Abstract
Betel leaves have deep roots in India's cultural heritage and have long been known for their use in traditional diets as a masticatory agent. These leaves contain beneficial compounds like antimicrobials, antioxidants, and anticancer properties. This study aims to investigate how betel leaf essential oil (BLEO) can act as a preservative for orange, grape, watermelon, and sugarcane juices. To make sure BLEO doesn't affect the taste, a sensory study was done using fuzzy logic analysis to determine the right concentration. Physicochemical and microbial properties in control and BLEO-treated juices were evaluated at weeks 0 and 2. Results showed that the addition of BLEO in fruit juices yielded a significant reduction in microbial populations, in total plate count and yeast and mold count. The decrease in free radical scavenging activity observed in BLEO-treated juices was comparatively less pronounced than in the control. GC–MS analysis has identified the bioactive compounds present in BLEO, revealing high concentrations of bioactive compounds. The observation of the study demonstrates the remarkable potential of BLEO to serve as a natural preservative in a diverse range of fruit juices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
People worldwide commonly consume fruit juices on a large scale. Currently, the primary way to keep these juices from spoiling involves using chemical preservatives like Sodium benzoate (E211), Sulfur dioxide (E220), and Potassium sorbate (E202). However, various reports suggest that using chemical preservatives can lead to allergic reactions, especially in sensitive individuals. For instance, preservatives like sulfur dioxide may trigger asthma symptoms in people with existing respiratory conditions. Additionally, consuming excessive amounts of chemical preservatives has been linked to stomach issues such as diarrhea and bloating (Pongsavee 2015). As a result, there is a growing need for natural preservatives to reduce the health risks linked with the use of chemical preservatives in the juice production industry.
The antimicrobial properties of essential oils (EOs), obtained through various extraction methods, have gained widespread recognition. This makes them valuable as preservatives or additives in various applications (Swamy et al. 2016). EOs are considered Generally Recognized as Safe (GRAS) substances as approved by the US Food and Drug Administration (FDA). The present research investigation aims to explore the natural abilities of Betel leaf essential oil (BLEO) as an efficient natural preservative for a diverse range of fruit juices. Betel leaves (Piper betle L.) are commonly known as Paan in many parts of India and belong to the Piperaceae family. The leaves possess a springy chewable texture and a pungent palatable taste, while the cured leaves offer improved taste, color (green to yellow), and other appealing organoleptic properties in addition to their stimulating and refreshing effects (Biswas et al. 2022; Toprani and Patel 2013).
The antibacterial and antifungal characteristics of BLEO (Betel Leaf Essential Oil), betel leaf extracts, and their isolates were examined in a study conducted by Nayaka et al. (2020). The research revealed that BLEO exhibited fungicidal effects against various pathogens such as Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, Candida albicans, Trichophyton rubrum, Microsporum canis, and more. Additionally, BLEO-based nanoemulsions demonstrated effectiveness against five strains of foodborne pathogens, highlighting their potential in combating microbial threats.
Basak (2018a) undertook a study to assess the preservative potential of BLEO in raw apple juice, employing an innovative fuzzy logic approach for sensory analysis. The outcomes unveiled the remarkable effectiveness of BLEO as a preservative, even at lower concentrations, significantly prolonging the shelf life of raw apple juice. Furthermore, BLEO-treated tomato paste samples were deemed highly acceptable in terms of sensory qualities and substantial extension in the shelf life of the samples (Basak 2018b). Similarly, Madhumita et al. (2021) conducted a comprehensive study on pasteurized sapodilla juice, investigating the effects of incorporating BLEO at varying concentrations. The research tracked sapodilla juice's physicochemical properties, demonstrating BLEO's significant efficacy in prolonging shelf life. However, there's a lack of information on BLEO's preservation effects on orange, grape, watermelon, and sugarcane juices. To address this gap, our study investigates BLEO's preservative impact on these juices, thoroughly examining physicochemical changes, microbial activity, and sensory analysis during storage.
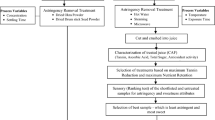
Materials and methods
Sample collection
The Betel leaf, sugarcane juice, watermelon, orange, and grape samples utilized in this study were procured from a local market in Thanjavur. All the chemicals and media employed were of analytical grade and sourced from Hi Media Laboratories Pvt. Ltd. in Mumbai, India, via local suppliers. The reagents were freshly prepared prior to use.
