Abstract
We present a review of the documented fungal colonizations of presumably symbiotic nature in lycophytes and ferns (“pteridophytes”). The sampling covers ca. 11 % (1287 spp.) of the estimated global diversity of these taxa (ca. 12,000 spp.) and shows an average presence of fungal endophytes of 68 %, which is significantly lower than the average presence of mycorrhiza of 80–85 % for the remaining tracheophytes. Above-average colonization rates up to 100 % among ferns are mainly found in phylogenetically old lineages, whereas below-average mycorrhization characterizes the Polypod I clade and the Aspleniaceae of the derived leptosporangiate ferns. Arbuscular Mycorrhizal Fungi (AMF) are found in 54 % of the species, to which 6 % of unspecified records of mycorrhizae should probably be added. Dark Septate Endophytes (DSE) are found in 13 % of the species, in about half the cases (6 %) together with AMF. Ectomycorrhizae have not been confirmed for pteridophytes so far, and basidiomycetes are found very rarely in mycoheterotropic gametophytes. Fungal endophytes are unevenly distributed across the life forms and most frequent with 75 % in the terrestrial species, followed with 69 % in saxicolous and with 58 % in epiphytic species. Although AMF have a low dispersal potential and thus are considered unreliable symbiotic partners for epiphytes, they are still present in 27 % of the investigated epiphytic pteridophytes. The occurrence of mycorrhizae across the taxa of pteridophytes bears a phylogenetic signal, as the derived ferns show a notable trend towards a growing independence from AM, in epiphytes more pronouncedly so than in terrestrial taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mycorrhizae represent the most important symbiosis that land plants partake in (Brundrett 2009). Estimates vary between 80 % and 85 % of all land plants being mycorrhizal (Brundrett 2002; Wang and Qiu 2006; Parniske 2008), ranging from 100 % in gymnosperms (Brundrett 2002, 2009) to 0 % in mosses (Bryopsida), for which occasional colonisations but no true fungal symbioses are confirmed (Brundrett 2002; Pressel et al. 2010). The prevalent fungal partners of land plants are the aseptate Glomeromycota (Schüßler et al. 2001), which have grown dependent on the green plants in the symbiosis called Arbuscular Mycorrhiza (AM) (Parniske 2008). Its origin lies in the era when plants first conquered the land some 400 Mya and thus predates the evolution of roots (Taylor et al. 1995, Brundrett 2002, Kenrick and Strullu-Derrien 2014). The oldest structures similar to extant AM in the earliest tracheophyte fossils from the Rhynie Chert (Remy et al. 1994) likely involved not only Glomeromycota, which are the prevalent symbionts today, but also Mucoromycotina, which are either sister to the rest of the Eumycota or to the Glomeromycota (Strullu-Derrien and Strullu 2007, Field et al. 2015a, Selosse et al. 2015). Recent anatomical and molecular studies have found Mucoromycotina as symbionts in the earliest diverging liverworts Treubia and Haplomitrium (Bidartondo and Duckett 2010; Pressel et al. 2010; Field et al. 2015) as well as in hornworts (Desirò et al. 2013) and some ferns (Rimington et al. 2015). Other important types of mycorrhizae involve mainly more derived Basidiomycota and Ascomycota, like the Ectomycorrhiaze (ECM), ericoid mycorrhizae, and orchid mycorrhizae (Brundrett 2002).
Plant genes controlling the mycorrhizal symbiosis can be traced back to the common ancestor of all land plants (Wang et al. 2010). This means the genes have retained their functionality during the switch from the gametophyte to the sporophyte as the generation involved in the symbiosis, in a comparable way as the genes coding for rhizoids and roots hairs (Jones and Dolan 2012). This is not surprising in the light of the many benefits mycorrhizae hold for the plant (Abbott and Robson 1984; van der Heijden 2015). In exchange for providing assimilates to its heterotrophic fungal partner, the plant normally receives an improved supply of phosphorus, nitrogen, micronutrients, and water, resistance against pathogens, and also a higher recovery rate from herbivory due to a higher productivity (Willis et al. 2013). However, in the course of evolution some plants have disposed of the mycorrhiza or changed the fungal partner for various reasons (Wilkinson 2001; Brundrett 2002). An independence from mycorrhizae can be seen as a response to abiotic conditions when the costs outweigh the benefits to the plant (Wang and Qiu 2006), either when photosynthetic rates are low (e.g. under CO2 limitation) or when micronutrients are available in surplus. Changes of the fungal partners have also occurred several times in connection with the conquest of the epiphytic habitat (Brundrett 2009; Kottke and Nebel 2005). Spores and other propagules of glomeromycota do not disperse through air (Willis et al. 2013) and their uncommon establishment on high branches necessitates the presence of potentially mycorrhizal plant species combined with the transfer of inoculum from the soil by a vector (Janos 1993), like ants or birds. This makes AM a priori unsuitable for epiphytes, which instead have often recruited Basidiomycota and Ascomycota, ubiquitous and original decomposers, as fungal partners (Fröhlich-Nowoisky et al. 2012). This has been documented for liverworts (Hepaticae; see Kottke and Nebel 2005, and references therein), Ericaceae (Cullings 1996; Brundrett 2002; Selosse et al. 2007) and Orchidaceae (Yukawa et al. 2009).
Lycophytes and ferns (monilophytes; Pryer et al. 2004; Smith et al. 2006), a paraphylum also commonly known as pteridophytes (Kubitzki 1990), are especially interesting in mycorrhizal research because they combine two interesting aspects. First, they are the only extant tracheophytes whose gametophytes and sporophytes are living independently, with stark anatomical and ecological differences, but ultimately confined to the same spots (Page 2002). With the exception of the relatively well-studied mycoheterotrophic prothallia of Lycopodiaceae (Schmid and Oberwinkler 1993, 1994; Winther and Friedman 2007a) and some eusporangiate ferns (Ophioglossaceae, Psilotaceae; Winther and Friedman 2007b, Winther and Friedman 2009), information on the mycorrhizae of green pteridophyte gametophytes is scant (e.g., Schmid and Oberwinkler 1995; Turnau et al. 2005; Ogura-Tsujita et al. 2012) and their influence on the ecological and evolutionary fitness of the species hardly understood. However, there is evidence that both generations usually share the same type of mycorrhiza, but that the fungal symbiont has to colonize each generation consecutively, thus allowing for a change of the fungal taxa involved (Schmid and Oberwinkler 1995; Turnau et al. 2005; Reyes-Jaramillo et al. 2008).
Furthermore, pteridophytes boast a high percentage of epiphytes (24 % of ca. 12,000 species vs. 9 % of ca. 275,000 species of angiosperms) (Zotz 2013). Several phylogenetic studies have focused on the evolution of epiphytism in pteridophytes in connection with the advent of angiosperm-dominated forests (e.g., Schuettpelz and Pryer 2009; Hennequin et al. 2008), but have not included mycorrhizae as modulating factor in the adaptation of ferns to this stressful ecological niche. Studies on other epiphytic lineages have revealed significant impact of mycorrhizae: Among liverworts (Kottke and Nebel 2005), the presence or absence of mycorrhiza conditions which lineage radiated into the epiphytic habitat and which not; comparative studies between terrestrial and epiphytic orchids (Martos et al. 2012) have shown that epiphytes are more conservative in their mycorrhizal partner, which indicates a more pronounced parallel evolution between plant and fungus than in the terrestrial species.
We here compile the current information about the fungal endophytes in lycophytes and ferns with the aim to retrieve general patterns across taxa and substrates for the use in comparative phylogenetic studies and as a basis for developing evolutionary and ecological hypothesis that can then be tested.
