Abstract
Watermelon (Citrullus lanatus) is important in the fruit juice industry. The aim of this study was to determine the pH, titratable acidity, Brix, color properties, lycopene, ascorbic acid, total phenolic concentration, total flavonoid concentration, antioxidant activity, hydroxy methyl furfural, sensory properties and microbial safety after ultrasound treatments (4, 8, 12 and 16 min) and the pasteurization process in red watermelon juice and yellow watermelon juice. Red watermelon juice and yellow watermelon juice samples showed no statistically significant change in Brix and titratable acidity values at the end of the treatments (p > 0.05). Improvements in lycopene, phenolic concentration, flavonoid concentration and antioxidant capacity of fresh fruit juice samples was determined with US16 (sonication for 16 min). However, ultrasound treatments led to reductions in the concentration of ascorbic acid compared to fresh juice samples. The concentration of lycopene in both fruit juice samples was found to have a negative correlation with the HMF (hydroxymethyl-furfural) concentration. Due to the current findings presented in this study, ultrasound treatments applied to fruit juices were found to be generally microbiologically safe. A better understanding of the improvement in bioactive components, provision of microbial safety and increased consumer appreciation linked to ultrasound treatment of red watermelon juice and yellow watermelon juice was provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruits and vegetables are sources of antioxidants, minerals and fiber. Due to the bioactive substances they contain, they help to protect against various diseases [1]. Watermelon (Citrullus lanatus) belongs to the Cucurbitaceae family. Depending on the concentration of lycopene and β-carotene, it has different colors such as yellow, orange and red [2]. In particular, consumption of lycopene-containing foods was associated with reduced incidence of coronary heart disease and some types of cancer. Watermelon is important in terms of nutritional physiology due to its bioactive properties in in vivo and in vitro studies [3,4,5,6,7]. Watermelon is suitable for processing into fruit juice due to 93% water content. Therefore, watermelon juice has been gaining popularity worldwide in recent years due to its sensory, physical and nutritional properties. Thermal treatment of watermelon juice adversely affects its quality, nutritional value and organoleptic properties. At the same time, the typical irritating odor after heating watermelon juice causes problems in the industry [8, 9]. Another problem is that HMF is an undesirable condition in food due to the harm to human health, and its presence and content are also an important quality criterion in the processing of food [10]. Non-thermal treatments are used to obtain more acceptable watermelon juice [3, 5, 11, 12].
Nowadays, consumers are more interested in fresh, nutritious and safe foods. Thermal pasteurization processes were successful in increasing the shelf life of nutrients, but involve losses in terms of bioavailability [13, 14]. This encourages research into innovative non-thermal processing technologies that could be an alternative to thermal processes in food. The ultrasound method or sonication is one of the alternative non-thermal technologies in the food industry. The sound waves that people can hear are between 20 Hz and 20 kHz. Mechanical sound waves at frequencies outside the range of sound that can be heard by the human ear are called ultrasounds or ultrasound [15]. It is reported that ultrasound applications to fruit juices provide significant microbial and enzyme inactivation while simultaneously keeping nutritional losses to a minimum [14, 16, 17].
Previously, ultrasound was applied to different fruit juices; for example, casturi lime juice [18], apple-carrot juice [19], apple juice [20], strawberry juice [21] and mango juice [22]. On the other hand, optimization for watermelon juice was carried out only with the application of thermosonication [3]. When the literature was examined, ultrasound application and comparison of red and yellow watermelon juice was not performed. Here, red watermelon and yellow watermelon juice, which have significant potential thanks to the nutritional properties of processed products in addition to fresh consumption, were researched. The aim of this study was to investigate the effects of different ultrasound treatments on physicochemical properties (pH, titratable acidity, total soluble solids and color attributes), bioactive properties (total polyphenols, total flavonoids, total antioxidant activity, lycopene and ascorbic acid), sensory properties and microbiological safety of red and yellow watermelon juice. At the same time, HMF was investigated because it is considered to evaluate the process applied to fruit juice during processing and quality control.
