Abstract
Listeriosis is a severe disease caused by the foodborne pathogen Listeria monocytogenes, posing a significant risk to vulnerable populations such as the elderly, pregnant women, and newborns. While relatively uncommon, it has a high global mortality rate of 20–30%. Recent research indicates that smaller outbreaks of the more severe, invasive form of the disease occur more frequently than previously thought, despite the overall stable infection rates of L. monocytogenes over the past 10 years. The ability of L. monocytogenes to form biofilm structures on various surfaces in food production environments contributes to its persistence and challenges in eradication, potentially leading to contamination of food and food production facilities. To address these concerns, this review focuses on recent developments in epidemiology, risk evaluations, and molecular mechanisms of L. monocytogenes survival in adverse conditions and environmental adaptation. Additionally, it covers new insights into strain variability, pathogenicity, mutations, and host vulnerability, emphasizing the important events framework that elucidates the biochemical pathways from ingestion to infection. Understanding the adaptation approaches of L. monocytogenes to environmental stress factors is crucial for the development of effective and affordable pathogen control techniques in the food industry, ensuring the safety of food production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Listeria monocytogenes is a Gram-positive, facultative anaerobic bacterium that grows best at temperatures between − 0.4 °C and 44 °C, with an optimal temperature of 37 °C. It is motile between 22 and 28 °C but non-motile above 30 °C (Won et al. 2020). It can able to survive with a wide pH range between 4.6 and 9.5, a relatively moderate water activity (aw 0.90), and salinity levels of up to 20% (Te Giffel and Zwietering 1999). These bacterial growth parameters allowed them to survive and proliferate in unfavorable environmental circumstances, which are frequently encountered at food production plants. Consequently, L. monocytogenes is a significant food-borne pathogen that causes the illness known as listeriosis, which can manifest as sporadic infections or disease outbreaks (Buchanan et al. 2017).
Listeria monocytogenes is still a major contributor to mild to severe food poisoning. It might present as a mild, febrile sickness with few problems, or it can present as systemic listeriosis, which is much more serious and often results in hospitalization and even death (Osek et al. 2022). Despite the widespread presence of bacteria in the environment and the relatively frequent isolation of the bacterium in foods, the incidence of listeriosis is normally low in the general population (Ricci et al. 2018). Systemic listeriosis is uncommon in healthy people but is more common in pregnant women, the elderly, and those with compromised immune systems (Mateus et al. 2013). Efforts to control L. monocytogenes in various food categories, particularly meats and meat products, have improved since the 1990s, leading to a decrease in its prevalence. However, over the past decade, the rate of disease has remained consistent, and new outbreaks have raised concerns about our understanding of the factors influencing foodborne illness, including virulence, hosts, and food matrix. These outbreaks have also cast doubt on previous risk assessments (Desai et al. 2019). According to the most recent report from the European Food Safety Authority (EFSA) and the European Centre for Disease Prevention and Control (ECDC) in 2020, there were 1,876 confirmed cases of invasive listeriosis in humans in the member states of the European Union. The notification rate was 0.42 cases per 100,000 people, with a high hospitalization rate of 97.1% (Osek et al. 2022). In the United States, the Centers for Disease Control and Prevention (CDC) reported approximately 1600 cases of L. monocytogenes infections each year, with a hospitalization rate of around 94% (Carstens et al. 2019).
Overall, this review highlights the gravity of listeriosis, the challenges posed by L. monocytogenes in food safety, and the importance of gaining knowledge about its adaptation strategies. By comprehensively addressing epidemiological aspects, risk assessments, and molecular mechanisms, this review aims to contribute to the development of effective control measures for L. monocytogenes in the food industry.