Extraction of betel leaf essential oil
The extraction of the essential oil was conducted using the Hydrodistillation process with the aid of the renowned Clevenger's apparatus. The established methodology outlined by Jadhav et al. (2020) was meticulously adhered to, involving a series of steps. Firstly, the betel leaves were subjected to a thorough cleansing and dried in a regulated hot air oven, set at an optimal temperature range of 80 to 90 ℃ for 30 to 40 min in 200 g batches, ensuring the preservation of their botanical constituents. Once desiccated to perfection, the leaves were shredded into finely fragmented pieces. These shredded leaves were then carefully introduced into a round bottom flask (RBF), and distilled water in a ratio of 1:5 was added. The entire setup was positioned over a heating mantle and subjected to a controlled heating regimen for a duration of three hours. The essential oil isolated on Clevenger's arm was collected and stored under controlled conditions, maintained at a temperature of 4 ℃, for future analysis.
Preparation of fruit juice
Juices from orange, grape, and watermelon fruits were extracted using a blender and filtered through a muslin cloth. Juices were diluted with pure drinking water in a ratio of 1:1. The vials were completely sterilized in hot water and 10 mL of juices from each fruit were filled in each vial under aseptic conditions. To explore the potential influence of Betel Leaf Essential Oil (BLEO) on the fruit juices, varying concentrations of 0.3 μL/mL, 0.6 μL/mL, and 0.9 μL/mL were carefully added to the corresponding vials containing the fruit juices. Furthermore, a separate set of control samples for each fruit juice without using BLEO was also kept. This was done to compare and analyze the results. The samples were stored at 4 °C in a refrigerator.
Sensory analysis by fuzzy logic
Sensory evaluation of the juice samples incorporated with betel leaf essential oil was carried out using fuzzy logic analysis described by Jaya and Das (2003). The sensory assessment involved the participation of 10 untrained panelists, who provided their evaluations for varying concentrations of individual fruit juice samples, including a control, at hourly intervals to prevent sensory fatigue. After identifying the BLEO concentrations that were well-received by the panelists, analysis was done for two weeks. It's important to note that the control juices didn't have BLEO, while the sample juices had BLEO in approved concentrations.
Physicochemical analysis
pH, total soluble solids (TSS), and titratable acidity (TA)
The pH of the sample was measured by pH meter (Model: FP20, Mettler Toledo, Greifensee, Switzerland) and TSS was measured using a refractometer (Labart, EHR503), and data was reported as °Brix. TA was determined using the methodology provided by the Food Safety and Standards Authority of India (FSSAI). An aliquot ranging from 1 to 5 g of the test sample was diluted at a ratio of 1:10 with distilled water within a conical flask and a phenolphthalein indicator was added. Titration against a standardized 0.1N sodium hydroxide (NaOH) solution was carried out. The appearance of a distinctive pink hue signified the attainment of the endpoint. Thereafter, the titratable acidity was precisely determined using Eq. 1, as provided below (Eq. 1).
where N = Normality of titrant (mEq/mL); V1 = Volume of Titrant (mL); Eq.wt = Equivalent weight of citric acid (64 mg/mEq); V2 = Volume of Sample (mL).
Ascorbic acid (AA)
Quantification of ascorbic acid content within the experimental samples was done using the AOAC (Association of Official Agricultural Chemists) method specifically AOAC 961.27. This method utilizes a titrimetric technique in conjunction with the 2,6-dichlorophenol indophenol (DCPIP) dye. Multiple spectrophotometric experiments were conducted on the fruit juice samples to ensure accuracy and reliability of the results. The quantification of ascorbic acid was expressed as milligrams per 100 ml of the juice. The calculation procedure employed for this determination is outlined as follows:
Total phenolic content (TPC)
Singleton et al. (1999) method with slight modifications was followed for the determination of Total Phenolic Content. A standard graph was plotted with various concentrations of gallic acid ranging from 10 to 60 µg/mL, with each concentration diluted to 3 mL using distilled water. Subsequently, 0.5 mL of Folin Ciocalteu Reagent was added to each test tube containing the different gallic acid concentrations. The test tubes were then incubated at room temperature for a duration of 30 min. The absorbance readings of the gallic acid were recorded using a UV Spectrophotometer set at 650 nm. A similar procedure was followed for the evaluation of the fruit juice samples and the controls. For analysis, 0.1 mL of the fruit juice samples and the controls were used. The concentration of phenolic compounds in the juices was determined by using the equation derived from the standard graph. The TPC values were expressed as milligrams of Gallic Acid Equivalent (mg GAE) per 100 mL of the fruit juice samples.
