Abstract
Gametophytes of Pellaea viridis that appeared spontaneously on the surface of substratum originating from an ultramafic area were found to form mycothallic symbiosis with arbuscular mycorrhizal fungi (AMF) under laboratory conditions. In gametophytes and sporophytes grown with Glomus tenue, abundant arbuscule formation was observed at both stages. In gametophytes, the fungus was found in the region where the rhizoids are initiated. If G. intraradices was added to the soil, the gametophytes were colonised mostly by G. tenue, and roots of sporophytes were colonised by G. intraradices. The presence of AM fungi in both gametophytes and sporophytes of P. viridis resulted in the development of larger leaf area and root length of the sporophyte. The analysis of gametophytes from the Botanical Garden in Krakow (Poland) showed that cordate gametophytes of Pteridales, namely Pellaea viridis (Pellaeaceae), Adiantum raddianum and A. formosum (Adiantaceae), were also mycothallic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mycorrhizal fungi have probably played a role in the evolution of land plants (Pirozynski and Malloch 1975). Mutualism in terrestrial, multicellular and parenchymatous plants seems to be ancestral and to have occurred during the Devonian era (Taylor et al. 1995; Stubblefield and Taylor 1988). A wide range of present Hepatophyta, Anthocerotophyta and Tracheophyta have been found to form mycothalli, mycorrhizomes and mycorrhizas (Read et al. 2000).
Pteridophytes are of ancient origin. Their life cycle involves two multicellular generations, the gametophyte and the sporophyte. Investigations on the occurrence of mycorrhiza in fern sporophytes (Janse 1897; Gallaud 1905; Rayner 1927; Burgeff 1938; Boullard 1957; Fontana 1959; Hepden 1960; Cooper 1976; Mishra et al. 1980; Iqbal et al. 1981; Laferière and Koske 1981; Berch and Kendrick 1982; Gemma and Koske 1990; Gemma et al. 1992; Zhi-wei 2000; Kottke 2002), indicate a considerable diversity of interactions, ranging from lack of symbiosis to facultative and obligate associations. Two fundamentally different growth forms exist among gametophytes of ferns and fern-allies: (1) green, epiterrestrial, short-lived forms commonly forming cordate lamellae, and (2) achlorophyllous, thick, fleshy, long-lived, subterranean forms. While the presence of fungal endophytes in the second group of fern gamethophytes (Bruchmann 1904; Boullard 1979; Peterson et al. 1981; Schmidt and Oberwinkler 1994) appears to be a common feature, the green photosynthetic gametophytes are generally considered to be fungus-free (Read et al. 2000). Endophytic fungi have been described in a few species of Marattiaceae, Gleicheniaceae and Osmundaceae (Campbell 1908; Bower 1923; Schmidt and Oberwinkler 1995), groups that are considered basal fern families (Rothwell 1999).
Recent years have brought increasing interest in the interactions between plants occurring on heavy-metal-rich substrata and associated soil microbiota (Leyval et al. 1997; Carlot et al. 2002; Jeffries et al. 2003). Pellaea viridis belongs to the heavy-metal-tolerant ferns (Wild 1968). The development of arbuscular mycorrhiza is often considered as a strategy that can alleviate the toxic effect on the plants of metal excess in soil, either directly by decreasing metal uptake or indirectly by improving mineral nutrition and alleviating drought stress (Smith and Read 1997).
The main objective of the present study was to investigate mycorrhizal associations of a fern that appeared abundantly on soil samples collected at the Agnes Mine, Mpumalanga Province in South Africa. In particular, we aimed at determining: (1) whether the gametophytes of Pellaea viridis are mycothallic, (2) whether mycorrhiza has any impact on sporophyte growth, and (3) whether the gametophytes host the same fungal species as the sporophytes. Mycorrhiza of gametophytes and sporophytes of a few fern taxa from greenhouses of the Botanical Garden of the Jagiellonian University in Krakow, Poland, were also examined for comparison.