2 Materials and methods
Records on mycorrhizae in lycophytes and ferns were gleaned from the literature (Berch and Kendrick 1982; Bhat and Kaveriappa 2003; Boullard 1958; Cooper 1976; Dhillion 1993; Fernández et al. 2010; Gemma et al. 1992; Gemma and Koske 1995; Iqbal et al. 1981; Lara-Pérez et al. 2015; Lesica and Antibus 1990; Moteetee et al. 1996; Muthukumar and Udaiyan 2000; Muthukumar and Prabha 2012, 2013; Ragupathy and Mahadevan 1993; Schmid et al. 1995; Sudha and Ammani 2010; Wäckers 1998; Zhang et al. 2004; Zhao 2000; Zubek et al. 2010) and included our own accounts (Kessler et al. 2010a, 2010b, 2014; Lehnert et al. 2009).
In the summary of the different records of mycorrhization, we distinguished simply between presence/absence in the following categories: Fungal colonisation, Arbuscular Mycorrhizal Fungi (AMF), unspecified colonisation, Dark Septate Endophytes (DSE), and mixed colonisation (aseptate and septate endophytes). Unspecified colonisations are from studies that did not specify which phenotype of fungal colonisation they regarded as mycorrhiza (e.g. Cooper 1976), but may be attributed entirely to AM because no other type was recognized in pteridophytes as mycorrhiza back then. The unequal approaches of the incorporated studies in the quantification of the colonisations precluded a distinction between obligate and facultative mycorrhizae.
All taxa were classified according to their substrate affinity (epiphyte, terrestrial, saxicolous, aquatic). We allowed multiple assignments per species in order to be able to interpret contradictory reports of mycorrhization in the light of substrate specifity. When possible, epiphytes were further divided into low and high epiphytes. Hemiepiphytes were included in low epiphytes; climbers were included in the terrestrial species because they at least start their life rooting in the soil, which is the key aspect for this study. If the information of the substrate was not provided in the original sources, it was obtained from literature (Jones and Clemensha 1976; Brownsey and Smith-Dodsworth 2000; Krömer and Kessler 2006), online specimen databases (www.tropicos.org), and personal experience. Percentages of fungal colonisation are given without decimals in the text because they would invoke a false sense of resolution that is not innate to the data pool.
The species were sorted into families following currently accepted classifications (Smith et al. 2006, amended with Christenhusz et al. 2011 and Rothfels et al. 2012; but see Christenhusz and Chase 2014), then grouped according to their phylogenetic position (Schuettpelz and Pryer 2007, 2009; Knie et al. 2015) and numbered consecutively. The Polypodiaceae were split into grammitid and non-grammitid ferns (Schneider et al. 2004b) to open the possibility to examine these ecologically distinct groups separately.
3 Results
Our sampling covers 1287 taxa of lycophytes and ferns, including true species as well as some hybrids and distinct varieties. This represents about 11 % of the estimated global diversity of lycophytes and ferns (ca. 12,000 species). Terrestrial species form the largest group (71 %) followed by epiphytic (24 %), saxicolous (12 %) and aquatic species (3 %); a considerable percentage of species occurred on more than one substrate (16 %). A further comparison of high and low epiphytes across all pteridophyte lineages was not possible because available data allowed this distinction only for a fraction of the epiphytic records.
The summary shows an average presence of putative mycorrhizal fungi in 67 % of the taxa. Terrestrial and saxicolous species are frequently colonized by fungi (75 % and 69 %, respectively), epiphytes notably less (55 %) and aquatics rather occasionally (18 %). Among the colonisations, AMF dominates in all cases (terrestrial 85 %; saxicolous 72 %; epiphytic 47 %; aquatic 100 %); within the non-aquatic species, unspecified records make up 11–14 % of the colonisations (Table 1). Only in 6–8 % of the colonized species per substrate category, AMF occur mixed with DSE. As pure colonisations, DSE are most frequent in epiphytes (40 %) but rather scarce in terrestrial and saxicolous species (5 % and 14 %, respectively) and absent in aquatics (Table 1).
At the family level (Table 2), almost all 48 pteridophyte families are represented in the data pool except for three species-poor leptosporangiate fern families (Matoniaceae, Thyrsopteridaceae, Rhachidosoraceae). Below-average mycorrhization is found in the lycophytes (14–66 %) and most families of the Polypod I-group (families 43, 45–48 with 40–62 %; Table 2) whereas the rest of the leptosporangiate and eusporangiate ferns predominantly have an average or above-average mycorrhization rate (≥ 67 %). Exceptions are the aquatic families Salviniaceae (0 %) and Marsileaceae (6 %), as well as the terrestrial-saxicolous Gleicheniaceae (65 %), Plagiogyriaceae (50 %), Diplaziopsidaceae (0 %), Woodsiaceae (0 %), and two diverse families with epiphytic radiations, the Pteridaceae (63 %) and Aspleniaceae (43 %). Families retrieved as fully mycorrhizal (100 %) are mostly represented with few taxa (1–5 spp.) but in the cases of Anemiaceae (18 of ca. 100 spp.), Schizeaceae (13 of ca. 30 spp.) and Nephrolepidaceae (10 of ca. 19 spp.) with a higher number of taxa; fully recovered as mycorrhizal are also small families comprising just 1–2 species each (Culcitaceae, Loxsomataceae, Hemidictyaceae).
4 Discussion
Representativeness of sampling
The sampling covers ca. 11 % of the extant diversity of the lycophytes and ferns, which seems substantial but the question remains if it is equally representative of all included groups. It is estimated that ca. 2865 spp. or 24 % of pteridophytes grow at least potentially as epiphytes (Zotz 2013), which matches the percentage of 24 % epiphytes in the sampling of this study. Similarly, aquatic species sum up to ca. 220 species (Smith et al. 2006) or ca. 2 % of the total diversity, and make up 3 % of our sample size. Thus, the spreading of the surveyed taxa does not show any over-emphasis of one particular substrate type.
Regarding the phylogenetic groups, our sampling is skewed towards basal lineages: While the proportion of lycophytes (8 %) in our sampling comes near to the estimated 9 % among all pteridophytes, polypods (64 %) are slightly underrepresented against the remaining ferns and horsetails (28 %) compared to the estimated proportions (75 % and 16 %, respectively).
The way of gathering and processing of samples may have an impact on the evaluation of mycorrhizae as well. Mycorrhizal colonisations can change in the course of the year (Iqbal et al. 1981), and may be undetectable if the roots are not sampled at the right time. Also, AM have a restricted lifespan in a given part of a root and have to be formed anew as the roots grow (Brundrett 2002). Standard procedures for sampling and screening of roots usually guarantee to get these informative parts (e.g., Grace and Stribley 1991). However, in some cases, most mycorrhizal roots of a plant are probably not retrievable from the substrate. Studies on angiosperms with AM (Tisserant et al. 1996) have shown that mostly finer roots of higher branching order are developed for fungal colonization but that at the same time root growth and mass is reduced compared to non-mycorrhizal species (Brundrett 2002). A plant highly dependent on mycorrhizae can thus be expected to have few roots that are infected only in a small percentage of their length. Fine roots are hard to preserve during sampling if the plants grow in compact mineral soil, like most Gleicheniaceae, or develop dense root systems as many tree ferns (Cyatheaceae, Dicksoniaceae), which may account for the relatively low mycorrhization rates in these families compared to their next relatives (Table 2). Several repetitions of a species are desirable in order to avoid sampling bias (Brundrett 2009), and a low percentage of fungal colonisations in a sample does not necessarily mean a low dependence of the plant on the symbiosis. A distinction between facultative and obligate mycorrhizae is not detectable by visual screening but only in comparative case studies using ecophysiological methods on the species level (e.g., Jurkiewicz et al. 2010). As this has not been done for any fern yet, it is uncertain whether most observed associations are really symbiotic or functionally neutral (Brundrett 2004, 2009). It seems that under high inoculum pressure in the substrate, typical non-host (or non-mycorrhizal) species may actually present colonized roots but without noteworthy symbiotic interaction (Lekberg et al. 2015).