Total antioxidant capacity (TAC)
The determination of total antioxidant activity involved the utilization of a technique based on the inhibition of DPPH (2,2-diphenyl-1-picrylhydrazyl) (Sigma-Aldrich, USA) (Konwar and Kardong 2019). Initially, a mixture was prepared by combining 2.9 mL of a 0.1 mM DPPH solution (prepared in methanol) with 0.1 mL of the fruit juice samples and controls. The mixture was vortexed and allowed to incubate at room temperature in darkness for a duration of 30 min. The absorbance was measured at a wavelength of 517 nm using a UV–VIS spectrophotometer. The % Free Radical Scavenging Activity (FRSA) was calculated by the Eq. 3.
where
Abs control = Absorbance of control; Abs sample = Absorbance of sample.
Microbial study
The total plate (bacterial) count (TPC) and total yeast and moulds count of fruit juice samples were analyzed by the pure culture technique (spread plate technique—0.1 ml sample was added into the respective agar plates). The Plate Count Agar and Potato Dextrose Agar were used for the bacterial and fungal counts, respectively. The bacterial sample plates were incubated at 35 ± 1 ̊ C whereas fungal plates were incubated at 28 ̊C for 24–48 h. All the trials were performed in triplicate. After incubation, the bacterial and fungal loads were calculated, and the mean value was reported (Chavan Priyanka Yashwant et al. 2022).
GC–MS analysis of BLEO
To analyze the active compounds, present in the BLEO, the following procedure was carried out. An aliquot of the extracted essential oil, specifically 1 μL, was injected into the GC–MS system as per manufacturers instruction. The GC–MS system used for analysis was Agilent's 8890GC/5977B GC/MSD equipment, equipped with an Rtx-5MS column (5% Diphenyl / 95% Dimethyl polysiloxane) measuring 30 m × 0.25 mm ID × 0.25 mm df. The GC running time ranged between 40 and 50 min. Finally, the resulting chromatograms and mass spectra were analyzed to determine the composition of the BLEO.
Statistical analysis
All values were obtained in triplicate and expressed as mean ± standard deviation (SD). The significant differences between mean values of samples were determined by Student’s t test at a significance level of p < 0.05. Statistical analysis was conducted using GraphPad PRISM® program.
Result and discussion
Sensory analysis by fuzzy logic
The sensory evaluation for fruit juices (orange, grape, watermelon, and sugarcane) added with BLEO were analyzed with fuzzy logic as mentioned by Jaya and Das (2003). The data related to the ranks for each juice is given in Online Resource 1.
In orange and sugarcane juices, the control was preferred over the samples added with BLEO indicating the strong dominance of the flavor of BLEO in the orange and sugarcane juices. S2 was preferred in grape juice over control, S3 and S4, and the presence of small amount of BLEO in grape juice was acceptable to the participants. For watermelon juice, adding 0.6μL/mL of BLEO was acceptable, as observed in sensory studies. The results of the sensory evaluation were similar to the results obtained by Basak (2018a) in the sensory evaluation of raw apple juice fortified with BLEO where the control yielded overall preference followed by the samples with the least amount of BLEO. As mentioned by Madhumita et al. (2021) dealing with the use of BLEO as a natural preservative for sapodilla juice with a hedonic rating, the samples with higher concentrations of essential oil were not preferred by the panelists. Basak (2018b) also revealed that the acceptability of tomato paste decreased as the concentration of BLEO increased. Other studies with essential oil from different sources added to fruit juices yielded similar results. Almeida et al. (2019) studied the addition of Mentha piperita essential oil (MPEO) to cashew, guava, mango, and pineapple juices. The sensory scores for taste and aftertaste were very low for the samples added with 1.85µL/mL of MPEO when compared to the control juice. Based on the sensory results, the samples (S1, S2, S3) with highest ranking in each fruit juice was chosen for further analysis, which was 0.3μL/mL of BLEO concentration in the case of grape, orange, and sugarcane juices and 0.6μL/mL of BLEO concentration in case of watermelon juice. In subsequent sections, the fruit juice samples and controls for further analysis are labeled OC (Orange Control), OS (Orange Sample—0.3μL/mL BLEO), GC (Grape Control), GS (Grape Sample—0.3μL/mL BLEO), WC (Watermelon Control), WS (Watermelon Sample—0.6μL/mL BLEO), SC (Sugarcane Control), and SS (Sugarcane Sample—0.3μL/mL BLEO). All the fruit juice controls and samples were held in sterilized vials at 4 °C in a refrigerator and studies were carried out at room temperature.
Change in physicochemical properties
Changes in pH
The pH of the fruit juices is an indication of the acidity of the juices which is an important parameter due to its influences in the taste of the fruit juices, its flavor profile, and perceived sweetness. The change in pH is denoted in Fig. 1. The pH of the control and sample decreased from Week 0 to Week 2. A slight reduction in pH of OC, OS, GC, GS, SC, and SS was observed in Week 2 while in WC and WS, a significant reduction in pH was observed compared to Week 0 samples. The reduction in the pH from Week 0 to Week 2 might be due to the conversion of sugars such as fructose, glucose, and other sugars into acid during the storage period. This conversion happens due to enzymatic reactions (Viuda-Martos et al. 2011).