Materials and methods
Soil samples were collected in February 2002 from sites in Agnes Mine, Mpumalanga Province, South Africa (Morrey et al. 1989, 1992). The soil, characterized by 1,070 mg kg−1 total Ni content, 300 mg kg−1 exchangeable Ni content (extracted in 0.02 M di-ammonium EDTA), and a mean pH value of 6.5, was mixed with granulated expanded clay (3:1) (Turnau and Mesjasz-Przybyłowicz 2003). In order to eliminate indigenous fungi, soil was pasteurised by heating twice at 80°C for 1 h separated by a 24 h cooling period (Morton 1990) and subsequently stabilised for 2 weeks (sprayed occasionally with distilled water) before use. Non-pasteurised soil contained crude inoculum consisting of spores, extraradical mycelium and pieces of plant roots. Pasteurised and non-pasteurised soil samples were deposited in 500 ml pots. The cultures were kept in sealed sunbags (Sigma, St. Louis, Mo.) at a light intensity ca. 78 μmol s−1 m−2 and a light regime of 12/12 h, and were watered according to Walker and Vestberg (1994). P. viridis gametophytes appeared spontaneously on both non-pasteurised and pasteurised soil samples. Randomly selected gametophytes from each pot (5–10 specimens per pot) were checked for mycorrhization. If no colonisation was detected, the pot was assumed to be devoid of arbuscular mycorrhizal fungi (AMF). Gametophytes originating from these pots were placed on the surface of substratum containing Glomus intraradices (BB-E-1-99; Biorize SARL, France, isolated from non-polluted soil) and/or Glomus tenue (UNIJAG. PL.18, Krakow, Poland, isolated from soil collected in Mpumalanga, Republic of South Africa,) propagules, in order to study the ability of these fungi to colonise both gametophytes and sporophytes.
Gametophytes used for the experiment on the growth response to mycorrhiza were carefully removed from pots that were previously confirmed to be AMF-free and planted in 200 ml pots filled with pasteurised soil. Gametophytes were divided into three groups. The first group (control) was left without inoculation and no AMF were found at the end of the experiment (G−S− plants). The second group was not inoculated but, due to failed sterilisation, mycorrhiza developed in sporophyte roots (G−S+ plants). The third group was inoculated with crude inoculum containing G. tenue (G+S+ plants). The presence/absence of AMF was confirmed on the basis of microscopic observations of remnants of the gametophytes that remained attached to the sporophyte for almost 2 months. Shoot and root samples for the estimation of shoot size and mycorrhizal parameters were collected after 3 months of growth; 5 control (non-mycorrhizal) plants and 10–15 mycorrhizal plants were used.
In total, almost 300 specimens of P. viridis were analysed, either at the point when the sporophyte had not yet developed or at different stages of sporophyte development, ranging in height from a few millimetres to 4 cm. To visualise arbuscular mycorrhiza, the plant material was carefully picked up from the substratum and, after the removal of the sporophyte shoots (if they were bigger than a few millimetres), prepared as follows. After careful washing in tap water, whole plants were softened in 10% KOH for 24 h at room temperature, washed in tap water, bleached in H2O2/NH3 (10:1) for a few minutes followed by washing in tap water, acidified in 5% lactic acid in water for 1 h, stained with 0.01% aniline blue in lactic acid for 24 h at room temperature and eventually stored in pure lactic acid. To avoid destruction of the fragile gametophytes, bleaching was applied only in cases when a well-developed root system was present. In the case of gametophytes, only the presence/absence of glomalean fungi was noted. In the case of sporophytes, standard mycorrhizal parameters: relative mycorrhizal root length (M%), intensity of colonisation within individual mycorrhizal roots (m%), relative arbuscular richness (A%), and arbuscule richness in root fragments where arbuscules were present (a%), were assessed according to Trouvelot et al. (1986), http://www.dijon.inra.fr/bbceipm/Mychintec/Mycocalc-prg/download.html, in whole roots placed on microscope slides without cutting, to enable measurements of whole root length. The length of the roots was measured separately for each plant using a binocular microscope and a computer image analysis system. Fern shoots/leaves were dried between filter paper sheets and scanned separately for each plant. The data were processed using Image-Pro Plus (ver. 4.0) software. Statistical data analysis was performed with the non-parametric Kruskal-Wallis and Mann-Whitney tests (P<0,05) using STATISTICA (ver. 5.0) software, while the correlation analysis was done with STATGRAPHICS (ver. 5.0).