A further drawback is the different approaches in determining the mycorrhizal associations and the inconsistent definitions for describing the types over the decades (Brundrett 2002). The same fungus may play different roles (neutral endophyte, symbiont, or necrophyte) in different species or at different life stages of the same plant (Brundrett 2002). This makes a direct critical comparison of the studies impossible and is the reason why we resorted to report only presence/absence of fungal endophytes that might represent a kind of symbiosis.
In most of the surveys focussing on pteridophytes used in our study, it becomes clear from the context that only AMF were considered. Colonizations by Mucoromycota, which quite recently have been recognized as true symbionts (Bidartondo et al. 2011; Field et al. 2015) are very similar in structure to Glomeromycota, having aseptate hyphae that may form coils and vesicle-like swellings but no arbuscules (Field et al. 2015). Consequently, they may have been mistaken for older Glomeromycota AM (Brundrett 2009; Strullu-Derrien et al. 2014) and may actually be more common functioning symbionts among ferns than currently recognized (Rimington et al. 2015). Colonisations of other fungal types like DSE (Jumpponen 2001) and Ascomycetes (Schmid et al. 1995) were mostly ignored and not reported. This is unfortunate, because Boullard (1979) already pointed out a high frequency of septate endophytes in epiphytic Hymenophyllum species without formally classifying them, although he did consider them to be symbionts. This dismissal probably explains for many accounts of non-mycorrhizal epiphytic ferns (Lesica and Antibus 1990; Schmid et al. 1995). However, there is limited, yet growing evidence that ascomycetes of the Hymenoscyphus/Rhizoscyphus alliance, which form the Ericoid mycorrhizae, can also be found in epiphytic liverworts (Kottke and Nebel 2005; Pressel et al. 2008) and ferns (Lehnert et al. 2009). The first report of ectomycorrhiza in ferns by Cooper (1976) was never reconfirmed and was dismissed by Brundrett (2002) as a likely contamination because the illustrated root resembled that of an angiosperm tree of the genus Fagus, presumably belonging to a member of the New Zealand native Nothofagus.
In summary, most reports on the mycorrhization of pteridophytes, especially of ferns, need reconfirmation using updated procedures and standards, and until then should be considered potential mycorrhizal fungal infections only. The patterns revealed by our compilation are rough estimates at best, but they already point to the ecological and taxonomic groups that most promise interesting results in more detailed studies.
Mycorrhizae related to phylogenetic position
Mycorrhizae are very old (Strullu-Derrien and Strullu 2007, Strullu-Derrien et al. 2014) and evidently primordial to all groups of land plants (Wang and Qiu 2006; Field et al. 2015). Evolutionary novelties would consequently encompass either independence from mycorrhizae, including the loss of the symbiosis, or the change to a different type of fungus (Brundrett 2002). Several changes of the mycorrhizal type can be retraced in the phylogeny of the spermatophytes (Wang and Qiu 2006). Within the gymnosperms, several independent changes from the ancestral AM to ectomycorrhizae occurred, most notably in the Pinaceae and Gnetaceae (Wang and Qiu 2006). Similarly, in the angiosperms several switches were made independently (Brundrett 2002, 2009) to basidiomycetes (e.g. orchid-mycorrhizae in Orchidaceae; ectomycorrhizae in arborescent taxa of temperate zones and some Ericaceae) and ascomycetes (e.g., ericoid mycorrhizae in Diapensiaceae and Ericaceae). These changes apparently occurred once in each lineage and triggered further diversification (Wang and Qiu 2006; Selosse et al. 2007; Yukawa et al. 2009).
However, there seems to be no clearly detectable general evolutionary trend among spermatophytes towards a diminishing importance or loss of mycorrhizae (Wang and Qiu 2006). Low percentages or absence of the symbiosis can be related to the aquatic (e.g., Butomaceae, Limnocharitaceae, Menyanthaceae) or parasitic (Loranthaceae) life form (Wang and Qiu 2006; Brundrett 2009). Other non-mycorrhizal families have been studied only with one or few species so far (e.g., Cyclanthaceae, Bataceae, Erythroxylaceae), so their sampling may not be representative (Wang and Qiu 2006). Proteaceae, Cyperaceae and Brassicaceae have long been quoted as examples for plants that generally lack mycorrhizae (Brundrett 2009), but there is an increasing body of evidence that the majority of plant species can simply modulate the mycorrhizal colonization depending on their needs (Cornwell et al. 2001; Bonfante and Genre 2008; Genre and Bonfante 2010), which may change with the substrate. Contrary to this, there appears to be a general trend in the more derived lineages of liverworts (Kottke and Nebel 2005) to reduce the influence of mycorrhizae, and we see similar tendencies among pteridophytes.
The colonisation rate of lycophytes is surprisingly low (58 %). However, their ancient looks belie the fact that the extant diversity had more time to evolve as any other tracheophyte lineage (Pryer et al. 2004; Qiu et al. 2007). The almost exclusively aquatic Isoetaceae mostly lack mycorrhiza because of their restriction to aquatic habitats, as outlined below. Lycopodiaceae are rated as 63 % mycorrhizal but this account is based on sporophytes only. With a few exceptions, their gametophytes are non-chlorophyllous and mycoheterophic involving AMF; Winther and Friedman (2007a) and Leake et al. (2008) also hypothesized that the sporophytes may nourish their gametophytes via the fungal mycelium. Considering both generations, the family should probably be treated as fully dependent on mycorrhizae, even if the sporophytes may be independent from the symbiosis under sufficient nutrient supply. Similarly, the relatively low colonisation rates of Selaginellaceae (Table 2) by may be explained the preference of most species for the deep shade of angiosperm dominated tropical forests, especially in spots like gorges and ravines where nutrients are accumulated (Wilcke et al. 2001).
Within ferns, we can observe most examples of above-average presence of endophytic fungi in the basal grade from the eusporangiate ferns up to the dennstaedtioid ferns (families 4–28; Table 2), and a below-average percentage mainly in the more derived Pteridaceae and eupolypods (families 30–48; Table 2). Exceptions are again the aquatic lineages (Azollaceae, Salviniaceae) in the basal leptosporangiates, which show only occasional colonisations by AMF. A large proportion of non-mycorrhizal species can be attributed to the epiphytic radiations, which have mainly occurred in the Pteridaceae (vittarioid ferns; Schuettpelz et al. 2007) and eupolypods (Polypodiaceae, Dryopteridaceae, Aspleniaceae) and only once in the basal leptosporangiate ferns (Hymenophyllaceae). Here, the species probably followed the same steps as the liverworts (Kottke and Nebel 2005), and first became independent from the symbiosis before being able to conquer the epiphytic habitat and eventually switching to a different fungal partner.