Materials and methods
Juice sample preparation
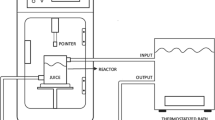
Fresh red watermelon and yellow watermelon were collected from local producers (Tekirdag, Turkey) and kept at 4 °C until the experiments were performed. Shells, stems, seeds and ripened parts were discarded. A blender (Waring Commercial Blender Model HGB2WTS3, USA) was used for crushing to extract the juice. Juice was then filtered through sterilized double-layer muslin fabric. Red watermelon juice was mixed with a vortex and selected as control (CRW). It was filled into sterilized 100 mL airtight bottles. This juice was pasteurized at 90 °C for 30 s in a water bath and cooled to 20 °C, and then named pasteurized red watermelon juice (PRW). Other samples were treated with ultrasound. In the study, the UP200St- ultrasound device by Hielscher Ultrasonics (Berlin, Germany) was used. Samples were stored at − 20 °C until analysis. The same procedures were applied to the yellow-flesh watermelons. Pasteurized yellow watermelon juice (PYW) and fresh water watermelon (CYW) were coded. As shown in Table 1, the processing time and operating conditions were set at 4 min (US4), 8 min (US8), 12 min (US12) and 16 min (US16). The parameters used for ultrasound were determined by modifying previous studies and performing preliminary trials [21, 23]. The samples were stored in glass bottles at a temperature of − 18 °C until the tests were carried out. All treatments were carried out in triplicate.
Microbiological analysis
Serial dilutions of juice were prepared in peptone water solution for microbial counts. Colony forming units (CFU) were determined by standard spreading and pouring plate methodologies. PCA (Plate Count Agar-Merck) was used for total aerobic plate count (TAPC). Samples were incubated at 30 °C for 48 h. For yeast and mold count (YMC), PDA (Potato Dextrose Agar-Merck) was used. Samples were incubated at 24 °C for 3–5 days. Total Enterobacteriaceae count (TEC) was determined in Violet Red BileGlucose Agar-Merck (VRBG) incubated at 37 °C for 24 h. Results are given as log colony forming units (CFU) per milliliter of juice [24].
Determination of pH, total soluble solids (°Brix) and titratable acidity
pH was measured using a potentiometer (Hanna Instruments HI 2002 pH/ORP, Romania). Measurements were made at 20 °C. Soluble solids were measured using a refractometer (ATAGO brand RX-7000α model, Japan) and the results were expressed in Brix. The titration acidity was potentiometrically determined by titration of the samples with 0.1 N NaOH (Sigma-Aldrich, USA) solution to pH 8.1. From the sample, 5 mL was taken and 50 mL of distilled water was added and 10 mL of the sample was taken from the filtrate. The results were calculated as g malic acid/L [25].
Color analysis
L*, a* and b* values of fruit juices were measured with a Hunter colorimeter (Color Measuring Device PCE-CSM 5, Germany). L* is a measure of light and darkness between 0 and 100. 0 corresponds to black and 100 corresponds to white. In the color measurement system, positive (+) values of the a* value indicate redness and negative (−) values indicate greenness. The positive (+) values of the b* value indicate yellow and the negative (−) values represent blue. Chroma (C) and hue angle (h) were expressed according to the following Eqs. (1–2) [26];
Determination of total polyphenols, total flavonoids and antioxidant activity
Total phenolic concentration analysis was performed according to the Folin-Ciocalteu method [27]. Juice samples of 0.5 mL were diluted with an appropriate proportion of purified water (1:10), and 2.5 mL of 0.2 N Folin-Ciocalteu reagent was added and after 3 min 2 mL of 7.5% (w/v) Na2CO3 solution was added. After incubation for 30 min in a dark environment at room temperature, the tubes were vortexed and absorbance measurements were performed with a spectrophotometer at 760 nm wavelength. The total phenolic concentration agent is expressed in gallic acid equivalent (mg GAE/L). The analyses were performed in three parallels.
The total flavonoid concentration was measured with the aluminum chloride colorimetric analysis method [28]. Each diluted juice sample of 1 mL was added to each tube, then 4 mL of purified water and 0.3 mL of 5% sodium nitrite (NaNO2) solution was added, after 5 min 0.3 mL of 10% aluminum chloride (AlCl3) solution was added and mixed and left to stand for 6 min. At the end of the time, 2 mL of 1 M NaOH was added and the total volume was completed to 10 mL with distilled water. The absorbance values of the samples were read on a UV–VIS spectrophotometer (SP-UV/VIS-300SRB, Australia) at a wavelength of 510 nm. The analyses were performed in three parallels. Total flavonoid concentration was expressed as mg catechin equivalents (mg CE/L) per liter.
Determination of antioxidant capacity was completed with the DPPH method, where the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical occurs based on inhibition with some modifications [29]. First, 2.9 mL of a 0.1 mM DPPH solution (prepared in ethanol) was added to 0.1 mL of fruit juice sample and mixed in a vortex and left at room temperature for 30 min in the dark. The absorbance was then measured on a UV–VIS spectrophotometer (SP-UV/VIS-300SRB, Australia) at a wavelength of 517 nm. The DPPH radical scavenging activity was calculated as:
where A0 is the absorbance of the control, and A1 is the absorbance of the juice.