Modern epidemiology breakthroughs
Since 2001, the reporting of listeriosis cases has been mandated in the United States. To effectively track pathogenic strains, identify associations and outbreaks of foodborne illnesses, and establish connections between clinical cases and specific food products, public health, and regulatory agencies have developed and implemented molecular typing methods such as whole genome sequencing (WGS) and pulsed-field gel electrophoresis (PFGE) (Maury et al. 2019). WGS provides a comprehensive nucleotide sequence of the entire bacterial genome, enhancing the precision of data used in routine epidemiological investigations by public health organizations. To analyze and compare WGS data, various bioinformatics tools have been developed (Brown et al. 2019). In the United States, regulatory bodies like the Food and Drug Administration (FDA) and the Center for Food Safety and Applied Nutrition (CFSAN) under the FDA, along with the Centers for Disease Control and Prevention (CDC), utilize WGS for their investigations. The FDA's Center for Food Safety and Applied Nutrition examines variations in Single Nucleotide Polymorphisms (SNPs) and utilizes whole genome multi-locus sequence typing (wgMLST) to assess the relatedness of different isolates and identify allele differences (Chen et al. 2017). The CDC, FDA, and the Food Safety and Inspection Service (FSIS) have incorporated or are in the process of implementing WGS as a regular practice. The FDA has developed the GenomeTrakr Database, a publicly accessible database that contains numerous sequences of L. monocytogenes genomes, which continues to expand (Miro et al. 2020). The USDA FSIS and FDA collect isolates of L. monocytogenes, sequence them, and compare them for matching with the assistance of the CDC. This approach has led to the discovery of new outbreaks with fewer cases and previously uninvestigated food sources (Parida and Mohapatra 2016). Currently, the CDC is working on a 5-year molecular monitoring project to enhance the connection between clinical and food isolates, aiming to replace conventional testing methods. WGS is also valuable in researching the virulence, evolution, population diversity, and global epidemiology of L. monocytogenes over both short and long terms (Miro et al. 2020).
Food vehicle and epidemics
There have been more outbreaks with fewer cases as a result of recent breakthroughs in detecting methods. Certain implicated foods have not been considered as likely vectors based on past experience or risk assessments (Houlihan and Whitworth 2019). Recent epidemics have brought to light the importance of considering the risk of infection from even small doses and of expanding the population of susceptible persons to include children. The number of listeriosis outbreaks and isolated cases reported across the European Union has grown. There were 1763 confirmed cases of listeriosis in humans in 2013, as reported by 27 EFSA member states (EFSA, 2015). The rate of infections increased by 8.6% from 2012 to 2014 reaching 0.44 cases per 100,000 individuals, the majority of reported cases were reportedly acquired at home (EFSA, 2022). The vast majority of reported cases reportedly originated from within the United States. For any zoonosis under EU observation, the average rate of hospitalized cases is 99.1%. The European Union was responsible for 156 deaths out of a total of 1228 confirmed cases (or 69.7%). Seven incidents of food poisoning were reported by five Member States, and all of them were confirmed. (EFSA, 2015; EFSA, 2022) One of the foods used as a test subject was a mixed salad. Cheese, meat, pig meat, and vegetables and juices and their products rounded out the food carriers.
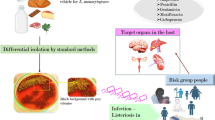
The number of foodborne illness outbreaks in the United States attributed to ready-to-eat (RTE) red meats and poultry decreased between 1998 and 2008, a result of both industry and government efforts. However, there was no decline in the frequency of listeriosis outbreaks that were linked to dairy products. Foods currently classified as “moderate risk” or “low risk” have been connected to several listeriosis outbreaks in the United States since 2010 (Brusa et al. 2021). Besides this, fruits and vegetables including celery, lettuce, cantaloupe, sprouts, stone fruit, and caramel apples were also included. In March 2015, a listeriosis outbreak linked to ice cream was uncovered using routine surveillance. The route of transmission of Listeria is represented in Fig. 1. After looking back through the PulseNet database and the WGS data, we were only able to link nine of the patients to this outbreak (Pouillot et al. 2016). All of the patients were hospitalized (at least eight of them took the product while being treated for an existing illness), and two of them tragically lost their lives there. Ten cases of listeriosis have been connected to machine-cut, diced celery served at five Texas hospitals. All of the people who contracted listeriosis were over the age of 55, with an average age of 80, and they all had preexisting medical issues that necessitated hospitalization. In a total of five cases, listeriosis was the underlying cause of death (Gaul et al. 2013).