Mycorrhizae related to substrate
This study is the first attempt to correlate the substrate preference of lycophytes and ferns with their mycorrhizal status in a phylogenetic context. There is the general notion stemming from a multitude of practical physiological experiments that mycorrhizae are of advantage (Brundrett 2002; Cairney 2000). The aquatic habitat is apparently not well suited for mycorrhizae, although AMF have been found in the roots of water-lilies (Nymphaea; Wang and Qiu 2006). The advantages of the symbiosis are levelled here because there is no shortage of water and soluble minerals are easily accessible, but productivity of photosynthesis underwater is limited (e.g. Nielsen 1993) due the low partial pressure of CO2 (e.g. Raven et al. 1985) and the extinction of light (Kirk 1994). So it is plausible to find no mycorrhizae in floating Salviniaceae, and submerged taxa of Pteridaceae (Ceratopteris) and Isoetaceae. The few AM colonisations in Isoetaceae (Beck-Nielsen and Madsen 2001; Radhinka and Rodrigues 2007) and Marsileaceae (e.g. Bhat and Kaveriappa 2003; Sudha and Ammani 2010) are accounted for by individuals that root in soil that has fallen dry. Their ability to form mycorrhizae shows how deeply this symbiosis is embedded genetically among embryophytes (Wang et al. 2010). Similarly, all Equisetaceae with the exception of Equisetum arvense L., can be regarded as at least potentially growing in water-logged soils, with some species growing partially submerged along the shores of lakes (E. fluviatile L., E. palustre L.) while others prefer seepage areas in gravelly gorges and ravines (E. ramosissimum Desf., E. bogotense Kunth) or wet meadows (E. sylvaticum L., E. pratense L.). Consequently, there are diverging reports of the mycorrhization in this family on the species level, with samples from relatively well-drained soils being regularly and sometimes highly infected by AMF while those from water-logged soils and the littoral always show lower colonisation rates (Dhillion 1993).
Of the ca. 12,000 species of ferns (Smith et al. 2006), a proportion of 2865 sp. (Zotz 2013) or ca. 24 % (maybe up to 29 %; Dubuisson et al. 2009) grow either potentially or obligately as epiphytes. This number is contrasted by 24,748 sp. (Zotz 2013) or ca. 9 % of the estimated total diversity of angiosperms (ca. 275,000 sp.; Zotz 2013). Several phylogenetic studies have focused on the evolution of epiphytism in pteridophytes in connection with the advent of angiosperm-dominated forests (Hennequin et al. 2008; Schuettpelz and Pryer 2009; Sundue et al. 2015), but have not included mycorrhizae as modulating factor in the evolution of ferns within this stressful ecological niche. Comparative studies between terrestrial and epiphytic orchids (Martos et al. 2012) have shown that epiphytes are more conservative in their mycorrhizal partner, which indicates a more pronounced parallel evolution between plant and fungus than in the terrestrial species. With this in mind, we took a closer look at the epiphytic radiations in ferns.
First, the definition of the epiphytic habitat is important. As AMF are mostly soil-bound (i.e., every stage from germination to sporulation happens below the ground) and their spores and propagules retain no or very low viability once transported through the air (Willis et al. 2013), they are not reliable fungal partners in the high canopy. The establishment of documented occurrences of AM on canopy branches is hypothesized to require the presence of a facultatively mycorrhizal host plant prior to the establishment of the symbiosis (Janos 1993). However, the bases of the trunks of tress are often connected to the soil by a layer of dead and living organic matter through which the AMF can spread vegetatively; this is especially true for the fibrous root mantle that tree ferns and many palms develop. From a mycorrhizal point of view, these low-epiphytic habitats (Fig.1) are more similar to the terrestrial substrate than to the upper trunk and branches (Janos 1993). A categorization into different zones of the chorophyte as done in ecological studies (Johansson 1974; Hietz and Hietz-Seifert 2009; Zotz 2013) must be considered in order be able to distinguish between substrates with high and low presence of AMF (Fig.1). Similarly, the inclusion of the saxicolous habitat in the epiphytic category as done by some authors (Hennequin et al. 2008) seems flawed in the context of mycorrhization, too, because this habitat is also directly connected to the soil. Furthermore, saxicolous tracheophyte species grow either in cracks or in moss cushions, which basically represent two different substrate types (one more mineral based, the other more organic). From our experience, saxicolous fern species growing in stone fissures are more likely to thrive also as terrestrials, whereas we observed many species growing in moss cushions irrespectively of them being located on a rock or on a tree branch. Due to this ambivalence, recognition of the saxicolous habitat at least as one category seems important (as done here), and a further splitting may be sensible in future studies.
Diagram of the substrates available to lycophytes and ferns. The terrestrial and saxicolous habitat was available prior to the advent of the angiosperms, as well as the root buttresses and lower trunks of tree ferns and gymnosperms, which are usually scored as epiphytic in the zones 1 & 2a (i.e., trunk base and lower part of the trunk) according to Johansson (1974) and its modifications (e.g. Zotz 2013). These zones are contiguous with the soil and so more easily colonized by AMF than the structured angiosperm crowns (zones 3–5, i.e. inner, middle and outer crown, respectively), which harbour most of the extant diversity of vascular epiphytes
Having considered this, several exceptions from the rule that AM do not prevail in epiphytes can be corrected. The Psilotaceae, for example, are rootless eusporangiate ferns comprising two genera, Psilotum and Tmesipteris, that are 100 % associated with AMF as gametophytes and sporophytes, yet they are almost exclusively epiphytic. The explanation is that Tmesipteris is found typically in the root-mantle of tree ferns and Psilotum often in pockets of soil that accumulated in the axils of dead palm fronds at relatively low heights, so that a spread of AMF though a continuous substrate layer from the soil is ensured.
In filmy ferns (Hymenophyllaceae), we observe besides terrestrial and saxicolous species both high and low epiphytes (Krömer and Kessler 2006; Dubuisson et al. 2009), and correspondingly a mixture of species with AMF, DSE or non-mycorrhizal species (Fig.2). AMF are mostly present in the Trichomanes-clade, which not only contains many terrestrial and saxicolous species (mainly the genera Callistopteris, Cephalomanes, Abrodictyum and Trichomanes s.s.) but also low epiphytes, hemiepiphytes and climbers (Fig. 1). These non-terrestrials are quite specialized: Just as the above mentioned Tmesipteris, the genus Polyphlebium is primarily found in the root mantle of tree ferns; the genus Didymoglossum is found either on wet rocks or on smooth tree bark at low heights. Opposed to this, the Hymenophyllum-clade is characterized by many epiphytic species that can be found also (but not exclusively) in the canopy (Krömer and Kessler 2006). These species either have been found to largely lack mycorrhizae or to be colonized by DSE. AMF are rare in this clade and found in the few terrestrial taxa and some ecologically potent species that occur in diverse habitats. Not surprisingly, these taxa include the early diverging lineages of the Hymenophyllum clade, e.g. Hymenophyllum nephrophyllum Ebihara & K.Iwats. (= Cardiomanes reniforme (Forst.) C.Presl) (Hennequin et al. 2008).
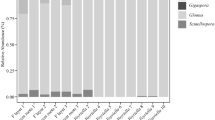
Diagram of the phylogeny of tracheophytes, families of lycophytes (1–3) and ferns (monilophytes, 4–48) shown in detail (after Smith et al. 2006; Rothfels et al. 2012; Knie et al. 2015) arranged in the same sequence as in Table 2. Some formal and commonly recognized informal groups are boxed: 1) lycophytes; 2) eusporangiate ferns; 3) basal leptosporangiate ferns; 4) water ferns; 5) tree fern alliance; 6) dennstaedtioid ferns; 7) Pteridaceae; 8) Eupolypods II; 9) Eupolypods I. Bars behind families represent total sampling; green = percentage without fungi; black boxes = percentage of fungal colonization (mycorrhizae & endophytes); light grey = percentage of AMF (Glomeromycota & Mucoromycota); yellow = undefined mycorrhizae; dark grey = septate endophytes including DSE and Ascomycota. Red dotted line marks average mycorrhization/colonization of 67 %
The change of mycorrhiza and substrate in the Hymenophyllaceae as indicated here is further correlated with changes of the body plan, from a monopodial rosette in terrestrial plants to colonial growth and diminishing size as adaptations to the epiphytic habitat (Dubuisson et al. 2013). These anatomical changes, decreasing the space in the roots for potentially symbiotic fungi, chronologically coincide with the other documented epiphytic radiations in the leptosporangiate ferns (Schuettpelz and Pryer 2009), which followed the radiation of angiosperms in the late Cretaceous/early Tertiary (Schneider et al. 2004a).