The CUPRAC (cupric reducing antioxidant capacity) method was used to determine antioxidant capacity [30]. Copper chloride of 1 mL (CuCl2, Sigma Aldrich, Germany), 1 mL of Neocuprin (Sigma Aldrich, Germany), 1 mL of ammonium acetate solution (NH4Ac, Merck, Germany), 0.1 mL of an appropriate diluted juice sample, and 1 mL of purified water were added to each tube in 3 parallel repetitions. These were vortexed and incubated for 1 h in a dark environment. The absorbance values of the samples were then measured at a wavelength of 450 nm. Calculations were made by using the standard calibration curve prepared with Trolox (Merck, Germany).
Determination of lycopene
Lycopene concentration was determined with the modified method [7]. Approximately 0.6 g of sample was weighed and added to 5 mL of 0.05% (w/v) BHT in acetone, 5 mL of 95% ethanol and 10 mL of hexane. The homogenate was centrifuged at 400 g for 15 min at 4 °C. After that, 3 mL of distilled water was added. The vials were agitated for 5 min and left at room temperature to allow phase separation to occur. The absorbance of the upper hexane layer was measured in a 1 cm-path length quartz cuvette at 503 nm using a spectrophotometer. Hexane was used as blank. The absorbance (Abs503) of the upper (hexane) layer was measured in a 1 cm path-length quartz cuvette at 503 nm blanked with hexane. The lycopene concentration (mg/L) of each sample was estimated as follows:
where MW is the molecular weight of lycopene (536.9 g/mol), DF is the dilution factor, L is the path length in cm, and ε is the molar extinction coefficient for lycopene (172,000 L/mol/cm).
Determination of ascorbic acid
Calculation of the ascorbic acid concentration of the samples was carried out with AOAC 961.27 vitamin preparation and ascorbic acid 2,6 dichlorophenol indophenol-titrimetric method in fruit juices (AOAC, 2000). The results obtained are expressed as milligrams of ascorbic acid per 100 mL sample, and the calculation was done as stated below:
Determination of HMF (hydroxymethyl-furfural)
The method used to measure the color intensity of HMF is based on the reaction of barbituric acid and p-toluene with the colorimetric method. Two milligrams of juice was transferred to two different glass tubes, and then 5 mL of p-toluidine 1 solution was added. After the mixture in the tubes was mixed with a vortex, 1 mL of distilled water was used as a witness for the tubes and 1 mL of barbituric acid solution was added to the second tube. After the tubes were again mixed with the vortex, the contents of the tube containing the barbituric acid solution were recorded after waiting until the tube reached maximum absorption at 550 nm in the spectrophotometer (SP-UV/VIS-300SRB, Australia) against the contents of the tube prepared as a blind [31].
Sensory analysis
The acceptance test was performed for color, texture (viscosity), taste and aroma and overall acceptance, using a 9-point structured hedonic scale ranging from (1) extremely dislike to (9) like extremely. A total of 30 (17 female, 13 male) panelists evaluated the juices. Scale scores were excellent 9; very good 8; good, 7; acceptable, 6; and poor (first odorless, tasteless development) < 6. Lower points were accepted as 6. Prior to sensory evaluation, the juice samples were cooled, randomly coded with three-digit numbers, and the order of presentation was completely randomized for each panelist. The evaluation was carried out in the Department of Nutrition and Dietetics of Tekirdağ Namık Kemal University (Tekirdag, Turkey).
Statistical analysis
All values were obtained in triplicate and expressed as mean ± standard deviation (SD). The significant differences between mean values of watermelon juice samples were determined by analysis of variance (one way-ANOVA) using Tukey’s HSD (Honestly Significant Difference) test at a significance level of p < 0.05. Statistical analysis was conducted using SPSS 22.0 software (SPSS Inc., Chicago, USA). Pearson correlation coefficients were obtained using OriginPro version 2017 (OriginLab, Northampton, Massachusetts, USA.).