Whole peaches, nectarines, plums, and pluots were among the stone fruits recalled in July 2014 by a California packaging company due to concerns about L. monocytogenes contamination. After uploading the PFGE types from the stone fruits to PulseNet, researchers obtained four exact PFGE matches from patients in August (Chen et al. 2016). Subsequent investigation linked nectarines to two of the cases; in one patient it was nectarines; in the other, it was nectarines and peaches. WGS research (Chen et al. 2016) concluded that the other two incidents were unrelated to the recalled fruit. In 2014, five people feel unwell after eating sprouts; all five were hospitalized, and two died. Listeria monocytogenes was discovered in sprout and irrigation water samples during a routine FDA examination. In addition, L. monocytogenes was found in the environment after further testing (Garner and Kathariou 2016). According to the WGS results, there was a close connection between all of the isolates. L. monocytogenes was still present in the workplace after that year, according to a follow-up investigation (Garner and Kathariou 2016; Moura et al. 2017).
Potential impact on food safety
More than 200 diseases are known to spread through food and food products. According to annual reports from the World Health Organization (WHO) for the years 2007–2015, more than a million people contract food-borne infections annually (Mehlhorn 2016). The Centers for Disease Control and Prevention (CDC) reported that 76 million people in the USA are infected from food-borne infections every year, and 5000 of them die. Despite advancements in food product manufacture, the risk of food borne illnesses has increased over the past 20 years. According to estimates, about a quarter of the world's population is susceptible to illnesses brought on by tainted food products, making mortality from such illnesses the primary cause of public health concern (CDC 2018). The WHO (2007–2015) recommendations state that 420,000 people die each year from food-borne infections, which cause one-third of all deaths in children under the age of five (Mehlhorn 2016).
Foodborne illnesses are caused by several different agents, including bacteria, viruses, and parasites, with bacteria being the most common. One of the main factors responsible for catastrophic illnesses in humans and animals is L. monocytogenes, which is spread through the eating of meat, poultry, and RTE foods like milk and other dairy products (Bintsis 2017). Milk only accounts for roughly 3% of the daily energy intake of Asian and African people, compared to estimates for Europe and Oceania countries where milk is a far more common staple. This explains why Asian and African populations receive only 6–7% of their dietary protein from animal sources (Górska-Warsewicz et al. 2019), whereas European nations receive 19%. Milk and milk products are high in protein, minerals like calcium, magnesium, selenium, riboflavin, vitamin B5, and B12, and are therefore essential for human growth and function, especially in the case of pregnant women and young children. However, these nutrients may also provide the ideal environment for the growth of contaminating bacteria like Listeria spp. and other slow-growing bacteria (Shamloo et al. 2019).
In 1983, Wechsler et al. (1983) discovered that 2% of pasteurized milk in Massachusetts had L. monocytogenes. Cheng and Han (2020) reported that L. monocytogenes causes mastitis in dairy cows and that this disease can taint the milk that the cows produce. Multiple studies on L. monocytogenes in milk and dairy products have been conducted since then. Raw milk contamination rates as high as 45% in Spain and 12% in the United States have been observed (Fenlon and Wilson 1989). A Brazilian researcher studied the prevalence of Listeria spp. in raw and pasteurized milk between October 1989 and 1990 and identified that the bacteria in 12.7% of raw milk samples and 0.9% of pasteurized milk samples (Moura et al. 1993). Another study conducted over a year at a milk processing facility in Northern Ireland and published in 2019 found that 4.4% of raw milk samples and 5.6% of pasteurized milk samples were infected with Listeria spp., (Shamloo et al. 2019). A survey conducted in Latvia found that Listeria spp. was most commonly found in raw milk that had been prepared using traditional methods. Bulk milk from an organic dairy farm was most likely to have L. monocytogenes, while the rate of infection was found to be three times higher in milk samples from conventional dairy farms, with 33 samples infected compared to 211 samples (Konosonoka et al. 2012). Researchers in Ethiopia found that raw milk and other dairy products contained 5.6% of Listeria spp., which includes L. monocytogenes. In a surprising finding, scientists determined that raw milk had the lowest contamination rate (18.9%) compared to all other types of milk (Seyoum et al. 2015).