For comparison, gametophytes and sporophytes of P. viridis (Forssk.) Prantl (Pteriditae, Pteridales, Pellaeaceae), Adiantum raddianum C. Presl, Adiantum formosum R. Br. (Pteriditae, Pteridales, Adiantaceae), Asplenium viviparum (L. f) C. Presl (Polypoditae, Aspleniales, Aspleniaceae) and Pteris cretica L. (Pteriditae, Pteridales, Pteridaceae) were collected from the Botanical Garden in Krakow. The presence of mycorrhizal fungi was checked as described above. The mycorrhizal parameters in sporophyte roots were not estimated.
Results
Mycorrhizal status of Pellaea viridis
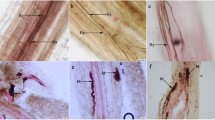
Most gametophytes of P. viridis that developed on the surface of ultramafic soil samples were mycothallic (Fig. 1A). Only a few samples were found to be devoid of mycorrhizal fungi; in others a fine endophyte, G. tenue, was detected (Fig. 1B, C, E). Fern gametophytes and roots of sporophytes were usually strongly colonised. Fine fungal mycelium with extraradical hyphae of 0.8–1.3 µm, hyphae developing within the gametophyte of 0.4–0.7 µm, often forming typical fan-shaped structures, and vesicles of 3–7 µm diameter were found. Arbuscules densely filling the gametophyte cells (Fig. 1E), strongly stained in aniline blue, were visible either in the basal part of the rhizoids or within cells of the cord-like gametophyte, where the rhizoids were initiated. The mycelium was also observed within the elongated part of the rhizoids (Fig. 1C). The fungus was spreading from one cell to another without the development of the intercellular phase. As G. tenue did not autofluoresce, it was possible to observe the symbiosis only after staining. G. tenue was the only fungus colonising gametophytes on the pasteurised substrata; on unheated substratum coarse mycorrhizal fungi were also present (Fig. 1D). The roots of sporophytes developing from mycothallic gametophytes were always mycorrhizal (Fig. 2A–C). The mycelium never colonised the sporophyte through the junction between the gametophyte and the sporophyte. The colonisation of roots was observed either by the mycelium originating from the rhizoids or from the extraradical hyphal net.
Gametophytes of Pellaea viridis. A Dorsal side of the gametophytes observed under binocular microscope. B Side view of the gametophyte (ga) and sporophyte (sp) with the endophyte (e) stained distinctly with aniline blue. C Gametophyte rhizoids (rh) colonised by Glomus tenue mycelium (e). D Rhizoids (rh) of gametophyte colonised by coarse mycorrhizal fungi (e). E Gametophyte (ga) colonised by G. tenue (e), which, however, do not invade the root (rt) of the sporophyte directly through the gametophyte-sporophyte junction
Mycorrhizal colonisation of P. viridis. A Dense formation of arbuscules by G. tenue within roots. B–C Formation of small vesicles (v) and arbuscules (a) by G. tenue. D P. viridis grown on Ni-rich soil: both gametophytes and sporophytes colonised by arbuscular mycorrhizal fungi (AMF) (G+S+), only sporophytes colonised by AMF (G−S+), and plants cultivated without AMF (G−S−)
In some samples, the fungus was found only in roots of the sporophytes, while the gametophytes that remained attached to the sporophyte root base for a few weeks had no visible signs of fungal colonisation.