Results and discussion
Microbiological analysis
Table 2 shows the effect of ultrasound treatment on microorganisms in red watermelon juice and yellow watermelon juice. In US4 treatment of fresh red watermelon juice, a decrease in TAPC content of 1.2 log CFU/mL was detected. Total Enterobacteriaceae could not be detected in other treatments. A significant reduction (p < 0.05) was observed in the TAPC of yellow watermelon juice samples subjected to the ultrasound treatments of US4, US8, US12 and US16 compared to the CYW juice samples. US4 treatment of yellow watermelon juice showed a decrease of 1.2 log CFU/mL in the content of YMC compared to the CYW sample. Currently, the Food and Drug Administration (FDA) requires a 5-log CFU/mL reduction for a relevant pathogen target in fruit juice, but there is no specific criterion for the total plate count. Therefore, ultrasound treatments applied to red watermelon juice and yellow watermelon juice were successful in the general microbiological evaluation. In addition, the increase in the duration of treatment with the reduction of microbial load showed similarities with the literature indicating that microbial inactivation increased more with time [18]. Similar studies reported successful use of ultrasound treatment for pear juice, apple juice, mango juice and purple cactus juice for inactivation of microbial cells [20, 22, 32, 33]. The causes of the decrease in microbial population include the increased pressure in the liquid with the effect of ultrasonication and cavitation. Shock waves, cavitation and hydroxyl radicals have destructive effects on microorganisms [34, 35].
Effect of ultrasound on pH, total soluble solids (°Brix), titratable acidity and color attributes
The results about the effect of treatments on pH, titratable acidity (TA), total soluble solids (TSS), and color attributes in red watermelon juice and yellow watermelon juice are shown in Table 3. In the study, minimal decreases were determined in red watermelon juice pH (5.60–5.59) and yellow watermelon juice pH (5.37–5.36). Red watermelon juice and yellow watermelon juice samples showed no statistically significant change in TSS and TA values at the end of the treatment (p > 0.05). Non-thermal treatments generally have no significant effect on pH, TA and TSS values [36]. The pH of red watermelon juice and yellow watermelon juice are closely related to changes in TSS and TA values, genotype, growing conditions and maturity of the raw material used. Similar results regarding pH, TA and TSS were reported for ultrasound-treated strawberry [37], orange [38], carrot [39], casturi lime [18], carrot [40] and mango juice [22].
The color of fruit juice is generally important for consumer choice. Results regarding the effect of ultrasound treatments on color values of red watermelon juice and yellow watermelon juice are given in Table 3. At the end of different ultrasound treatments, statistically significant differences in L*, a* and b*, C and h values between red watermelon and yellow watermelon juice samples were determined (p < 0.05). At the end of the treatment, there was no statistical difference in CYW and PYW samples for yellow watermelon juice for L* value (p > 0.05), but statistically significant differences were found when compared with other samples (p < 0.05). Different ultrasound treatments applied to carrot juice were reported to increase L* in the first minutes and then to decrease it [39]. In this study, it was determined that the increase in the L* value of yellow watermelon juice, especially with the US4 treatment, decreased later. The a* and C color values of red and yellow watermelon juice in the US4 samples were found to be statistically different from other samples (p < 0.05). The PYW sample for red watermelon juice was found to be statistically significant when compared with other samples for hue angle (p < 0.05). This is considered to be a result of oxidation/isomerization of carotenoids leading to small changes in redness and yellowness in fruit juices [41]. Increases in L* values may result from collapse of unstable particles [42]. For a decrease in the a* values of fruit juices, anthocyanin degradation and the formation of maillard reaction products were reported [43]. In a study related to the decrease in color values, it can be concluded that the total phenolic concentration in fruit juice samples may increase as a result of cavitation [44]. On the other hand, one of the reasons for color changes is the formation of hydroxyl radicals as a result of cavitation and collapse due to high pressure effects during ultrasound treatment [45]. Although ultrasound treatments caused changes in L*, a* and b* values of red watermelon juice and yellow watermelon juice, these changes were not easily seen by the naked eye. Therefore, ultrasound treatment can be used in the processing of watermelon juices.
