochar soil amendment on alleviation of drought and salt stress in plants: a critical review. Environ Sci Pollut Res 24:12700–12712
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotech Adv 27:84–93
Aziz A, Larher F (1995) Changes in polyamine titers associated with the proline response and osmotic adjustment of rape leaf discs submitted to osmotic stresses. Plant Sci 112:175–186
Babu MA, Singh D, Gothandam KM (2012) The effect of salinity on growth, hormones and mineral elements in leaf and fruit of tomato cultivar PKM1. J Anim Plant Sci 22:159–164
Bankaji I, Sleimi N, López-Climent MF, Perez-Clemente RM, Gomez-Cadenas A (2014) Effects of combined abiotic stresses on growth, trace element accumulation, and phytohormone regulation in two halophytic species. J Plant Growth Regul 33:632–643
Cao WH, Liu J, He XJ, Mu RL, Zhou HL, Chen SY, Zhang JS (2007) Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol 143:707–719
Chapman HD (1965) Cation-exchange capacity. Methods of soil analysis. Part 2. Chemical and microbiological properties, (methods of soil anb), pp 891–901
Cheng Y, Cai ZC, Chang S, Wang J, Zhang JB (2012) Wheat straw and its biochar have contrasting effects on inorganic N retention and N2O production in a cultivated Black Chernozem. Biol Fertil Soil 48:941–946
Concepcion M, Lizada C, Yang SF (1979) A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem 100:140–145
Djanaguiraman M, Sheeba JA, Durga DD, Bangarusamy U (2009) Cotton leaf senescence can be delayed by nitrophenolate spray through enhanced antioxidant defence system. J Agron Crop Sci 195:213–224
Downie A, Crosky A, Munroe P (2009) Physical properties of biochar. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology, 2nd edn. Earthscan, London, pp 13–32
Du H, Wu N, Chang Y, Li X, Xiao J, Xiong L (2013) Carotenoid deficiency impairs ABA and IAA biosynthesis and differentially affects drought and cold tolerance in rice. Plant Mol Biol 83:475–488
Duan J, Li J, Guo S, Kang Y (2008) Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol 165:1620–1635
Enders A, Hanley K, Whitman T, Joseph S, Lehmann J (2012) Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour Technol 114:644–653
Farhangi-Abriz S, Torabian S (2017) Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol Environ Saf 137:64–70
Flores HE (1991) Changes in polyamine metabolism in response to abiotic stress. In: Slocum RM, Flores HE (eds) Biochemistry and Physiology of Polyamines Plants. CRC Press, Boca Raton, pp 213–228
George S, Venkataraman G, Parida A (2010) A chloroplast-localized and auxin induced glutathione S-transferase from phreatophyte Prosopis juliflora confer drought tolerance on tobacco. J Plant Physiol 167:311–318
Ghanem ME, Albacete A, Martínez-Andújar C, Acosta M, Romero-Aranda R, Ian CD, Lutts S, Pérez-Alfocea F (2008) Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.). J Exp Bot 59:3039–3050
Grattan SR, Grieve CM (1998) Salinity–mineral nutrient relations in horticultural crops. Sci Hortic 78:127–157
Grieve CM, Grattan SR, Maas EV (2012) Plant salt tolerance. Agricultural salinity assessment and management, 2nd edn. ASCE Manual Reports on Engineering Practice, vol 71, pp 405–459
Groppa MD, Benavides MP (2008) Polyamines and abiotic stress: recent advances. Amino Acids 34:35–45
Hasanuzzaman M, Nahar K, Fujita M (2013) Plant response to salt stress and role of exogenous protectants to mitigate saltinduced damages. In: Ahmad P, Azooz MM, Prasad MNV (eds) Ecophysiology and responses of plants under salt stress. Springer, New York, pp 25–87
Horvath E, Szalai G, Janda T (2007) Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul 26:290–300