The derived Polypodiaceae are probably an even better example for this pattern of concerted evolution. They are interpreted as being primordially epiphytic (Schuettpelz and Pryer 2009; Sundue et al. 2015) and concordantly have a below-average mycorrhization rate (45 %) and an even lower occurrence of confirmed AMF (16 %; Table 2). The Polypodiaeae show many anatomical features that can be interpreted as adaptations that compensate for the loss of the symbiotic partner: The root hairs are well developed and long-lived; most species have thick, long-lasting rhizomes that store carbohydrates; species can be very drought resistant, with thick fronds, strong cuticles and dense indument of scales on the laminae; niche-forming leaves that act as leaf litter collectors have developed several times independently (Aglaomorpha, Drynaria, Platycerium) (Watkins and Cardelús 2012); domatia for ants, which not only defend their host plant but also fertilize it with their faeces, occur among pteridophytes only in this family (Lecanopteris, Solanopteris) (Kramer et al. 1995; Dubuisson et al. 2009). In few words, most Polypodiaceae can be characterized as non-mycorrhizal nutrient savers. The exception is the grammitid ferns, a monophylum that was long treated as distinct family because of many anatomical features that diverge from the remainder of the Polypodiaceae, e.g., small plant size and green spores (Schneider et al. 2004b; Dubuisson et al. 2009). In our survey, we further found grammitid Polypodiaceae to have a much higher fungal colonization rate (73 %) and occurrence of DSE (48 %) than the rest of the family (28 % and 5 %, respectively; Fig. 2). The evolutionary pressure of the common ancestor towards the grammitid habitus probably was forced onto the size of the plants. The saving-and-storing strategy leads to relatively large plants that are not able to colonize thin branches, thus putting the light-endowed outer canopy out of reach. In order to colonize this favourable niche, the ancestor of the grammitid ferns presumably formed mycorrhiza anew with easily available wind-dispersed ascomycetes (Lehnert et al. 2009), which are primordially free-living decomposers but also potential symbiotic partners, with more than 40 % of the named species also being lichenized (Schoch et al. 2009). With a symbiotic partner functioning as an extended root system (compare Allen et al. 2003), the grammitid ferns could reduce the size of their whole body plan, including the root mass, while ensuring a constant supply of water and nutrients. It appears that grammtid ferns have become highly dependence on their mycorrhizae, as they are one of the few fern groups that are almost impossible to cultivate (Hoshizaki and Moran 2001) and even to transplant in situ (Lehnert 2013). While direct evidence is lacking, we suspect that the intolerance of grammitids to displacement is due to their tight association with fungal partners.
The influence of angiosperms on ecological niche evolution in ferns is not restricted to providing room for epiphytes in their open-structured canopy; it can also be seen in the fast nutrient turnover. Compared to extant gymnosperms, deciduous angiosperm trees produce leaf litter that is easily decomposed and has a high nutrient release (Klemmedson 1992) even in comparison to that of other broad-leafed, non-angiosperm taxa, as it is found e.g. in forests dominated by ferns (Allison and Vitousek 2004; Amatangelo and Vitousek 2008). As ferns and lycophytes originated in an angiosperm-free world (Schuettpelz and Pryer 2007), it may be hypothesized that these taxa today find an abundance of freely accessible nutrients in the soils under most angiosperm-dominated vegetation types, whose exploitation triggered the evolution of other key innovations, e.g. the new photoreceptor in derived ferns that allow them to grow under the deeply shading angiosperm canopies (Schneider et al. 2004a). Under such conditions, mycorrhizae may have become optional or dispensable for terrestrial taxa (Allen et al. 2003; Kessler et al. 2014), providing the prerequisite for the evolution of high epiphytes, as postulated for liverworts (Kottke and Nebel 2005).
Among the derived leptosporangiate ferns, the Pteridaceae (Schuettpelz et al. 2007) provide a good example that supports this assumption. The genus Adiantum is predominantly terrestrial and mycorrhizal (88 % of the species with AMF). Field studies have shown that the species can be ecologically separated along a soil nutrient gradient, but that they avoid soils that are categorized as nutrient deficient (Tuomisto et al. 1998). This suggests a low reliance of the genus on mycorrhiza for improved nutrient supply. The species of its sister clade, the vittarioid ferns, are mostly found on trees, from the roots to the upper branches. Accordingly, only 28 % of the vittarioid ferns are mycorrhizal (Table 2), with low epiphytes like the genera Anetium and Antrophyum often showing strong fungal colonization and high epiphytes being non-mycorrhizal (e.g. Radiovittaria stipitata (Kunze) E.H.Crane). In both Adiantum and the vittarioid ferns, only AMF have been recorded. Although vittarioid ferns can be abundant epiphytes in a community, they are less diverse than syntopic epiphytic Hymenophyllaceae and grammitid ferns (Polypodiaceae), which often are associated with DSE (Table 2; Fig. 2).
5 Conclusions and outlook
The occurrence of mycorrhizae across the taxa of pteridophytes seems to show a phylogenetic signal, as phylogenetically derived ferns show a notable trend towards a growing independence from AM, in epiphytes more pronouncedly so than in terrestrial taxa. As the independence is rated here mainly as the absence of mycorrhizal fungi and not as a function over time in relation to external factors, it is assumed that this pattern will come out clearer once a closer look has been taken into the mutual functionality of this symbiosis across a larger set of taxa. We recognize four groups that offer themselves as study objects for a more detailed molecular study focussing on the evolutionary change of fungal symbionts with substrate, i.e. Hymenophyllacae, vittarioid ferns of the Pteridaceae, the genus Elaphoglossum within the Dryopteridaceae, and Polypodiaceae.
In the last two decades the understanding of the evolution of fungi has experienced major advances, with great reverberations on the taxonomy of the Mycobionta (e.g., Schüßler et al. 2001; Kroon et al. 2004; Fitzpatrick et al. 2006; Rossman and Palm 2006; Hibbett et al. 2007; Tian et al. 2015; Wijayawardene et al. 2016). However, the molecular characterization of endophytic fungi from lycophytes and ferns is still in its infancy (Rimington et al. 2015) and has so far focused on lineages with mycoheterotropic life stages, such as Lycopodiaceae (Winther and Friedman 2007a), Ophioglossaceae (Winther and Friedman 2007b), and Psilotaceae (Winther and Friedman 2009). With the latest advances in Next Generation Sequencing (Wei et al. 2014), it may be possible to simultaneously retrieve multiple copies of targeted genome regions from several fungal lineages with one analytical step. The greatest hindrance to overcome is still the extraction of endophyte DNA from fern roots, which are usually thin, tough, and darkened by largely unknown secondary metabolites. These factors diminish the yield and quality of the isolated DNA, as we know from first-hand experience.
The survey of pteridophytes under floristic, ecological and phylogenetic aspects (Lehtonen 2011, and references therein) has seen a surge in recent years, and the impact of mycorrhizae on pteridophytes at the community level is just emerging (Kessler et al. 2010b, 2014). With the fast increasing body of data on the diversely adapted taxa, especially ferns, which show a manageable absolute diversity, will certainly be the first large land plant group for which pattern of niche evolution can be retraced convincingly. Indeed, a recent ecological study in Ecuador found that while mycorrhizal fern species are more abundant, non-mycorrhizal fern species have higher growth rates, perhaps because they lose fewer carbohydrates to the fungi (Kessler et al. 2014). There thus appears to be a balance in ferns between the benefits and costs of having mycorrhizal partners, which so far has been little studied. Whether this balance differs between ferns and angiosperms based on their different physiological adaptations remains to be explored.