Change in pH in untreated and treated (with BLEO) juices for Week 0 and Week 2 at 4° C storage. *—untreated juice 0th week vs untreated juice 2th week, #—treated juice 0th week vs treated juice 2th week, @—untreated juice 2th week vs treated juice 2th week, *, # and @ symbols over the bars represent statistically significant differences (p < 0.05) between the means of each group as determined by unpaired student’s t test. ns—non-significant between the experimental groups. Values are mean ± SD (n = 3)
Changes in titratable acidity (TA)
TA serves as a crucial parameter for assessing the overall acid content in food products. In Fig. 2, the change in TA is presented for both the control and samples. By the end of the experiment, the titratable acidity of each fruit juice had increased, resulting in an elevation of acid content and a decrease in pH. OC and GC exhibited higher values at the 2nd week compared to the 0th week. Specifically, OC displayed a noteworthy increase of 58.781%, while GC demonstrated a rise of 27.553% in titratable acidity at the 2nd week. Conversely, OS and GS had relatively lower increases in titratable acidity compared to OC and GC, with values of 26.406% and 11.657% respectively at the 2nd week. Regarding watermelon and sugarcane, both the control and samples exhibited increases in titratable acidity. WC experienced a rise of 17.012%, while WS had a slightly higher increase of 22.439% by the end of the 2nd week. Similarly, SC displayed a significant rise of 446.32%, while SS exhibited a comparable increase of 441.578%. The study by Yadav and Chakravarty (2013) also found the increase in TA during storage for different fruit juices which was attributed to the reason of microbial growth. Consequently, the inclusion of BLEO in orange and grape juice samples contributed to better resistance against changes in titratable acidity, potentially due to its ability to control microbial growth in OS and GS.
Change in Titratable Acidity (TA) in untreated and treated (with BLEO) juices for Week 0 and Week 2 at 4° C storage. *—untreated juice 0th week vs untreated juice 2th week, #—treated juice 0th week vs treated juice 2th week, @—untreated juice 2th week vs treated juice 2th week, *, # and @ symbols over the bars represent statistically significant differences (p < 0.05) between the means of each group as determined by unpaired student’s t test. ns—non-significant between the experimental groups. Values are mean ± SD (n = 3)
Change in total soluble solids
Total Soluble Solids (TSS) levels in juice indicate the concentration of dissolved solids, primarily sugars. The TSS content of both untreated and treated samples exhibited decreases from the 0th Week to the 2nd Week, as depicted in Fig. 3. A slight increase in TSS was observed for juice treated with BLEO compared to the untreated sample. However, the increase in TSS in the treated juice samples was not significant. This finding aligns with the results reported by Anaya-Esparza et al. (2017) and Jerry and Bright (2019), where no significant changes in total soluble solids were observed at 4 °C in fruit juices. Bhardwaj and Pandey (2011) have emphasized that the slight increase in TSS of fruit juices during storage was intended to preserve quality. The microorganisms utilize the monosaccharides to produce acids, which further promote their growth and proliferation in untreated juice (Singh and Sharma 2017). It is evident that a correlation was observed between pH, titratable acidity, and TSS during the 2-week storage of fruit juices.
Change in Total Soluble Solids (TSS) in untreated and treated (with BLEO) juices for Week 0 and Week 2 at 4° C storage. *—untreated juice 0th week vs untreated juice 2th week, #—treated juice 0th week vs treated juice 2th week, @—untreated juice 2th week vs treated juice 2th week, *, # and @ symbols over the bars represent statistically significant differences (p < 0.05) between the means of each group as determined by unpaired student’s t test. ns—non-significant between the experimental groups. Values are mean ± SD (n = 3)
Change in ascorbic acid (AA) content
Ascorbic acid is highly sensitive to light and temperature, leading to degradation over time. Figure 4 presents the quantification of ascorbic acid (AA) content over time. A remarkable alteration was observed in the AA levels of the treated juice samples during storage. Throughout the storage period, both the sample and control fruit juice groups exhibited a decline in AA content. Faizi and Mahen (2020) found a decrease in ascorbic acid in a cultivar of Mandarin juice, possibly due to oxidation and enzymatic conversion. Storage, preservatives, packaging air, and temperature were identified as influencing factors. For the controls, the degradation of ascorbic acid was measured as 13.52% (OC), 15.782% (GC), 47.947% (WC), and 36.066% (SC), while for the samples, the degradation values were determined as 5.956% (OS), 3.773% (GS), 39.929% (WS), and 30.421% (SS) after storage. The results clearly indicate that the addition of BLEO in fruit juices preserved the AA for considerable amount of time which sustained the activity of AA when compared the controls. This sustained activity was particularly significant in the grape and watermelon groups even after a storage period of two weeks, preventing the loss of ascorbic acid thereby inhibiting enzymatic browning. These findings align with the previous research conducted by Madhumita et al. (2021) in sapodilla juice.