A Finnish study found that milk could get contaminated after pasteurization. Bottled raw milk had higher L. monocytogenes (4.8% vs. 1.7%) than fresh bulk tank milk (Rodríguez-Díaz et al. 2022). Milk filter socks had 39% germs. They found that refrigerating milk reduced Listeria growth. The latest Iranian report from Isfahan found Listeria spp. in raw milk, ice cream, cream, and porridge at 5.49%, 19.04%, 11.11%, and 4%, respectively (Shamloo et al. 2019). However, they found no Listeria spp. in the yogurt, butter, kashk, or cheese. L. innocua and L. monocytogenes were the two most dominant species, with prevalence rates of 5.44% and 1.36%, respectively, according to earlier data from Iran (Sayevand et al. 2018).
Survival strategies of L. monocytogenes in adverse environmental conditions
Temperatures
Listeria monocytogenes, a versatile Gram-positive bacterium, possesses adaptive mechanisms that enable it to grow and survive in a wide range of temperatures. The bacterium can withstand low temperatures without significant changes in its live population, allowing it to persist in refrigerated environments (Buchanan et al. 2017). When exposed to cold stress, L. monocytogenes exhibit distinct responses to ensure its survival. To accumulate compatible solutes and enhance its cold tolerance, L. monocytogenes utilizes a chill-activated transport system that facilitates the uptake of glycine betaine and carnitine from the surrounding environment (Angelidis and Smith 2003). These organic osmolytes, found in various foods, provide support for the bacterium's ability to multiply and survive at lower temperatures. The gbu operon encodes the glycine betaine transporter (gbu), while the opuC ABC transporter, derived from the opuC operon, is responsible for the transport of carnitine in response to cold shock (Angelidis and Smith 2003).
In response to cold stress, the sigma factor protein σB (SigB) in L. monocytogenes plays a crucial role. SigB is activated upon temperature shift and enables the bacterium to accumulate solutes like betaine and carnitine (Dorey et al. 2019). The absence of SigB impairs the bacterium's ability to adapt to lower temperatures, particularly in stationary-phase cells (Jaishankar and Srivastava 2017). SigB is also involved in regulating specific genes, such as the opuCA gene, which encodes the opuCA protein with ATPase-coupled transmembrane transporter activity (Osek et al. 2022). At higher temperatures, L. monocytogenes responds by producing heat shock proteins (HSPs). These proteins, categorized into three classes, play roles in stabilizing, repairing, and preventing the aggregation of denatured proteins within the bacterial cell (Buchanan et al. 2017). Class I HSPs, including grpE, dnaK, dnaJ, groEL, and groES, act as intracellular chaperones and are upregulated when heat-induced protein denaturation occurs. The regulation of class I HSP genes involves the HrcA repressor. In growth-restricting conditions, the alternative sigma factor SigB is essential for the transcription of class II HSP genes (Buchanan et al. 2017). Class III HSP genes, such as clpP, clpE, and clpC operons, are negatively regulated by the ctsR regulator. The mcsB gene within the clpC operon produces mcsB kinase, which prevents the binding of ctsR to gene promoters, facilitating gene expression upon temperature increase (Buchanan et al. 2017).