References
Abbott LK, Robson AD (1984) The effect of mycorrhizae on plant growth. In: Powell CL, Bagyaraj DJ (eds) VA mycorrhiza. CRC Press, Boca Raton
Allen MF, Swenson W, Querejeta JI, Egerton-Warburton LM, Treseder KK (2003) Ecology of mycorrhizae: a conceptual framework for complex interactions among plants and fungi. Annu Rev Phytopathol 41:271–303
Allison SD, Vitousek PM (2004) Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i. Oecologia 141(4):612–619
Amatangelo KL, Vitousek PM (2008) Stoichiometry of ferns in Hawaii: implications for nutrient cycling. Oecologia 157:619–627
Beck-Nielsen D, Madsen TV (2001) Occurrence of vesicular-arbuscular mycorrhiza in aquatic macrophytes from lakes and rivers. Aquat Bot 71:141–148
Berch SM, Kendrick B (1982) Vesicular-arbuscular mycorrhizae of southern Ontario ferns and fern-allies. Mycologia 74:769–776
Bhat PR, Kaveriappa KM (2003) Occurrence of vesicular arbuscular mycorrhizal fungi in Marsilea minuta L. Mycorrhiza News 15(4):11–13
Bidartondo MI, Duckett JG (2010) Conservative ecological and evolutionary patterns in liverwort-fungal symbioses. P Roy Soc B-Biol Sci 277:485–492
Bidartondo MI, Read DJ, Trappe JM, Merckx V, Ligrone R, Duckett JG (2011) The dawn of symbiosis between plants and fungi. Biol Letters 7(4):574–577
Bonfante P, Genre A (2008) Plants and arbuscular mycorrhizal fungi: an evolutionary-developmental perspective. Trends Plant Sc 13:492–498
Boullard B (1958) La mycotrophie chez pteridophytes: sa frequence, ses caracteres. sa signification. Drouillard, Bordeaux
Boullard B (1979) Considerations Sur la symbiose fongique chez les pteridophytes. Syllogeus 19:1–61
Brownsey PJ, Smith-Dodsworth J (2000) New Zealand ferns and allied plants. New revised edition, Bateman, Wellington, NZ
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154:275–304
Brundrett MC (2004) Diversity and classification of mycorrhizal associations. Bot Rev 79:473–495
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77
Cairney JW (2000) Evolution of mycorrhiza systems. Naturwissenschaften 87:467–475
Christenhusz MJ, Chase MW (2014) Trends and concepts in fern classification. Ann Bot 113(4):571–594
Christenhusz MJM, Zhang X-C, Schneider H (2011) A linear sequence of extant families and genera of lycophytes and ferns. Phytotaxa 19:7–54
Cooper KM (1976) A field survey of mycorrhizas in New Zealand ferns. New Zeal J Bot 14:169–181
Cornwell WK, Bedford BL, Chapin CT (2001) Occurrence of arbuscular mycorrhizal fungi in a phosphorus-poor wetland and mycorrhizal response to phosphorus fertilization. Am J Bot 88:1824–1829
Cullings KW (1996) Single phylogenetic origin of ericoid mycorrhizae within the Ericaceae. Can J Bot 74:1896–1909
Desirò A, Duckett JG, Pressel S, Villarreal JC, Bidartondo MI (2013) Fungal symbioses in hornworts: a chequered history. P Roy Soc Lond B Bio 280(1759):20130207
Dhillion SS (1993) Vesicular-arbuscular mycorrhizas of Equisetum species in Norway and the USA: occurrence and mycotrophy. Mycol Res 97:656–660
Dubuisson JY, Schneider H, Hennequin S (2009) Epiphytism in ferns: diversity and history. CR Biol 332:120–128
Dubuisson JY, Bary S, Ebihara A, Carnero-Diaz E, Boucheron-Dubuisson E, Hennequin S (2013) Epiphytism, anatomy and regressive evolution in trichomanoid filmy ferns (Hymenophyllaceae). Bot J Linn Soc 173:573–593
Fernández N, Fontenla S, Messuti MI (2010) Mycorrhizal status of obligate and facultative epiphytic ferns in a Valdivian temperate forest of Patagonia, Argentina. Amer Fern J 100:16–26
Field KJ, Rimington WR, Bidartondo MI, Allinson KE, Beerling DJ, Cameron DD, Duckett JG, Leake JR, Pressel S (2015) First evidence of mutualism between ancient plant lineages (Haplomitriopsida liverworts) and Mucoromycotina fungi and its response to simulated Palaeozoic changes in atmospheric CO2. New Phytol 205(2):743–756
Fitzpatrick DA, Logue ME, Stajich JE, Butler G (2006) A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol Biol 6:99
Fröhlich-Nowoisky J, Burrows SM, Xie Z, Engling G, Solomon PA, Fraser MP, Mayol-Bracero OL, Artaxo P, Begerow D, Conrad R, Andreae MO, Després VR, Pöschl U (2012) Biogeography in the air: fungal diversity over land and oceans. Biogeosciences 9:1125–1136
Gemma JN, Koske RE (1995) Mycorrhizae in Hawaiian epiphytes. Pac Sci 49:175–180
Gemma JN, Koske RE, Flynn T (1992) Mycorrhizae in Hawaiian pteridophytes: occurrence and evolutionary significance. Am J Bot 79:843–852
Genre A, Bonfante P (2010) The making of symbiotic cells in arbuscular mycorrhizal roots. In: Koltay H, Kapulnik Y (eds) Arbuscular mycorrhizas: physiology and function. Springer, Netherlands, pp. 57–71
Grace C, Stribley DP (1991) A safer procedure for routine staining of vesicular-arbuscular mycorrhizal fungi. Mycol Res 95:1160–1162
Hennequin S, Schuettpelz E, Pryer KM, Ebihara A, Dubuisson JY (2008) Divergence times and the evolution of epiphytism in filmy ferns (Hymenophyllaceae) revisited. Int J Plant Sci 169:1278–1287
Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, Lumbsch HT (2007) A higher-level phylogenetic classification of the fungi. Mycol Res 111:509–547
Hietz P, Hietz-Seifert U (2009) Structure and ecology of epiphyte communities of a cloud forest in Central Veracruz, Mexico. J Veg Sci 6:719–728
Hoshizaki BJ, Moran RC (2001) Fern Grower's manual. Timber Press, Portland, Oregon, Revised and Expanded Edition
Iqbal SH, Yousaf M, Younus M (1981) A field survey of mycorrhizal associations in ferns of Pakistan. New Phytol 87:69–79
Janos DP (1993) Vesicular-arbuscular mycorrhizae of epiphytes. Mycorrhiza 4:1–4
Johansson D (1974) Ecology of vascular epiphytes in west African rain forest. Acta Phytogeog Suec 59:1–136
Jones DL, Clemensha SC (1976) Australian ferns and fern allies. A.H. and A.W, Reed, Sidney
Jones VAS, Dolan L (2012) The evolution of root hairs and rhizoids. Ann Bot 110:205–212
Jumpponen ARI (2001) Dark septate endophytes–are they mycorrhizal? Mycorrhiza 11(4):207–211
Jurkiewicz A, Ryszka P, Anielska T, Waligórski P, Białońska D, Góralska K, Tsimilli-Michael M, Turnau K (2010) Optimization of culture conditions of Arnica montana L.: effects of mycorrhizal fungi and competing plants. Mycorrhiza 20(5):293–306
Kenrick P, Strullu-Derrien C (2014) The origin and early evolution of roots. Plant Physiol 166(2):570–580
Kessler M, Jonas R, Cicuzza D, Kluge J, Piatek K, Naks P, Lehnert M (2010a) A survey of the mycorrhization of southeast Asian ferns and lycophytes. Plant Biol 12:788–793