Change in ascorbic acid in untreated and treated (with BLEO) juices for Week 0 and Week 2 at 4° C storage. *—untreated juice 0th week vs untreated juice 2th week, #—treated juice 0th week vs treated juice 2th week, @—untreated juice 2th week vs treated juice 2th week, *, # and @ symbols over the bars represent statistically significant differences (p < 0.05) between the means of each group as determined by unpaired student’s t test. ns—non-significant between the experimental groups. Values are mean ± SD (n = 3)
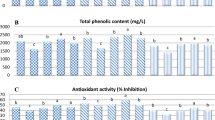
Change in total phenolic content (TPC)
The changes in the TPC were provided in Fig. 5. The TPC value of orange juice was increased in 2nd Week of the storage period. A 12.651% increase in TPC was observed in the OC while in the OS, a raise of 16.105% was observed. Similarly, in sugarcane juice, TPC values increased from Week 0 to Week 2. 14.696% and 23.882% of raise in TPC values were observed in the SC and SS respectively. In grape and watermelon fruit juices control and samples exhibit a remarkable reduction in TPC values after 2 weeks. This decrease in these samples might also be due to reactions such as oxidation and polymerization of phenolic compounds with the proteins present in the fruit juices and degradation of the phenolic compounds in the watermelon (Cao et al. 2012). In the grape juice control, a noticeable decline of 2.81% in TPC was observed from the initial week to the second week. Similarly, the grape juice sample exhibited a comparable reduction of 14.568% in TPC. However, both reductions, were determined to be statistically non-significant. Likewise, a decrease in TPC value was observed in watermelon juice sample and control. The WC displayed a substantial drop of 56.613% in TPC, whereas the WS exhibited a decline of 23.145%. This phenomenon could potentially be attributed to the degradation of phenolic compounds within the watermelon. During the storage period, alterations in the TPC value of fruit juices can be ascribed to various factors, including the type of processing employed, storage temperature conditions, enzymatic activities, microbial proliferation, occurrence of the Maillard reaction, and degradation of amino acids (Castro-López et al. 2016). The lower value in TPC during the storage in grape and watermelon samples and controls could be attributed to the gradual degradation of phenolic compounds.
Change in total phenolic contents (TPC) in untreated and treated (with BLEO) juices for Week 0 and Week 2 at 4° C storage. *—untreated juice 0th week vs untreated juice 2th week, #—treated juice 0th week vs treated juice 2th week, @—untreated juice 2th week vs treated juice 2th week, *, # and @ symbols over the bars represent statistically significant differences (p < 0.05) between the means of each group as determined by unpaired student’s t test. ns—non-significant between the experimental groups. Values are mean ± SD (n = 3)
Change in total antioxidant capacity (TAOC)
The antioxidant capacity of fruit juices is reliant upon the quantity and composition of bioactive compounds present in each specific fruit (Singh and Sharma 2017). Figure 6 presents the changes in Total Antioxidant Capacity (TAOC) over time. In control and sample of fruit juices, a decline in antioxidant activity was observed after a two-week period of refrigerated storage. This decline in antioxidant activity within fruit juices suggests the possibility of antioxidant antagonism, where various bioactive molecules in the juices engage in interactions that lead to the reduction of antioxidant activity (Zielinski et al. 2014).
Change in total antioxidant capacity (TAOC) in untreated and treated (with BLEO) juices for Week 0 and Week 2 at 4° C storage. *—untreated juice 0th week vs untreated juice 2th week, #—treated juice 0th week vs treated juice 2th week, @—untreated juice 2th week vs treated juice 2th week, *, # and @ symbols over the bars represent statistically significant differences (p < 0.05) between the means of each group as determined by unpaired student’s t test. ns—non-significant between the experimental groups. Values are mean ± SD (n = 3)
Upon comparing the TAOC values of control and sample fruit juices, it became evident that the samples with BLEO displayed significantly higher TAOC values after storage. For controls, the decrease in TAOC was recorded as 67.021% (OC), 35.955% (GC), 39.622% (WC), and 73.217% (SC), while for samples, the decrease in TAOC was observed as 36.483% (OS), 16.391% (GS), 9.838% (WS), and 59.499% (SS). The enhanced TAOC in the samples can be attributed to the addition of BLEO. Study by Arambewela et al. (2006) highlighted that BLEO exhibited a higher percentage of free radical scavenging activity compared to the synthetic antioxidant Butylated Hydroxy Toluene (BHT). Consequently, the rise in TAOC observed in the samples, in comparison to the controls, could be attributed to the combined contributions of various terpene, terpenoid molecules, and phenolic compounds present in the essential oil of betel leaf, alongside natural antioxidants.