Iqbal N, Umar S, Khan NA, Khan MIR (2014) A new perspective of phytohormones in salinity tolerance: regulation of proline metabolism. Environ Exp Bot 100:34–42
Javid MG, Sorooshzadeh A, Moradi F, Modarres Sanavy S, Allahdadi I (2011) The role of phytohormones in alleviating salt stress in crop plants. Aust J Crop Sci 5:726–734
Jia W, Wang Y, Zhang S, Zhang J (2002) Salt-stress-induced ABA, accumulation is more sensitively triggered in roots than in shoots. J Exp Bot 53:2201–2206
Jimenez-Bremont JF, Ruiz OA (2007) Modulation of spermidine and spermine levels in maize seedlings subjected to long-term salt stress. Plant Physiol Biochem 45:812–821
Kazan K, Manners JM (2009) Linking development to defense: auxin in plant pathogen interactions. Trends Plant Sci 14:1360–1385
Keskin BC, Sarikaya AT, Yuksel B, Memon AR (2010) Abscisic acid regulated gene expression in bread wheat. Aust J Crop Sci 4:617–625
Khan NA, Khan MIR (2014) The ethylene: from senescence hormone to key player in plant metabolism. J Plant Biochem Physiol 2, e124
Kim HS, Kim KR, Yang JE, Ok YS, Owens G, Nehls T, Wessolek G, Kim KH (2016) Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 142:153–159
Kovac M, Piskernik D, Ravnikar M (2003) Jasmonic acid-induced morphological changes are reflected in auxin metabolism of beans grown in vitro. Biol Plant 47:273–275
Kramell R, Atzorn R, Schneider G, Miersch O, Bruckner C, Schmidt J, Sembdner G, Parthier B (1995) Occurrence and identification of jasmonic acid and its amino acid conjugates induced by osmotic stress in barley leaf tissue. J Plant Growth Regul 14:29–36
Lashari MS, Liu Y, Li L, Pan W, Fu J, Pan G, Zheng J, Zheng J, Zhang X, Yu X (2013) Effects of amendment of biochar-manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from Central China Great Plain. Field Crop Res 144:113–118
Lashari MS, Ye Y, Ji H, Li L, Kibue GW, Lu H, Zheng J, Pan G (2014) Biocharmanure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from Central China: a twoyear field experiment. J Sci Food Agric 95:1321–1327
Lashari MS, Ye Y, Ji H, Li L, Kibue GW, Lu H, Zheng J, Pan G (2015) Biochar–manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: a 2-year field experiment. J Sci Food Agric 95:1321–1327
Lehmann J, Joseph S (2015) Biochar for environmental management: science, technology and implementation. Routledge, London
Li XJ, Meng FJ (1996) Study on the photoperiodic-induced flowering in soybean: changes of plant hormones and assimilates of the first leaves. J China Agric Univ 1:35–39
Malamy J, Carr JP, Klessig DF, Raskin I (1990) Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250:1002–1004
Monteiro CC, Rolão MB, Franco MR, Peters LP, Cia MC, Capaldi FR, Carvalho RF, Gratão PL, Rossi ML, Martinelli AP, Peres LEP, Azevedo RA (2012) Biochemical and histological characterization of tomato mutants. An Acad Bras Cienc 84:573–585
Moons A, Prinsen E, Bauw G, Montagu M (1997) Antagonistic effects of abscisic acid and jasmonate on salt stress inducible transcripts in rice roots. Plant Cell 12:2243–2259
Munns R, Tester M (2008) Mechanism of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nadeem SM, Zahir ZA, Naveed M, Ashraf M (2010) Microbial ACC-deaminase: prospectsand applications for inducing salt tolerance in plants. Crit Rev Plant Sci 29:360–393
Nazar R, Iqbal N, Masood A, Syeed S, Khan NA (2011) Understanding the significance of sulfur in improving salinity tolerance in plants. Environ Exp Bot 70:80–87
Nazar R, Khan MIR, Iqbal N, Masood A, Khan NA (2014) Involvement of ethylene in reversal of salt-inhibited photosynthesis by sulphur in mustard. Physiol Plant 152:331–344