Kessler M, Jonas R, Strasberg D, Lehnert M (2010b) Mycorrhizal colonizations of ferns and lycophytes on the island of La Réunion in relation to nutrient availability. BAE 11:329–336
Kessler M, Güdel R, Salazar L, Homeier J, Kluge J (2014) Impact of mycorrhization on the abundance, growth and leaf nutrient status of ferns along a tropical elevational gradient. Oecologia 175:887–900
Kirk JT (1994) Light and photosynthesis in aquatic ecosystems. Cambridge University Press
Klemmedson JO (1992) Decomposition and nutrient release from mixtures of Gambel oak and ponderosa pine leaf litter. Forest Ecol Manag 47:349–361
Knie N, Fischer S, Grewe F, Polsakiewicz M, Knoop V (2015) Horsetails are the sister group to all other monilophytes and Marattiales are sister to leptosporangiate ferns. Mol Phylo Evol 90:140–149
Kottke I, Nebel M (2005) The evolution of mycorrhiza-like associations in liverworts: an update. New Phytol 167:330–334
Kramer KU, Schneller JJ, Wollenweber E (1995) Farne und Farnverwandte: Bau, Systematik, Biologie. Georg Thieme Verlag, Stuttgart
Krömer T, Kessler M (2006) Filmy ferns (Hymenophyllaceae) as high-canopy epiphytes. Ecotropica 12:57–63
Kroon LP, Bakker FT, Van Den Bosch GB, Bonants PJ, Flier WG (2004) Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genet Biol 41:766–782
Kubitzki K (ed) (1990) The families and genera of vascular plants, vol 1. In: Kramer KU, Green PS (vol eds) Pteridophytes and Gymnosperms. Springer-Verlag, Berlin
Lara-Pérez LA, Valdés-Baizabal MD, Noa-Carrazana JC, Zulueta-Rodríguez R, Lara-Capistrán L, Andrade-Torres A (2015) Mycorrhizal associations of ferns and lycopods of Central Veracruz, Mexico. Symbiosis 65(2):85–92
Leake JR, Cameron DD, Beerling DJ (2008) Fungal fidelity in the mycoheterotroph to autotroph life cycle of Lycopodiaceae: a case of parental nurture? New Phytol 177:572–576
Lehnert M (2013) Grammitid Ferns I (Polypodiaceae): Melpomene. Flora Neotropica Monograph 112, New York Botanical Garden, USA, 122 pp.
Lehnert M, Kottke I, Setaro S, Pazmiño LF, Suárez JP, Kessler M (2009) Mycorrhizal associations in ferns from southern Ecuador. Am Fern J 99:292–306
Lehtonen S (2011) Towards resolving the complete fern tree of life. PLoS One 6:e24851. doi:10.1371/journal.pone.0024851
Lekberg Y, Rosendahl S, Olsson PA (2015) The fungal perspective of arbuscular mycorrhizal colonization in ‘nonmycorrhizal’plants. New Phytol 205(4):1399–1403
Lesica P, Antibus RK (1990) The occurrence of mycorrhizae in vascular epiphytes of two costa Rican rain forests. Biotropica 1990:250–258
Martos F, Munoz F, Pailler T, Kottke I, Gonneau C, Selosse MA (2012) The role of epiphytism in architecture and evolutionary constraint within mycorrhizal networks of tropical orchids. Mol Ecol 21:5098–5109
Moteetee A, Duckett JG, Russell AJ (1996) Mycorrhizas in the ferns of Lesotho. In: Gibby M, Johns RJ (eds) Camus JM. Pteridology in perspective. Royal Botanic Gardens, Kew, UK, pp. 621–631
Muthukumar T, Prabha K (2012) Fungal associations in gametophytes and young sporophytic roots of the fern Nephrolepis exaltata. Acta Bot Croat 71(1):139–146
Muthukumar T, Prabha K (2013) Arbuscular mycorrhizal and septate endophyte fungal associations in lycophytes and ferns of South India. Symbiosis 59(1):15–33
Muthukumar T, Udaiyan K (2000) Arbuscular mycorrhizas of plants growing in the western Ghats region, southern India. Mycorrhiza 9:297–313
Nielsen SL (1993) A comparison of aerial and submerged photosynthesis in some Danish amphibious plants. Aquat Bot 45(1):27–40
Ogura-Tsujita Y, Sakoda A, Ebihara A, Yukawa T, Imaichi R (2012) Arbuscular mycorrhiza formation in cordate gametophytes of two ferns, Angiopteris lygodiifolia and Osmunda japonica. J Plant Res 126:1–10
Page CN (2002) Ecological strategies in fern evolution: a neopteridological overview. Rev Paleobot Palynol 119(1):1–33
Parniske M (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbil 6:763–775
Pressel S, Ligrone R, Duckett JG (2008) The ascomycete Rhizoscyphus ericae elicits a range of host responses in the rhizoids of leafy liverworts: an experimental and cytological analysis. Fieldiana Bot 47:59–72
Pressel S, Bidartondo MI, Ligrone R, Duckett JG (2010) Fungal symbioses in bryophytes: new insights in the twenty first century. Phytotaxa 9:238–253
Pryer KM, Schuettpelz E, Wolf PG, Schneider H, Smith AR, Cranfill R (2004) Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. Am J Bot 91:1582–1598
Qiu YL, Li L, Wang B, Chen Z, Dombrovska O, Lee J, Kent L, Li R, Jobson RW, Hendry TA, Taylor DW (2007) A nonflowering land plant phylogeny inferred from nucleotide sequences of seven chloroplast, mitochondrial, and nuclear genes. Int J Plant Sci 168(5):691–708
Radhinka KP, Rodrigues BF (2007) Arbuscular mycorrhizae in association with aquatic and marshy plants in Goa, India. Aquat Bot 86:291–294
Ragupathy S, Mahadevan A (1993) Distribution of vesicular-arbuscular mycorrhizae in the plants and rhizosphere soils of the tropical plains, Tamil Nadu, India. Mycorrhiza 3:123–136
Raven JA, Osborne BA, Johnston AM (1985) Uptake of CO2 by aquatic vegetation. Plant Cell Environ 8(6):417–425
Remy W, Taylor TN, Hass H, Kerp H (1994) Four hundred-million-year-old vesicular arbuscular mycorrhizae. PNAS 91(25):11841–11184
Reyes-Jaramillo I, Camargo-Ricalde SL, Aquiahuatl-Ramos MDLÁ (2008) Mycorrhizal-like interaction between gametophytes and young sporophytes of the fern Dryopteris muenchii (Filicales) and its fungal endophyte. Rev Biol Trop 56:1101–1107
Rimington WR, Pressel S, Duckett JG, Bidartondo MI (2015) Fungal associations of basal vascular plants: reopening a closed book? New Phytol 205(4):1394–1398
Rossman AY, Palm ME (2006) Why are phytophthora and other oomycota not true fungi? Outlook Pest Manag 17:217
Rothfels CJ, Sundue MA, Schuettpelz E, Kato M, Larsson A, Kuo LY, Pryer KM (2012) A revised classification for eupolypod II ferns (Polypodiidae: Polypodiales). Taxon 61:515–533
Schmid E, Oberwinkler F (1993) Mycorrhiza-like interaction between the achlorophyllous gametophyte of Lycopodium clavatum L. And its fungal endophyte studied by light and electron-microscopy. New Phytol 124:69–81
Schmid E, Oberwinkler F (1994) Light and electron microscopy of the host– fungus interaction in the achlorophyllous gametophyte of Botrychium lunaria. Can J Bot 72:182–188
Schmid E, Oberwinkler F (1995) A light-and electron-microscopic study on a vesicular–arbuscular host-fungus interaction in gametophytes and young sporophytes of the Gleicheniaceae (Filicales). New Phytol 129:317–324