pH
Foods that have undergone acidification, a common preservation technique for dairy products, meat, and vegetables, create a low pH environment through fermentation by bacteria present in the raw food or added as starter cultures (Osek et al. 2022). L. monocytogenes encounters such acidic conditions in the digestive system of its host. The low pH environment leads to an increase in hydrogen proton concentration, inhibiting microbial growth. Interestingly, low pH also enhances the virulence of L. monocytogenes and protects against other environmental stressors, allowing the bacteria to persist (Chlebicz and Śliżewska 2018). To maintain optimal intracellular pH for growth and survival, L. monocytogenes employs various metabolic and homeostatic mechanisms. It utilizes the glutamate decarboxylase (GAD) system and an internal proton pump to enhance cytoplasmic buffer capacity (Wiktorczyk-Kapischke et al. 2023). The GAD mechanism is considered a primary mechanism for intracellular homeostasis. Most L. monocytogenes strains possess five genes associated with GAD, including three decarboxylases (gadD1, gadD2, and gadD3) and two antiporters (gadT1 and gadT2) (Karatzas et al. 2012). These genes are located in three distinct genetic loci: gadD1T1, gadT2D2, and gadD3. Glutamate decarboxylase converts cytosolic glutamate to the neutral molecule γ-aminobutyrate (GABA), resulting in a drop in internal proton levels and an increase in the intracellular pH of L. monocytogenes cells (Osek et al. 2022).
In the food production environment, there are several sub-lethal alkaline stress factors, such as detergents and disinfectants that L. monocytogenes encounters. The bacterium has developed mechanisms to tolerate high pH-related environments, leading to cross-resistance against more severe stress factors like heat, alkali, ethanol stresses, and cleaning operations (Buchanan et al. 2017). L. monocytogenes responds to alkaline stress through various strategies to maintain cytoplasmic pH. These include deamination of amino acids and fermentation of carbohydrates to increase intracellular acid production. Additionally, the bacteria activate transporters and enzymes crucial for cell surface modifications and proton retention (Diether and Willing 2019). Research has shown that monovalent cation-proton antiporters play a vital role in maintaining neutral cytoplasmic pH, enabling bacterial growth under alkaline conditions (Osek et al. 2022).
Osmotic shock
The food-borne bacterium L. monocytogenes is remarkably resistant to osmotic stress and high salt concentrations. It grows in a 12% NaCl medium and can survive in a 20% NaCl environment (Osek et al. 2022). Plasmolysis and a drop in intracellular turgor pressure result from the high amounts of NaCl present in the environment, which limits bacterial growth. Increased osmotic pressure and decreased electrochemical potential across the cell membrane caused by NaCl effect on L. monocytogenes by disrupting the ATP-generating process of oxidative phosphorylation (Osek et al. 2022). Listeria monocytogenes use both primary and secondary response mechanisms during osmoadaptation, its reaction to osmotic stress. The high-affinity KdpABC transporter system and the low-affinity system produced by the lmo0993 gene are essential for the organism to adjust to high salt concentrations (Buchanan et al. 2017). To survive in environments with a lot of salt, L. monocytogenes relies heavily on these transporters. Intriguingly, L. monocytogenes activates many osmotolerance-associated genes not only in response to osmotic stress but also to other stressful environmental circumstances such as low temperature, low pH, and artificial food acidification. These genes aid the virus in replicating and adapting to its environment (Angelidis and Smith 2003). Genes involved in the uptake of b-glucoside, galactose, fructose, and cellobiose are downregulated in L. monocytogenes under osmotic stress conditions. When bacteria are subjected to osmotic stress, their growth rate slows down and they take in less glucose (Angelidis and Smith 2003).
Strain variation and pathogenicity
Listeria monocytogenes exhibit resistance and tolerance to both phages and quaternary ammonium disinfectants (quats). The ability to tolerate disinfectants can arise from the acquisition of new genes or gene mutations (Møretrø et al. 2017). Multiple efflux mechanisms, acquired through horizontal gene transfer, mediate resistance to quats. One such mechanism is mediated by Tn6188, typically found in serotype 1/2a strains, which carries qacH and facilitates the efflux of quaternary ammonium disinfectants (Müller et al. 2014). Another mechanism involves the gene bcrABC, carried by a separate transposon and commonly found on plasmids in strains belonging to various clonal groups and serotypes, which also mediates quat tolerance via efflux. Additionally, ermB, located on a chromosomal island, mediates quat resistance in the CC8 clone associated with the 2008 outbreak of listeriosis in Canada caused by deli meats (Partridge et al. 2018).