G. intraradices, originating from pot cultures used to inoculate sterilised soil samples on which the fungus-free gametophytes were introduced, was found to colonise the gametophytes and the sporophyte roots. In cases where both G. intraradices and G. tenue were present, almost 90% gametophytes were colonised by G. tenue, while sporophyte roots hosted G. intraradices, colonising up to 90% of the root length and producing abundant arbuscules (A% up to 60%). When G. intraradices was forming arbuscules within cortical cells of the roots, G. tenue was present only in the form of mycelium, often with fan-like structures and small vesicles but no arbuscules of this fungus were found.
Cyanobacteria were present on the surface of gametophytes, mycothallic or not. Their threads were present mostly on the underside of the gametophytes, lining the grooves between cells.
Influence of mycorrhiza on plant growth
Observation of gametophytes that were transferred into sterilised substratum at a similar development stage showed that the fern is not obligatory mycorrhizal. Mycorrhizal plants (G+S+) had well branched roots, statistically longer than non-mycorrhizal plants (G−S+ and G−S−), and developed larger leaves (Figs. 22D, 3). G−S− plants were less green (yellowish) than G−S+ and G+S+ plants. The mycorrhizal colonisation of the roots that had developed from nonmycorrhizal gametophytes was much higher than if roots were already colonised at the gametophyte stage (Fig. 3C).
Growth and mycorrhizal parameters of P. viridis cultivated in the presence or absence of AMF. A Lateral root length. B Mean leaf area. C Intensity of colonisation within individual mycorrhizal roots (m%), relative mycorrhizal root length (M%), arbuscule richness in root fragments where the arbuscules were present (a%) and relative arbuscular richness (A%) of 3-month-old plants. G+S+ Both gametophytes and sporophytes colonised by AMF, G−S+ only sporophytes colonised by AMF, G−S− plants cultivated without AMF. Different letters above bars indicate statistically significant differences
Occurrence of mycorrhizal fungi in fern gametophytes and sporophytes collected in the Botanical Garden in Krakow
AMF colonisation was observed in both life stages of Adiantum raddianum (Fig. 4A), Adiantum formosum, and P. viridis. In gametophytes, coarse and fine fungal mycelium was found to form arbuscules within the region of rhizoid initiation (Fig. 4A–E). The coarse endophyte showed strong autofluorescence (Fig. 4B) upon excitation with UV light (455–490 nm wavelength). Only coarse mycelium was detected in the darkly pigmented sporophyte roots. Chloroplasts were present in the cells where arbuscules had developed, but the strength of chlorophyll autofluorescence was much lower than in non-colonised cells (Fig. 4C). Thread-like cyanobacteria were observed lining the grooves between cells of the gametophytes on the underside of the leaves, similarly to P. viridis. No AM fungi were found in the gametophytes and sporophytes of Asplenium viviparum and Pteris cretica.
Gametophyte of Adiantum raddianum. A Gametophyte with attached sporophyte (sp) and the endophyte (e) stained distinctly with aniline blue. B Endophyte (e) showing autofluorescence induced by UV light. C Weak autofluorescence of the chloroplasts (ch) within gametophyte cells colonised by AMF. D–E Fine endophyte (e) within the gametophyte stained with aniline blue
Discussion
In this investigation, Pellaea viridis spores germinated and sporophytes grew spontaneously enabling the estimation of the symbiosis at all stages of the fern’s life cycle.