Schmid E, Oberwinkler F, Gomez LD (1995) Light and electron microscopy of a host-fungus interaction in the roots of some epiphytic ferns from Costa Rica. Can J Bot 73:991–996
Schneider H, Schuettpelz E, Pryer KM, Cranfill R, Magallón S, Lupia R (2004a) Ferns diversified in the shadow of angiosperms. Nature 428:553–557
Schneider H, Smith AR, Cranfill R, Hildebrand TE, Haufler CH, Ranker TA (2004b) Unraveling the phylogeny of polygrammoid ferns (Polypodiaceae and Grammitidaceae): exploring aspects of the diversification of epiphytic plants. Mol Phylogenet Evol 31:1041–1063
Schoch CL, Sung GH, López-Giráldez F, Townsend JP, Miadlikowska J, Hofstetter V, et al. (2009) The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst Biol 58:224–239
Schuettpelz E, Pryer KM (2007) Fern phylogeny inferred from 400 leptosporangiate species and three plastid genes. Taxon 56:1037–1050
Schuettpelz E, Pryer KM (2009) Evidence for a Cenozoic radiation of ferns in an angiosperm-dominated canopy. PNAS 106:11200–11205
Schuettpelz E, Schneider H, Huiet L, Windham MD, Pryer KM (2007) A molecular phylogeny of the fern family Pteridaceae: assessing overall relationships and the affinities of previously unsampled genera. Mol Phylogenet Evol 44:1172–1185
Schüßler A, Schwarzott D, Walker C (2001) A new fungal phylum, the glomeromycota: phylogeny and evolution. Mycol Res 105:1413–1421
Selosse MA, Setaro S, Glatard F, Richard F, Urcelay C, Weiß M (2007) Sebacinales are common mycorrhizal associates of Ericaceae. New Phytol 174:864–878
Selosse MA, Strullu-Derrien C, Martin FM, Kamoun S, Kenrick P (2015) Plants, fungi and oomycetes: a 400-million year affair that shapes the biosphere. New Phytol 206(2):501–506
Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG (2006) A classification for extant ferns. Taxon 55:705–731
Strullu-Derrien C, Strullu DG (2007) Mycorrhization of fossil and living plants. Comptes Rendus Palevol 6(6):483–494
Strullu-Derrien C, Kenrick P, Pressel S, Duckett JG, Rioult JP, Strullu DG (2014) Fungal associations in Horneophyton ligneri from the Rhynie Chert (c. 407 million year old) closely resemble those in extant lower land plants: novel insights into ancestral plant–fungus symbioses. New Phytol 203(3):964–979
Sudha K, Ammani K (2010) Arbuscular mycorrhizal fungi in medicinal plants in Thrissur district, Kerala. Mycorrhiza News 21:13–18
Sundue M, Testo W, Ranker TA (2015) Morphological innovation, ecological opportunity, and the radiation of a major vascular epiphyte lineage. Evolution: Online first. doi:10.1111/evo.12749
Taylor TN, Remy W, Hass H, Kerp H (1995) Fossil arbuscular mycorrhizae from the Early Devonian. Mycologia 87(4):560–573
Tian Q, Liu JK, Hyde KD, Wanasinghe DN, Boonmee S, Jayasiri SC, Luo ZL, Taylor JE, Phillips AJ, Bhat DJ, Li WJ (2015) Phylogenetic relationships and morphological reappraisal of Melanommataceae (Pleosporales). Fungal Divers 74:267–324
Tisserant B, Gianinazzi S, Gianinazzi-Pearson V (1996) Relationships between lateral root order, arbuscular mycorrhiza development, and the physiological state of the symbiotic fungus in Platanus acerifolia. Can J Bot 74:1947–1955
Tuomisto H, Poulsen AD, Moran RC (1998) Edaphic distribution of some species of the sern genus Adiantum in western Amazonia. Biotropica 30:392–399
Turnau K, Anielska T, Jurkiewicz A (2005) Mycothallic/mycorrhizal symbiosis of chlorophyllous gametophytes and sporophytes of a fern, Pellaea viridis (Forsk.) Prantl (Pellaeaceae, Pteridales). Mycorrhiza 15:121–128
van der Heijden MG, Martin FM, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205(4):1406–1423
Wäckers, E (1998) Untersuchungen zu Pilz-Wirtsinteraktionen in Wurzeln und Gametophyten von Pteridophyten. Ph.D. thesis, University of Tübingen, Germany. Published by the author; 156 pp
Wang B, Qiu YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363
Wang B, Yeun L, Xue JY, Liu Y, Ané JM, Qiu YL (2010) Presence of three mycorrhizal genes in the common ancestor of land plants suggests a key role of mycorrhizas in the colonisation of land by plants. New Phytol 186:514–525
Watkins JE Jr, Cardelús CL (2012) Ferns in an angiosperm world: cretaceous radiation into the epiphytic niche and diversification on the forest floor. Int J Plant Sci 173:695–710
Wei N, Bemmels JB, Dick CW (2014) The effects of read length, quality and quantity on microsatellite discovery and primer development: from Illumina to PacBio. Mol Ecol Res 14:953–965
Wijayawardene NN, Hyde KD, Wanasinghe DN, Papizadeh M, Goonasekara ID, Camporesi E, Bhat DJ, McKenzie EH, Phillips AJ, Diederich P, Tanaka K (2016) Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Divers 77:1–316
Wilcke W, Yasin S, Valarezo C, Zech W (2001) Nutrient budget of three microcatchments under tropical montane forest in Ecuador-preliminary results. Erde 132(1):61–92
Wilkinson DM (2001) Mycorrhizal evolution. Trends Ecol Evol 16:64–65
Willis A, Rodrigues BF, Harris PJC (2013) The ecology of arbuscular mycorrhizal fungi. Crit Rev Plant Sci 32:1–20
Winther JL, Friedman WE (2007a) Arbuscular mycorrhizal associations in Lycopodiaceae. New Phytol 177:790–801
Winther JL, Friedman WE (2007b) Arbuscular mycorrhizal symbionts in Botrychium (Ophioglossaceae). Am J Bot 94:1248–1255
Winther JL, Friedman WE (2009) Phylogenetic affinity of arbuscular mycorrhizal symbionts in Psilotum nudum. J Plant Res 122:485–496
Yukawa T, Ogura-Tsujita Y, Shefferson RP, Yokoyama J (2009) Mycorrhizal diversity in Apostasia (Orchidaceae) indicates the origin and evolution of orchid mycorrhiza. Am J Bot 96:1997–2009
Zhang Y, Guo LD, Liu RJ (2004) Arbuscular mycorrhizal fungi associated with common pteridophytes in Dujiangyan, Southwest China. Mycorrhiza 14:25–30
Zhao Z (2000) The arbuscular mycorrhizas of pteridophytes in Yunnan, Southwest China: evolutionary interpretations. Mycorrhiza 10:145–149
Zotz G (2013) The systematic distribution of vascular epiphytes–a critical update. Bot J Linn Soc 171:453–481
Zubek S, Piatek K, Naks P, Heise W, Wayda M, Mleczko P (2010) Fungal root endophyte colonization of fern and lycophyte species from the Celaque National Park in Honduras. Am Fern J 100:126–136
Acknowledgments
We thank Jürgen Kluge, Rayko Jonas, and Ramona Güdel for their participation in fieldwork and lab analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lehnert, M., Krug, M. & Kessler, M. A review of symbiotic fungal endophytes in lycophytes and ferns – a global phylogenetic and ecological perspective. Symbiosis 71, 77–89 (2017). https://doi.org/10.1007/s13199-016-0436-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-016-0436-5