Different strains of L. monocytogenes have different levels of virulence; however, serotype 4b is commonly linked to epidemics in the United States (Buchanan et al. 2017). The reported pathogenicity islands and genes found in L. monocytogenes are mentioned in Table 1. In animal and cell culture models, some strains are less infectious than others, and the processes driving this reduced virulence are not well known. Premature stop codons (PMSC) in the inlA gene of strains 1/2a, 12b, and 1/2c have been discovered through research, and this finding may have an impact on the strains' ability to invade human epithelial cells (Cruz et al. 2014). It takes a greater bacterial load (3 logs more cells) from PMSC isolates with inlA mutations compared to fully virulent cells (Cruz et al. 2014) to produce infection For some reason, certain strains of L. monocytogenes are more commonly discovered in food or processing environments than linked with clinical cases (Manuel et al. 2015). This may be because these strains have PMSC mutations in inlA. Serotype 1/2c PMSCs showed inlA but not inlB mutations, and no strains possessed lapB, aut, flopA, ami, or vip gene deletions. Although prfA gene changes that cause reduced virulence are infrequent, they have been connected to some strains (Buchanan et al. 2017). It appears that genes and gene cassettes conferring resistance to quaternary ammonium disinfectants and phages were acquired from other bacteria via horizontal gene transfer. Possible improved resistance and adaptability to industrial settings in strains harboring these genes. Although several virulence factors have been identified, their effect on the organism is poorly understood (Muhterem-Uyar et al. 2018).
Resistance and persistence
Listeria monocytogenes “persistent strains” are bacterial clones that have been cultured repeatedly from the same environmental setting. Genome-based methods, such as pulsed-field gel electrophoresis (PFGE) or, more recently, next-generation sequencing (NGS), reveal that the molecular backgrounds of such isolates are identical (Unrath et al. 2021). Tolerance to disinfectants, cold resistance, metal resistance, and the ability to produce biofilm all contribute to L. monocytogenes continued survival, among other characteristics. Food producers have a significant issue from persistent isolates since they are linked to cross-contamination of food items and are rarely if ever, eradicated from environments where food is produced (Mazaheri et al. 2021). The persistence of L. monocytogenes may be influenced by several genetic factors, although the nature of these factors' contributions to the persistence phenomenon is still unclear. This might be because it's challenging to set up in vitro research in a way that correctly replicates the natural environment seen in food-producing facilities (Osek et al. 2022). After cleaning and disinfection, persistent L. monocytogenes strains have been identified from settings associated with food production. There has been research on the connection between resistance to different biocides and the persistence of particular L. monocytogenes subtypes in various food processing environments, but no conclusive link has been found (Mazaheri et al. 2021). On the other hand, studies have revealed a connection between some strains' persistence and their ability to resist benzalkonium chloride, particularly those that are positive for the bcrABC gene cassette (Cherifi et al. 2018). Several researchers have looked into the connection between L. monocytogenes biofilm development and survival in contexts where food is produced. In comparison to non-persistent strains, persistent strains typically exhibit higher biofilm development (Wiktorczyk-Kapischke et al. 2022). The ability of two L. monocytogenes strains with and without persistent capacity to cling to stainless steel surfaces was studied. It was found that the biofilms created by persistent strains on stainless steel surfaces are thicker than those formed by strains found only intermittently. It was demonstrated that persistent strains had considerably larger mean adherent cell counts throughout a 24-h period at 25 °C (Borucki et al. 2003). Besides this, the mixed biofilms formed by persistent L. monocytogenes and other bacteria often exhibit higher resistance to disinfectants and antibiotics (Chen et al. 2023).
Previous studies have indicated that serotype 4b of L. monocytogenes, particularly the persistent strains, exhibits remarkable resistance to high temperatures and pressure. Persistent clones of L. monocytogenes have demonstrated stronger evidence of cadmium resistance compared to sporadic contaminating strains (Lee et al. 2013). However, both persistent and non-persistent populations of L. monocytogenes have shown similar frequencies of known cadmium resistance cassettes. The presence of the cadA1 gene, which provides lesser resistance to cadmium, was found to be more prevalent in persisters, while the cadA4 sequence was only present in non-persistent isolates (Palaiodimou et al. 2021). In environments associated with food production, persistent L. monocytogenes populations tend to over-express two stress survival islets (SSIs) known as SSI-1 and SSI-2, which contribute to growth and survival under adverse conditions such as low pH, alkaline pH, and oxidative stress (Taylor and Stasiewicz 2019).