To our knowledge, the presence of arbuscular colonisation of gametophytes of a member of Pteridales is reported for the first time. The ability to form arbuscular symbiosis by the gametophytes is probably not a rare event for the members of this genus and also not restricted to soils rich in Ni, as the same phenomenon was observed in gametophytes of a few other fern species growing in the greenhouses of the Botanical Garden in Krakow on typical horticultural substrata. P. viridis is a fern of Afro-Indian provenance, including the Mascarene and Comoro islands, Malagasy, Madagascar and Africa (from Yemen to Ethiopia, Sudan, tropical East Africa, Malawi, Mozambique, Zimbabwe and, in South Africa, from the Transvaal to the south-western Cape) (Jacobsen 1983). It is a widespread xeromorphic fern, common in the summer rainfall areas (Hancock and Lucas 1973). It is often found at the base of rocks and seems to be well adapted to exposed conditions at altitudes from sea level to about 1,900 m and an annual rainfall of 500 to over 1,200 mm (preferably 700–1,200 mm). It is common along wet forest edges, in dry or riverine forests, pine and eucalypt plantations, in montane and secondary grassland, on rocky hillsides among boulders, on cliff ledges and wet or dry sheetrock mats (Jacobsen 1983). It was recorded from nonmetaliferous soils and those rich in Cu (Wild 1968). Under all these conditions the formation of mycorrhiza might be of great importance. However, as shown in the present study, the development of the symbiosis between the gametophytes and the glomalean fungi is not obligatory, while the sporophyte, according to literature (Zhi-wei 2000) and as found in the present study, is usually colonised by a mycorrhizal fungus.
Preliminary data obtained in this study indicate that the presence of AM fungi at both the sporophyte and the gametophyte stage stimulates fern growth, although in order to draw definitive conclusions it would be necessary to obtain material enabling deliberate and controlled inoculation. The appearance of AM fungi at the gametophyte phase may significantly shorten the period when the small plants are especially susceptible to drought. As the nonmycorrhizal plants were always slightly yellowish, the fungi are probably also improving their nutritional status. This should be supported in the future by exact analysis of the plant material.
G. tenue was demonstrated to be a mycothallic symbiont. Although heat treatment of the soil is believed to eliminate most native fungi (Morton 1990), G. tenue belongs to the few species that are more resistant to heat stress (Warcup 1981; McGee 1989). This is one of the reasons why pasteurised soil samples used for culturing nonmycorrhizal plants should be carefully checked before conclusions are drawn. G. tenue is a common fungus in a wide range of soils (including agricultural and forest soils) and at a wide range of altitudes, often recorded in lowlands and in high mountain soils. Its presence in ultramafic soils has been already shown in Berkheya coddii roots, suggesting its tolerance to heavy metals (Turnau and Mesjasz-Przybyłowicz 2003). Several unpublished records are available from industrial wastes rich in Zn, Pb, Cd, As, Cu, and others (unpublished observations). Due to the small size of spores the species is difficult to isolate; thermal treatment of soil samples seems to be the only way to obtain it in pure culture. In natural stands or in culture conditions, a commonly observed feature is that G. tenue arbuscules are formed only in roots where there is no competition with other mycorrhizal fungi, and only the characteristic mycelium with the small vesicles is visible (unpublished observations). The interactions between coarse and fine endophytes have been investigated previously (Sainz et al. 1989; Wilson 1984; Powell 1979). The low competition ability might explain why this species is found in places such as industrial wastes, low pH forest soils or on tree stumps where ferns start to grow.
As discussed above, there are still many gaps in our knowledge concerning the occurrence and the role of mycorrhizal fungi in ferns. The sporophytes of e.g., tropical ferns were found to be able to form arbuscular, ericoid and orchid type of symbiosis (Cooper 1976; Kottke 2002). As shown here, gametophytes of ferns can form symbioses with the same fungi that are common symbionts of phanerogamic plants. At the same time the mycothalli strongly resemble the mycothalli of liverworts (Turnau et al. 1999) and mycothalli of the Gleicheniaceae family (Schmidt and Oberwinkler 1995). This would mean that the evolutionary trends were probably similar to those observed in vascular plants and in liverworts, where the most ancient was arbuscular symbiosis, later substituted by other types of symbiosis or even by losing the mycorrhizal association due to eutrophisation.