Effect of ultrasound on bioactive compounds
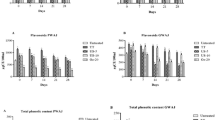
The results regarding the effects of treatments of red watermelon juice and yellow watermelon juice in terms of bioactive compounds are shown in Table 4. Different ultrasound applications were found to be effective on the bioactive compounds (p < 0.05). Phenolic compounds are very important and beneficial to human health as they play a role in controlling the risk of many chronic degenerative diseases occurring in the human body [46]. In this study, decreases in TPC (mg GAE/L) and TFC (mg CE/L) contents in both fruit juices were found in pasteurized samples. In contrast, increases in TPC and TFC levels were found in this study in ultrasound-treated fruit juice samples (Table 4). With US16 treatment of red watermelon juice, the TPC content was increased by 1.7% compared to the CRW sample. In yellow watermelon juice, US16 treatment showed a 5.7% improvement in TPC content compared to red watermelon juice CYW sample. With the US16 treatment of red watermelon juice, an increase of 0.016 CE mg/L was found compared to the CRW sample. For yellow watermelon juice, the US16 treatment was detected to cause a 0.012 CE mg/L increase compared to the CYW sample. Similar results were found in other fruit juices (carrot, casturi lime, grapefruit and Chokanan mango juices) as in this study [18, 22, 47, 48]. This increase in TPC and TFC contents can be attributed to the breakage of the bonded form of phenolic content due to cavitation pressure generated during the ultrasound process and cell wall breakage. In addition, ultrasound-generated hydroxyl radicals (OH−) can be explained by binding to the aromatic ring of phenolic compounds [47]. The TPC and TFC content in red watermelon juice and yellow watermelon juice showed positive correlation with antioxidant capacity of CUPRAC (mg TEAC/mL), and were statistically significant (p > 0.05). However, the contents of TPC and TFC in red watermelon and yellow watermelon juice were negatively correlated with HMF (mg/L). TPC and TFC values showed a low degree positive correlation with the vitamin C concentration (mg/100 mL) for fruit juice samples, but it was not statistically significant (p > 0.05) (Table 5).
The total antioxidant capacity (DPPH and CUPRAC) of fruit juices treated with different ultrasound processing conditions were shown in Table 4. Statistically significant differences were found in DPPH and CUPRAC antioxidant capacities after ultrasound treatment in red watermelon and yellow watermelon juices (p < 0.05). While antioxidant capacity of fresh fruit juices decreased with pasteurization, it was found to increase at the end of ultrasound processing (Table 4). At the end of the ultrasound treatments, the highest increase in DPPH amounts in red watermelon juice and yellow watermelon juice samples was 9.9% and 10.4% in US16 samples, respectively. CUPRAC amounts were also 6.6% and 7.5% in US16 samples, respectively. As in this study, several reports have shown positive correlations and improvements between the total phenolics, including flavonoids, and the antioxidant activities of fruit juices (castor lime, grapefruit, cactus pear, strawberry and carrot-grape juice) [16, 18, 32, 47, 49]. These increases can be attributed to the increase in phenolic substances associated with cavitation in processed fruit juices after ultrasound [47]. A significant positive correlation was found between TPC and TFC levels and total antioxidant content (CUPRAC and DPPH). There was a positive correlation between total antioxidant capacity and lycopene levels of fruit juice samples (p < 0.05), but it was found to have a negative correlation with HMF concentration (Table 5). Antioxidant compounds play an important role in preventing the risk of cancer and cardiovascular disease, which are believed to be associated with oxidative stress [50]. Therefore, it is thought that ultrasound-treated red watermelon juice and yellow watermelon juice will be very beneficial for human health with the increases in total phenols and antioxidants shown in this study.
Lycopene is a bright red pigment from carotenoid. Lycopene has strong antioxidant ability that can protect against cancer and other degenerative diseases [51]. Lycopene concentration (mg/100 mL) of fruit juices treated with different ultrasound processing conditions are shown in Table 4. In this study, yellow watermelon juice lycopene concentration was much lower than red watermelon juice. These results showed that lycopene is abundant in red watermelon juice and yellow watermelon has a lower concentration of lycopene, which is consistent with the literature [52]. There were statistically significant differences in the lycopene concentration after ultrasound treatment in red watermelon and yellow watermelon juices (p < 0.05). The lycopene concentration was decreased by pasteurization in both fruit juice samples but increases were detected after ultrasound treatments. The main causes of lycopene degradation with pasteurization are oxidation and isomerization [9]. The increase in lycopene in ultrasound-treated red watermelon juice was US4 = 1.4%, US8 = 2.5%, US12 = 3.0% and US16 = 4.5%, respectively, compared to CRW. In the thermosonication treatment applied to watermelon juice, the researchers found that the concentration of lycopene increased under low treatment conditions and decreased at high amplitude levels [3]. There was a positive correlation between lycopene concentration of red watermelon juice and yellow watermelon juice with TPC, TFC, and total antioxidant content (CUPRAC and DPPH) (p < 0.05). The concentration of lycopene in both fruit juice samples was found to have a negative correlation with the HMF concentration (Table 5).