Novel strategies to control L. monocytogenes
The food industry has developed and executed a number of strategies for eliminating L. monocytogenes from the food supply. One of these is the use of gamma radiation in an irradiation processing method (Osek et al. 2022). This method of food preservation is used all around the world because it is safe and effective. When food is irradiated, harmful bacteria like L. monocytogenes and spoilage-causing bacteria are eliminated (Munir and Federighi 2020). Another method for sterilizing food and production facilities against harmful microorganisms is the use of ozone (Panebianco et al. 2022). Gaseous ozone at a concentration of 50 ppm for 10 min has been found to kill planktonic cells and biofilm of reference and food-related L. monocytogenes strains. A significant reduction in biofilm biomass and full inactivation of planktonic cells after only 6 h of treatment (Panebianco et al. 2022). This environmentally friendly technique is generally regarded as safe (GRAS). It has been suggested that gaseous ozone be utilized to reduce the possibility of L. monocytogenes contamination on food-contact surfaces and in final products (Botta et al. 2020). Phages offer a fresh alternative biological approach to controlling L. monocytogenes in the food chain (Kawacka et al. 2020). The industrial guidelines to prevent Listeria contamination during food processing is mentioned in Fig. 2. Phages are useful in the fight against bacterial diseases since they are only harmful to their host bacteria and not to any other microbes. They are crucial in the creation of fermented meals because they do not detract from the final product's sensory qualities (Botta et al. 2020). Commercial phage-based therapies, such as ListShield™ (Intralytics, Columbia, MD, U.S.) a cocktail of six various lytic bacteriophages have been used to successfully decrease L. monocytogenes contamination in many food products, including RTE meats (Perera et al. 2015). It is reported that the ListShield™ successfully reduces L. monocytogenes levels in RTE foods by 82–98% (Perera et al. 2015).
Despite the promising results and suggestions, bacteriophages are only licensed in a few numbers of countries and laws often apply to specific bacteriophage products (Kawacka et al. 2020). Listex™ P100, for instance, sees extensive use on a global scale, particularly in the USA, Canada, and Switzerland. Połaska and Sokołowska, (2019) reported that its usage as a processing aid is legal in Australia, New Zealand, Israel, the Netherlands, and Switzerland, but that approval varies widely among countries. The existence of phage-resistant strains in food processing facilities should be monitored, and more study is needed to determine the efficacy and safety of phage-based therapies against L. monocytogenes in foods.
Conclusion
Listeria monocytogenes is a devastating food-borne illness that has a high global fatality rate, especially among the elderly, pregnant women, and newborns. Despite of relatively consistent infection rates over the past decade, a new study has indicated that smaller outbreaks of the invasive form of the disease occur more frequently than previously thought. L. monocytogenes can be difficult to eradicate because of its capacity to build biofilms in food production environments, which contributes to its persistence and poses issues in eradication, and may result in contamination of food and facilities. To ensure food safety, it is important to understand how L. monocytogenes adapt to environmental stress factors so that efficient and cost-effective pathogen control methods can be developed for the food sector.