Before a clear picture is drawn, further research on this subject, including the relations between fungi and gametophytes is necessary on a broader range of fern species. As pointed out by Read et al. (2000) investigations on the function of mycorrhiza in this group of plants are also required.
References
Berch M, Kendrick B (1982) Vesicular-arbuscular mycorrhizae of Southern Ontario ferns and fern-allies. Mycologia 74:769–776
Boullard B (1957) La mycotrophie chez les ptéridophytes. Sa fréquence, ses caractères, sa signification. Botaniste 41:5-185
Boullard B (1979) Consideration sur la symbiose fongique chez les Pteridophytes. Syllogeus 19:1–59
Bower FO (1923) The ferns, vol 1. Cambridge University Press, Cambridge
Bruchmann H (1904) Űber das Prothallium und die Keimpflanze von Ophioglossum vulgatum L. Bot Zeitung 62:227–248
Burgeff H (1938) Mycorrhiza. In: Verdoon F (ed) Manual of Pteridology, vol 1. Nijhoff, The Hague, pp 159–191
Campbell DH (1908) Symbiosis in fern prothallia. Am Nat 42:154–165
Carlot M, Giacomini A, Casella S (2002) Aspects of plant-microbe interactions in heavy metal polluted soil. Acta Biotechnol 22:13–20
Cooper KM (1976) A field survey of mycorrhizas in New Zealand ferns. N Z J Bot 14:169–181
Fontana A (1959) Ricerche sulla simbiosi micorrhizica nelle loro radici. Allionia 5:27–66
Gallaud I (1905) Etudes sur les mycorrhizes endotrophs. Rev Gén Bot 17:66–83
Gemma JN, Koske RE (1990) Mycorrhizae in recent volcanic substrates in Hawai. Am J Bot 77:1193–1200
Gemma JN, Koske RE, Flynn T (1992) Mycorrhizae in Hawaiian pteridophytes: occurrence and evolutionary significance. Am J Bot 79:843–852
Hancock FD, Lucas A (1973) Ferns of the Witwatersrand. Witwatersrand University Press, Johannesburg
Hepden PM (1960) Studies in vesicular-arbuscular endophytes. II. Endophytes in the Pteridophyta, with special reference to leptosporangiate ferns. Trans Br Mycol Soc 43:559–570
Iqbal SH, Yousaf M, Younus M (1981) A field survey of mycorrhizal associations in ferns of Pakistan. New Phytol 87:69–79
Jacobsen WBG (1983) The ferns and fern allies of Southern Africa. Butterworths, Durban/Pretoria
Janse JM (1897) Les endophytes radicaux de quelques plantes javanaises. Ann Jard Bot Buitenzorg 14:53–212
Jeffries P, Gianinazzi S, Perotto S, Turnau K, Barea JM (2003) The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fertil Soils 37:1–16
Kottke I (2002) Mycorrhizae—rhizosphere determinants of plant communities. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half, 3rd edn. Dekker, New York, pp 919–932
Laferière JL, Koske RE (1981) Occurrence of VA-mycorrhizas in some Rhode Island Pteridophytes. Trans Br Mycol Soc 76:331–332
Leyval C, Turnau K, Haselwandter K (1997) Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological and applied aspects. Mycorrhiza 7:139–153
McGee PA (1989) Variation in propagule numbers of vesicular-arbuscular mycorrhizal fungi in a semi-arid soil. Mycol Res 92:28–33
Mishra RR, Sharma GD, Gatphoh AR (1980) Mycorrhizas of ferns of North Eastern India. Proc Indian Acad Sci 46:546–551
Morrey DR, Balkwill K, Balkwill M-J (1989) Studies on serpentine flora: preliminary analyses of soils and vegetation associated with serpentinite rock formations in the south-eastern. Transvaal Afr J Bot 55:171–177
Morrey DR, Balkwill K, Balkwill M-J, Wiliamson S (1992) A review of some studies of the serpentine flora of southern Africa. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soil. Intercept, Andover, Hampshire, pp 147–157