Change in microbial count
The total plate count and yeast and mould count of the untreated and treated sample of each fruit juice were enumerated in Week 0 and Week 2. The number of colonies observed in total plate count and in yeast and mould count is given as additional data in Online Resource 2. As the fruit juices were not subjected to any thermal treatment prior to the study, the initial microbial count was higher even in the 105 and 106 dilutions in all four juices. The total plate count and yeast and mould count in the samples fruit juices were less compared to the control fruit juices in both 0th and 2nd weeks. This might be due to the reduction in pH which would hinder the growth of specific microbes. Kaddumukasa et al. (2017) states that the total plate count in fruit juices is influenced by various factors such as temperature, pH, and light exposure.
In the assessment of yeast and mould population at both Week 0 and Week 2, a declining pattern was observed across all samples treated with BLEO in contrast to the controls. At Week 0, the yeast and mould count in all BLEO treated samples exhibited a notable twofold to threefold reduction when compared to controls, and this pattern persisted through Week 2. Remarkably, in Week 2, the growth of yeast and mould was nearly suppressed at a dilution level of 104. Similar outcomes regarding the decrease in microbial count were observed in previous studies concerning apple juice (Basak 2018a) and sapodilla juice (Madhumita et al. 2021) subjected to BLEO treatment. The profound antimicrobial activity exhibited by the bioactive compounds present in BLEO is accountable for the discernible reduction in both total plate count and yeast and mould population. Although BLEO was added in lower amounts for consumer approval in sensory evaluations, there was a clear and substantial reduction in microbial count in fruit juices with BLEO.
GC–MS analysis of BLEO
GC–MS is a powerful tool for identifying bioactive compounds present in BLEO. The GC–MS Profile of the BLEO is given in Online Resource 3.
A total of 52 bioactive compounds were identified in GC–MS, out of which Safrole and 3-Allyl- 6-methoxy phenol (Chavibetol) were the compounds that were present predominantly in the BLEO. 16 compounds were present in percentages varying from 1 to 9 and other compounds present were lower than 1% in the BLEO. The GC–MS analysis revealed the presence of organic compounds belonging to the class of phenylpropanoid, sesquiterpene, sesquiterpenoid, and diterpene alcohol, etc. Most of the compounds possess antimicrobial and antioxidant, which explains the reduction in the total bacterial count and total fungal count observed in the juice supplemented with BLEO. Phytol is an acyclic diterpene alcohol and a constituent of chlorophyll is found in BLEO at a concentration of 2.37%, which is a precusor form of Vitamin E and Vitamin K is shown to have higher antioxidant properties (Santos et al. 2013). Interestingly, the GC–MS analysis revealed the presence of folic acid (0.12%) and 9,12,15- Octadecatrienoic acid (0.22%) in the BLEO. These compounds exert a strong antimicrobial activity which might have a synergistic effect along with the terpenes to inhibit microbial growth in the juices. According to a systematic review and meta-analysis conducted by Fu et al. (2021), there is evidence suggesting that folate intake may be linked to a reduced likelihood of developing pancreatic cancer. The BLEO also revealed the presence of various saturated fatty acids such as Hexadecanoic acid which is anti-microbial and anti-inflammatory in nature (Aparna et al. 2012) and other fatty acids such as Tetradecanoic acid, and Dodecanoic acid. All the bioactive compounds present in varying percentages in the BLEO provided the culminating antimicrobial effect in the fruit juice.
Conclusion
Adding BLEO to fruit juices at lower concentrations, determined by sensory analysis, effectively prevented microbial growth in the juices studied. The reduction in microbial count is attributed to the terpene group of bioactive compounds and essential fatty acids identified through GC–MS analysis of BLEO. Additionally, BLEO-treated juices maintained superior free radical scavenging capacity during storage. Based on robust data, it is confidently concluded that BLEO significantly mitigates microbial growth in all four tested fruit juices after storage, offering implications for extending shelf life. This highlights BLEO as a natural and eco-friendly alternative to conventional synthetic preservatives in the juice industry. The study also suggests essential oils, like BLEO, may have unexplored pleiotropic functions. Future research should further investigate BLEO's multifaceted impacts, considering potential interactions with various constituents and stability under different storage conditions, to establish its role as a reliable natural preservative for fruit juices.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Code availability
Not applicable.