Vitamin C concentration (mg/100 mL) of fruit juices treated with different ultrasound processing conditions was shown in Table 4. Statistically significant differences were found between the ascorbic acid concentrations in red watermelon and yellow watermelon juices (p < 0.05). A study found that ascorbic acid degradation increased when thermosonication was applied to watermelon juice at a higher temperature. At the same time, they reported that with the maximum amplitude and the longest processing time, % ascorbic acid retention decreased to 50% [3]. In this study, ascorbic acid retention in treatments of red watermelon juice was found to be PRW = 45.5%, US4 = 91.9%, US8 = 68.3%, US12 = 41% and US16 = 20.3%, respectively, compared to the CRW sample. Ascorbic acid retention in treatments compared to CYW sample was determined as PYW = 49.5% US4 = 91.8%, US8 = 75.4%, US12 = 50.2% and US16 = 19.8%, respectively. In the studies where different ultrasound conditions were applied, in parallel with this study, ascorbic acid concentration decreased at the end of the treatments [22, 53, 54]. Reductions in the amount of amino acids may be caused by the reduction in the concentration of ascorbic acid compound which is influenced by a number of factors such as the reaction of free radicals (especially hydroxyl), the presence of dissolved gases (O2), acoustic power and temperature [22, 55]. Red watermelon juice and yellow watermelon juice samples were found to have a negative correlation between the concentrations of vitamin C and HMF (p < 0.05) (Table 5).
HMF (mg/L) of fruit juices treated with different ultrasound processing conditions was shown in Table 4. Red watermelon juice and yellow watermelon juice showed significant differences in HMF levels after treatment (p < 0.05). At the end of ultrasound treatments in both fruit juices, the concentration of HMF increased as time increased. An increase in the concentration of HMF of 0.049 mg/L was detected in the maximum US16 sample compared to the CRW sample. In yellow watermelon juice, HMW concentration of 0.025 mg/L was found to be higher in US16 than CRW. When the fruit juice results were evaluated, the HMF levels were determined to be below 20 mg/kg maximum according to AINP (European Fruit Juice Association) and below 25 mg/kg maximum according to IFFJP (standards for International Federation of Fruit Juice Producers). In the study, cavitation occurring during ultrasound therapy may have caused an increase in maillard reaction products. There was a negative correlation between HMF, TPC (r = − 0.826) and vitamin C (r = − 0.848) in red watermelon juice (p < 0.05). In yellow watermelon juice, HMF was found to have a negative correlation with vitamin C (r = − 0.963).
Effect of ultrasound on sensory analysis
Sensory analysis of fruit juices treated with different ultrasound processing conditions are shown in Table 6 and Fig. 1. Statistically significant differences were found in color, texture, taste, aroma and overall acceptability evaluations (p < 0.05). In the general evaluation of the panelists, US16 (8.63 points) was the sample with highest points for red watermelon juice compared to CRW. For yellow watermelon juice US8 (7.50 points) was identified as the most popular. There were no statistically significant differences in the aroma evaluation of yellow watermelon juice (p > 0.05). When red watermelon juice and yellow watermelon juice were evaluated among themselves, red watermelon juice was appreciated more. Researchers found that the cranberry juice [56], orange juices [57] and carrot-grapes [16] products treated with ultrasound were generally acceptable. The positive effect of ultrasound on sensory properties is attributed to the removal of oxygen [57]. In this study, it was determined that the ultrasound procedure improved the sensory properties and showed parallels with the literature.
Conclusion
In this study, it was determined that sonication treatment improves total phenolic concentration, total flavonoid concentration, lycopene, total antioxidant capacity and color values in red watermelon juice and yellow watermelon juice. There was no significant variation in the physicochemical parameters (pH, titration acidity, °Brix). At the same time, it was determined that the microbial burden significantly reduced and the levels of HMF were increased at the end of treatments despite reliable levels. Vitamin C, a bioactive component, was negatively affected and decreased. The sensory evaluation of the panelists found both fruit juices were successful and generally improved after treatment. Compared to pasteurization, ultrasound-treated juice was found to be more successful.
When the results are evaluated, ultrasound therapy was shown to be successful for the health of the consumer because it promotes the improvement in general in terms of consumer appreciation and bioactive properties. New research is needed to develop models such as surface response methodology to optimize process variables during ultrasound procedures. It is also recommended to investigate the use of ultrasound technology with other non-thermal food processing technologies to improve the quality of red watermelon juice and yellow watermelon juice.