Morton JB (1990) INVAM policies. INVAM Newslett 1:1–8
Peterson RL, Howarth MJ, Whittier D (1981) Interactions between a fungal endophyte and gametophyte cells in Psilotum nudum. Can J Bot 59:711–720
Pirozynski KA, Malloch DW (1975) The origin of land plants: a matter of mycotrophism. Biosystems 6:153–164
Powell CL (1979) Inoculation of white clover and ryegrass seed with mycorrhizal fungi. New Phytol 83:81–85
Rayner MC (1927) Mycorrhiza. New Phytol 26:22–45
Read DJ, Duckett JG, Francis R, Ligrone R, Russell A (2000) Symbiotic fungal associations in ‘lower’ land plants. Phil Trans R Soc Lond B 355:815–831
Rothwell GW (1999) Fossils and ferns in the resolution of land plant phylogeny. Bot Rev 65:188–218
Sainz MJ, Vilarino A, Arines J (1989) Competition between Glomus tenue and some coarse fungi for colonizing red clover roots in acid soils. Agric Ecosyst Environ 29:337–340
Schmidt E, Oberwinkler F (1994) Light- and electron microscopy of the host-fungus interaction in the achlorophyllous gametophyte of Botrychium lunaria. Can J Bot 72:182–188
Schmidt E, Oberwinkler F (1995) A light- and electron-microscopic study on a vesicular-arbuscular host-fungus interaction in gametophytes and young sporophytes of the Gleicheniaceae (Filicales). New Phytol 129:317–324
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic Press, London
Stubblefield SP, Taylor TN (1988) Recent advances in palaeomycology. New Phytol 108:3–25
Taylor TN, Remy W, Hass H, Kerp H (1995) Fossil arbuscular mycorrhizae from the Early Devonian. Mycologia 87:560–573
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986) Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and genetical aspects of Mycorrhizae. INRA, Paris, pp 217–221
Turnau K, Mesjasz-Przybyłowicz J (2003) Arbuscular mycorrhiza occurrence in Berkheya coddii and other Ni-hyperaccumulating members of Asteraceae from ultramafic soils in South Africa. Mycorrhiza 13:185–190
Turnau K, Ronikier M, Unrug J (1999) Role of mycorrhizal links between plants in establishment of liverworts thalli in natural habitats. Acta Soc Bot Pol 68/1:63–68
Walker C, Vestberg M (1994) A simple and inexpensive method for producing and maintaining closed pot cultures of arbuscular mycorrhizal fungi. Agric Sci Finland 3:233–240
Warcup JH (1981) Effect of fire on the soil microflora and other non-vascular plants. In: Gill AM, Grove RH, Noble IR (eds) Fire and the Australian Biota. AAS, Canberra, pp 203–214
Wild H (1968) Geobotanical anomalies in Rhodesia. 1. The vegetation of copper-bearing soils. Kirkia 7:1–71
Wilson JM (1984) Competition for infection between vesicular-arbuscular mycorrhizal fungi. New Phytol 97:427–435
Zhi-wei Z (2000) The arbuscular mycorrhizas of pteridophytes in Yunnan, southwest China: evolutionary interpretations. Mycorrhiza 10:145–149
Acknowledgements
The present work was supported by the Jagiellonian University internal project no CRBW N-25/CRBW-VI-23/2003. The soil used in the experiments was generously supplied by Dr. J. Mesjasz-Przybyłowicz from iThemba Labs, South Africa. The equipment used was financed by the Foundation for Polish Science (FNP) REGLE 25/97 and SUBIN 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turnau, K., Anielska, T. & Jurkiewicz, A. Mycothallic/mycorrhizal symbiosis of chlorophyllous gametophytes and sporophytes of a fern, Pellaea viridis (Forsk.) Prantl (Pellaeaceae, Pteridales). Mycorrhiza 15, 121–128 (2005). https://doi.org/10.1007/s00572-004-0306-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-004-0306-5