References
Anaya-Esparza LM, Méndez-Robles MD, Sayago-Ayerdi SG, García Magaña ML, Ramírez-Mares MV, Sánchez-Burgos JA, Montalvo González E (2017) Effect of thermosonication on pathogenic bacteria, quality attributes and stability of soursop nectar during cold storage. J Food 15(4):1–9
Aparna V, Dileep KV, Mandal PK, Karthe P, Sadasivan C, Haridas M (2012) Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem Biol Drug Des 80(3):434–439
Arambewela L, Arawwawala M, Rajapaksa D (2006) Piper betle: a potential natural antioxidant. Int J Food Sci Technol 41(SUPPL. 1):10–14. https://doi.org/10.1111/j.1365-2621.2006.01227.x
Basak S (2018a) a) The use of fuzzy logic to determine the concentration of betel leaf essential oil and its potency as a juice preservative. Food Chem 240:1113–1120. https://doi.org/10.1016/j.foodchem.2017.08.047
Basak S (2018b) Shelf life extension of tomato paste through organoleptically acceptable concentration of betel leaf essential oil under accelerated storage. Environment J Food Sci 83(5):1396–1403. https://doi.org/10.1111/1750-3841.14133
Bhardwaj RJ, Pandey S (2011) Juice blends-a way of utilization of under-utilized fruits, vegetables, and spices: a review. Crit Rev Food Sci Nutr 51:563–570
Biswas P, Anand U, Saha SC, Kant N, Mishra T, Masih H, Bar A, Pandey DK, Jha NK, Majumder M, Das N, Gadekar VS, Shekhawat MS, Kumar M, Radha Proćków J, de la Lastra JMP, Dey A (2022) Betelvine (Piper betle L.): A comprehensive insight into its ethnopharmacology, phytochemistry, and pharmacological, biomedical and therapeutic attributes. J Cell Mol Med 26(11):3083–3119. https://doi.org/10.1111/jcmm.17323
Cao X, Bi X, Huang W, Wu J, Hu X, Liao X (2012) Changes of quality of high hydrostatic pressure processed cloudy and clear strawberry juices during storage. IFSET 16:181–190. https://doi.org/10.1016/j.ifset.2012.05.008
Castro-López C, Sánchez-Alejo EJ, Saucedo-Pompa S, Rojas R, Aranda-Ruiz J, Martínez-Avila GCG (2016) Fluctuations in phenolic content, ascorbic acid and total carotenoids and antioxidant activity of fruit beverages during storage. Heliyon 2(9):e00152. https://doi.org/10.1016/j.heliyon.2016.e00152
de Santos CC, M P, Salvadori M S, Mota V G, Costa L M, de Almeida A A C, de Oliveira G A L, Costa J P, de Sousa D P, de Freitas R M, de Almeida R N, (2013) Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. J Neurosci 2013:1–9. https://doi.org/10.1155/2013/949452
Faizi ZA, Mahen MY (2020) Effect of storage conditions, preservative levels and packing materials on ascorbic acid juice of mandarin cv. Nagpur Santra Turkish JAF Sci Tech 2(2):38–41
Fu H, Zeng J, Liu C, Gu Y, Zou Y, Chang H (2021) Folate intake and risk of pancreatic cancer: a systematic review and updated meta-analysis of epidemiological studies. Dig Dis Sci 66:2368–2379. https://doi.org/10.1007/s10620-020-06525-7
Jadhav NL, Garule PA, Pinjari DV (2020) Comparative study of ultrasound pretreatment method with conventional hydrodistillation method for extraction of essential oil from Piper betle L (Paan). Indian Chem. Eng. 64(2):132–140. https://doi.org/10.1080/00194506.2020.1828193
Jaya S, Das H (2003) Sensory evaluation of mango drinks using fuzzy logic. J Sens Stud 18(2):163–176
Jerry AA, Bright Q (2019) Effect of storage temperature on the physicochemical, nutritional and microbiological quality of pasteurised soursop (Annona muricata L.) Juice. Afr. J. Food Sci. 13(2):38–47. https://doi.org/10.5897/AJFS2018.1767
Kaddumukasa PP, Imathiu SM, Mathara JM, Nakavuma JL (2017) Influence of physicochemical parameters on storage stability: Microbiological quality of fresh unpasteurized fruit juices. Food Sci Nutr 5(6):1098–1105. https://doi.org/10.1002/fsn3.500
Konwar, Kardong (2019) Assessment of antioxidant activity in the fresh fruit juice and the brew fermented from Syzygium cumini (L) fruit. Curr Trends Pharmaceut Res 7(1). www.dibru.ac.in/ctpr
Latika Y, Archana C (2013) Effect of keeping time period on acidity of fruit juices and determination of fungal growth in fruit juices. Asian J Home Sci 8:166–169
Madhumita M, Guha P, Nag A, Prabhakar PK (2021) Natural preservative efficacy of cured betel leaf essential oil for sapota juice: Effect on physicochemical, microbial, and sensory properties. J Food Process Preserv. https://doi.org/10.1111/jfpp.15927