References
J.L. Slavin, B. Lloyd, Adv. Nutr. 3, 506 (2012)
H.E. Khoo, K.N. Prasad, K.W. Kong, Y. Jiang, A. Ismail, Molecules 16, 1710 (2011)
A. Rawson, B.K. Tiwari, A. Patras, N. Brunton, C. Brennan, P.J. Cullen, C. O’Donnell, Food Res. Int. 44, 1168 (2011)
T. Shahzad, I. Ahmad, S. Choudhry, M.K. Saeed, M.N. Khan, Int. J. Pharm. Pharm. Sci. 6, 223 (2014)
I. Aguiló-Aguayo, R. Soliva-Fortuny, O. Martín-Belloso, Innov. Food Sci. Emerg. Technol. 11, 299 (2010)
P. Perkins-Veazie, A. Davis, J.K. Collins, Isr. J. Plant Sci. 60, 395 (2013)
G. Oms-Oliu, I. Odriozola-Serrano, R. Soliva-Fortuny, O. Martín-Belloso, Food Chem. 115, 1312 (2009)
Y. Liu, C. He, H. Song, Food Res. Int. 107, 119 (2018)
J. Shi, M. Le Maguer, Crit. Rev. Biotechnol. 20, 293 (2000)
Z. Li, Y. Yuan, Y. Yao, X. Wei, T. Yue, J. Meng, Food Control 102, 56 (2019)
C. Zhang, B. Trierweiler, W. Li, P. Butz, Y. Xu, C.E. Rüfer, Y. Ma, X. Zhao, Food Chem. 126, 254 (2011)
Y. Liu, X.S. Hu, X.Y. Zhao, C. Zhang, J. Food Sci. Technol. 50, 317 (2013)
A. Rawson, A. Patras, B.K. Tiwari, F. Noci, T. Koutchma, N. Brunton, Food Res. Int. 44, 1875 (2011)
F. Chemat, Zill-e-Huma, M.K. Khan, Ultrason. Sonochem. 18, 813 (2011)
T.J. Mason, J.P. Lorimer, Applied Sonochemistry (Wiley, Weinheim, 2002)
M. Nadeem, N. Ubaid, T.M. Qureshi, M. Munir, A. Mehmood, Ultrason. Sonochem. 45, 1 (2018)
L.H. Cheng, C.Y. Soh, S.C. Liew, F.F. Teh, Food Chem. 104, 1396 (2007)
R. Bhat, N.S.B.C. Kamaruddin, L. Min-Tze, A.A. Karim, Ultrason. Sonochem. 18, 1295 (2011)
O. Kahraman, H. Lee, W. Zhang, H. Feng, Ultrason. Sonochem. 38, 820 (2017)
M. Abid, S. Jabbar, T. Wu, M.M. Hashim, B. Hu, S. Lei, X. Zhang, X. Zeng, Ultrason. Sonochem. 20, 1182 (2013)
J. Wang, J. Wang, J. Ye, S.K. Vanga, V. Raghavan, Food Control 96, 128 (2019)
V. Santhirasegaram, Z. Razali, C. Somasundram, Ultrason. Sonochem. 20, 1276 (2013)
S. Yıkmış, Food Sci. Technol. 1 (2019)
N. Cruz, M. Capellas, M. Hernández, A.J. Trujillo, B. Guamis, V. Ferragut, Food Res. Int. 40, 725 (2007)
AOAC, Official Methods of Analysis, 16th ed. (Association of Official Analytical Chemists, Washington, DC, 1995).
L.E. Ordóñez-Santos, J. Martínez-Girón, M.E. Arias-Jaramillo, Food Chem. 233, 96 (2017)
V. Singleton, A. Rossi, Am. J. Enol. Vitic. 16, 144 (1965)
J. Zhishen, T. Mengcheng, W. Jianming, Food Chem. 64, 555 (1999)
C. Grajeda-Iglesias, E. Salas, N. Barouh, B. Baréa, A. Panya, M.C. Figueroa-Espinoza, Food Chem. 194, 749 (2016)
R. Apak, K. Güçlü, M. Özyürek, S. Esin Karademir, E. Erçağ, Int. J. Food Sci. Nutr. 57, 292 (2006)
B.W. LeBlanc, G. Eggleston, D. Sammataro, C. Cornett, R. Dufault, T. Deeby, E. St, Cyr. J. Agric. Food Chem. 57, 7369 (2009)
Q.Y. Zafra-Rojas, N. Cruz-Cansino, E. Ramírez-Moreno, L. Delgado-Olivares, J. Villanueva-Sánchez, E. Alanís-García, Ultrason. Sonochem. 20, 1283 (2013)
M. Saeeduddin, M. Abid, S. Jabbar, B. Hu, M.M. Hashim, M.A. Khan, M. Xie, T. Wu, X. Zeng, Int. J. Food Sci. Technol. 51, 1552 (2016)
S. Koda, M. Miyamoto, M. Toma, T. Matsuoka, M. Maebayashi, Ultrason. Sonochem. 16, 655 (2009)
I. Oyane, T. Takeda, Y. Oda, T. Sakata, M. Furuta, K. Okitsu, Y. Maeda, R. Nishimura, Ultrason. Sonochem. 16, 532 (2009)
C. M. G. Charoux, C. P. O’Donnell, B.K. Tiwari, in Ultrasound Adv. Food Process. Preserv., edited by D. Bermúdez-Aguirre (Elsevier, Amsterdam, 2017), pp. 215–235.