Papadakis AK, Roubelakis-Angelakis KA (2005) Polyamines inhibit NADPH oxidase-mediated superoxide generation and putrescine prevents programmed cell death induced by polyamine oxidase generated hydrogen peroxide. Planta 220:826–837
Peng F, He PW, Luo Y, Lu X, Liang Y, Fu J (2012) Adsorption of phosphate by biomass char deriving from fast pyrolysis of biomass waste. Clean 40:493–498
Qian L, Chen B (2013) Dual role of biochars as adsorbents for aluminum: the effects of oxygen-containing organic components and the scattering of silicate particles. Environ Sci Technol 47:8759–8768
Ramzani PMA, Shan L, Anjum S, Ronggui H, Iqbal M, Virk ZA, Kausar S (2017) Improved quinoa growth, physiological response, and seed nutritional quality in three soils having different stresses by the application of acidified biochar and compost. Plant Physiol Bioch 116:127–138
Rhodes D, Nadolska-Orczyk A, Rich PJ (2004) Salinity, osmolytes and compatible solutes. In: Läuchli A, Lüttge U (eds) Salinity: environment-plants-molecules. Kluwer Academic Publishers, Dordrecht, pp 181–204
Seo PJ, Park CM (2010) MYB96-mediated abscisic acid signals induce pathogen resistance response by promoting salicylic acid biosynthesis in Arabidopsis. New Phytol 186:471–483
Shaharoona B, Arshad M, Zahir ZA, Khalid A (2006) Performance of Pseudomonas spp. containing ACC-deaminase for improving growth and yield of maize (Zea mays L.) in the presence of nitrogenous fertilizer. Soil Biol Biochem 38:2971–2975
Shi H, Chen L, Ye T, Liu X, Ding K, Chan Z (2014) Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiol Biochem 82:209–217
Shim IS, Momose Y, Yamamoto A, Kim DW, Usui K (2003) Inhibition of catalase activity by oxidative stress and its relationship to salicylic acid accumulation in plants. Plant Growth Regul 39:285–292
Smith TA (1972) Purification and properties of the polyamine oxidase of barley plants. Phytochemistry 11:899–910
Thomas SC, Frye S, Gale N, Garmon M, Launchbury R, Machado N, Melamed S, Murray J, Petroff A, Winsborough C (2013) Biochar mitigates negative effects of salt additions on two herbaceous plant species. J Environ Manage 129:62–68
Tognetti VB, Mühlenbock P, Van Breusegem F (2011) Stress homeostasis—the redox and auxin perspective. Plant Cell Environ 35:321–333
Van de Poel B, Smet D, Van Der Straeten D (2015) Ethylene and hormonal crosstalk in vegetative growth and development. Plant Physiol 169:61–72
Wang S, Xu L, Li G, Chen P, Xia K, Zhou X (2002) An ELISA for the determination of salicylic acid in plants using a monoclonal antibody. Plant Sci 162:529–535
Wang H, Liang X, Wan Q, Wang X, Bi Y (2009) Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta 230:293–307
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100:681–697
Xu Q, Burgess P, Xu J, Meyer W, Huang B (2016) Osmotic stress-and salt stress-inhibition and gibberellin-mitigation of leaf elongation associated with up-regulation of genes controlling cell expansion. Environ Exp Bot 131:101–109
Zhang C, Huang Z (2013) Effects of endogenous abscisic acid, jasmonic acid, polyamines, and polyamine oxidase activity in tomato seedlings under drought stress. Sci Hort 159:172–177
Zorb C, Geilfus CM, Muhling KH, Jutta Ludwig-Muller J (2013) The influence of salt stress on ABA and auxin concentrations in two maize cultivars differing in salt resistance. J Plant Physiol 170:220–224
Acknowledgements
We appreciate the University of Tabriz for providing greenhouse and laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farhangi-Abriz, S., Torabian, S. Biochar Increased Plant Growth-Promoting Hormones and Helped to Alleviates Salt Stress in Common Bean Seedlings. J Plant Growth Regul 37, 591–601 (2018). https://doi.org/10.1007/s00344-017-9756-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-017-9756-9