Nayaka NM, Sasadara MM, Sanjaya DA, Yuda PE, Dewi NL, Cahyaningsih E, Hartati, (2020) Piper betle (L.) recent review of antibacterial and antifungal properties, safety profiles, and commercial applications. Molecules 26(8):2321
Pongsavee M (2015) Effect of sodium benzoate preservative on micronucleus induction, chromosome break, and Ala40Thr superoxide dismutase gene mutation in lymphocytes. Biomed Res Int 2015:103512. https://doi.org/10.1155/2015/103512
Singh SK, Sharma M (2017) Review on biochemical changes associated with storage of fruit juice. Int J Curr Microbiol Appl Sci 6(8):236–245
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth Enzymol Academic Press 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Swamy MK, Akhtar MS, Sinniah UR (2016) Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid Based Complem Alternat Med 2016:3012462. https://doi.org/10.1155/2016/3012462
Tayse E, da Cruz Almeida, Geany Targino de Souza, Jossana Pereira de Sousa Guedes, Isabella Medeiros Barbosa, Cristina Paiva de Sousa, Lúcio Roberto Cançado Castellano, Marciane Magnani, Evandro Leite de Souza, (2019) Mentha piperita L. essential oil inactivates spoilage yeasts in fruit juices through the perturbation of different physiological functions in yeast cells. Food Microbiol 82:20–29. https://doi.org/10.1016/j.fm.2019.01.023
Toprani R, Patel D (2013) Betel leaf: revisiting the benefits of an ancient Indian herb. South Asian J Cancer 2(3):140–141. https://doi.org/10.4103/2278-330X.114120
Viuda-Martos M, Fernandez-Lopez J, Sayas-Barbera E, Sendra E, Perez-Alvarez JA (2011) Physicochemical characterization of the orange juice waste water of a citrus by-product. J Food Process Preserv 35(2):264–271
Yashwant CP, Rajendran V, Krishnamoorthy S, Nagarathinam B, Rawson A, Anandharaj A, Sivanandham V (2022) Antibiotic resistance profiling and valorization of food waste streams to starter culture biomass and exopolysaccharides through fed-batch fermentations. Food Sci Biotechnol 32:863–874. https://doi.org/10.1007/s10068-022-01222-9
Yin X, Chen K, Cheng H, Chen X, Feng S, Song Y, Liang L (2022) Chemical stability of ascorbic acid integrated into commercial products: a review on bioactivity and delivery technology. Antioxidants. https://doi.org/10.3390/antiox11010153
Zielinski A, Haminiuki C, Alberti A, Nogueira A, Demiate I, Granato D (2014) Comparative study of the phenolic compounds and the in vitro antioxidant activity of different Brazilian teas using multivariate statistical techniques. Food Res Int 60:246–254. https://doi.org/10.1016/j.foodres.2013.09.010
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AFB performed all the experiments and generated data. AFB and EA wrote the original draft. EA and AA reviewed the draft. AA and VS analyzed and interpreted the data. NB conceptualized, designed the methodology and supervised the whole project. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
No competing interests.
Ethics approval
This article does not contain any studies with human participants or animals.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13197_2024_5963_MOESM1_ESM.pdf
Supplementary file 1 The supplementary file contains data related to the results of sensory analysis. The ranks of each fruit juice added with BLEO are presented in the table (PDF 92 KB)
13197_2024_5963_MOESM2_ESM.pdf
Supplementary file 2 The supplementary file contains data related to the results of microbial analysis. The number of colonies observed in each control and sample for total plate count and total yeast and mold count is given in Table 2 and Table 3 for weeks 0 and 2 (PDF 85 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdul Kareem, F.B., Elumalai, A., Anandharaj, A. et al. Exploring the preservation efficiency of cured betel leaf essential oil in augmenting the quality of fruit juice: a comprehensive evaluation of physicochemical, microbial, and sensory parameters. J Food Sci Technol 61, 1862–1873 (2024). https://doi.org/10.1007/s13197-024-05963-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-024-05963-9