B. K. Tiwari, C.P. O'Donnell, A. Patras, P.J. Cullen, J. Agric. Food Chem. 56, 10071 (2008)
V.M. Gómez-López, L. Orsolani, A. Martínez-Yépez, M.S. Tapia, LWT Food Sci. Technol. 43, 808 (2010)
L. Chen, X. Bi, D. Guo, Y. Xing, Z. Che, Food Sci. Technol. Int. 25, 394–403 (2019)
S. Jabbar, M. Abid, T. Wu, M. Muhammad Hashim, B. Hu, S. Lei, X. Zhu, X. Zeng, Int. J. Food Sci. Nutr. 65, 28 (2014)
A. Adekunte, B.K. Tiwari, P.J. Cullen, A.G.M. Scannell, C.P. O’Donnell, Food Chem. 122, 500 (2010)
B.K. Tiwari, C.P. O'Donnell, K. Muthukumarappan, P.J. Cullen, Int. J. Food Sci. Technol. 44, 586 (2009)
I. Aguiló-Aguayo, R. Soliva-Fortuny, O. Martín-Belloso, J. Food Eng. 92, 37 (2009)
B. Tomadoni, L. Cassani, G. Viacava, M.D.R. Moreira, A. Ponce, J. Food Process. Eng. 40, e12533 (2017)
K. Aguilar, A. Garvín, A. Ibarz, P.E.D. Augusto, Ultrason. Sonochem. 37, 375 (2017)
M. L. Luna-Guevara, J. J. Luna-Guevara, P. Hernández-Carranza, H. Ruíz-Espinosa, and C. E. Ochoa-Velasco, in Stud. Nat. Prod. Chem., edited by Atta-ur-Rahman (Elsevier, Amsterdam, 2018), pp. 79–108.
R.M. Aadil, X.-A. Zeng, Z. Han, D.-W. Sun, Food Chem. 141, 3201 (2013)
M. Abid, S. Jabbar, T. Wu, M.M. Hashim, B. Hu, S. Lei, X. Zeng, Ultrason. Sonochem. 21, 93 (2014)
J. Šic-Žlabur, L. Jasenko, S. Voća, N. Dobričević, S. Pliestić, A. Galić, J. Process. Energy Agric. 23, 73 (2019)
T. Srdić-Rajić, A. Konić Ristić, Encyclopedia of Food and Health (Academic Press, 2016), pp. 227–233
A.-N. Li, S. Li, D.-P. Xu, X.-R. Xu, Y.-M. Chen, W.-H. Ling, F. Chen, H.-B. Li, Food Anal. Methods 8, 1207 (2015)
W.S. Choo, W.Y. Sin, Adv. Appl. Sci. Res. 3, 2779 (2012)
B.K. Tiwari, C.P. O’Donnell, P.J. Cullen, Trends Food Sci. Technol. 20, 137 (2009)
J. Wang, S.K. Vanga, V. Raghavan, LWT 107, 299 (2019)
S. Merouani, H. Ferkous, O. Hamdaoui, Y. Rezgui, M. Guemini, Ultrason. Sonochem. 23, 37 (2015)
A.R. Jambrak, M. Šimunek, M. Petrović, H. Bedić, Z. Herceg, H. Juretić, Ultrason. Sonochem. 38, 783 (2017)
B.H. Samani, M.H. Khoshtaghaza, Z. Lorigooini, S. Minaei, H. Zareiforoush, Innov. Food Sci. Emerg. Technol. 32, 110 (2015)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yıkmış, S. Sensory, physicochemical, microbiological and bioactive properties of red watermelon juice and yellow watermelon juice after ultrasound treatment. Food Measure 14, 1417–1426 (2020). https://doi.org/10.1007/s11694-020-00391-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00391-7