Future directions
There are still many unanswered questions about L. monocytogenes and listeriosis that need to be investigated. The first step in keeping up with any shifts in epidemiology and spotting new patterns caused by L. monocytogenes is constant surveillance and monitoring of outbreaks and infection rates. Identifying the causes of the uptick in localized invasions will allow for more precise measures to be taken against them. It is also important to study the biofilm development and persistence mechanisms of L. monocytogenes in food production environments at the molecular level. To do so requires investigating potential survival mechanisms, such as biofilm-related genes and surface structures. In order to reduce the likelihood of contamination and subsequent occurrences of listeriosis, it will be essential to develop novel anti-biofilm chemicals and improve cleaning and sanitation methods.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- EFSA:
-
European Food Safety Authority
- ECDC:
-
European Centre for Disease Prevention and Control
- CDC:
-
Centers for Disease Control and Prevention
- WGS:
-
Whole genome sequencing
- PFGE:
-
Pulsed-field gel electrophoresis
- FDA:
-
Food and Drug Administration
- CFSAN:
-
Center for Food Safety and Applied Nutrition
- SNPs:
-
Single Nucleotide Polymorphisms
- wgMLST:
-
Whole genome multi-locus sequence typing
- FSIS:
-
Food Safety and Inspection Service
- EU:
-
European Union
- RTE:
-
Ready-to-eat
- WHO:
-
World health organization
- NGS:
-
Next-generation sequencing
- SSIs:
-
Stress survival islets
- GRAS:
-
Generally regarded as safe
References
Alvarez-Domínguez C, Vázquez-Boland JA, Carrasco-Marín E et al (1997) Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect Immun 65:78–88. https://doi.org/10.1128/iai.65.1.78-88.1997
Angelidis AS, Smith GM (2003) Role of the glycine betaine and carnitine transporters in adaptation of Listeria monocytogenes to chill stress in defined medium. Appl Environ Microbiol 69:7492–7498. https://doi.org/10.1128/AEM.69.12.7492-7498.2003
Bintsis T (2017) Foodborne Pathogens. AIMS Microbiol 3:529–563. https://doi.org/10.3934/microbiol.2017.3.529
Borucki MK, Peppin JD, White D et al (2003) Variation in biofilm formation among strains of Listeria monocytogenes. Appl Environ Microbiol 69:7336–7342. https://doi.org/10.1128/AEM.69.12.7336-7342.2003
Botta C, Ferrocino I, Pessione A et al (2020) Spatiotemporal distribution of the environmental microbiota in food processing plants as impacted by cleaning and sanitizing procedures: the case of slaughterhouses and gaseous ozone. Appl Environ Microbiol 86:1–15. https://doi.org/10.1128/AEM.01861-20
Brown E, Dessai U, Mcgarry S, Gerner-Smidt P (2019) Use of whole-genome sequencing for food safety and public health in the United States. Foodborne Pathog Dis 16:441–450. https://doi.org/10.1089/fpd.2019.2662
Brusa V, Prieto M, Campos CA et al (2021) Quantitative risk assessment of listeriosis associated with fermented sausage and dry-cured pork shoulder consumption in Argentina. Food Control 123:107705. https://doi.org/10.1016/j.foodcont.2020.107705
Buchanan RL, Gorris LGM, Hayman MM et al (2017) A review of Listeria monocytogenes: an update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 75:1–13. https://doi.org/10.1016/j.foodcont.2016.12.016
Carstens CK, Salazar JK, Darkoh C (2019) Multistate outbreaks of foodborne illness in the United States associated with fresh produce from 2010 to 2017. Front Microbiol 10:1–15. https://doi.org/10.3389/fmicb.2019.02667
CDC (2018) Estimated annual number of episodes of illnesses caused by 31 pathogens transmitted commonly by food , United States * National Center for Emerging and Zoonotic Infectious Diseases. Cdc 228412
Chen Y, Burall LS, Luo Y et al (2016) Listeria monocytogenes in stone fruits linked to a multistate outbreak: enumeration of cells and whole-genome sequencing. Appl Environ Microbiol 82:7030–7040. https://doi.org/10.1128/AEM.01486-16
Chen Y, Luo Y, Carleton H et al (2017) Whole genome and core genome multilocus sequence typing and single nucleotide polymorphism analyses of Listeria monocytogenes isolates associated with an outbreak linked to cheese, United States, 2013. Appl Environ Microbiol 83:1–15. https://doi.org/10.1128/AEM.00633-17
Chen Q, Zhang X, Wang Q et al (2023) The mixed biofilm formed by Listeria monocytogenes and other bacteria: formation, interaction and control strategies. Crit Rev Food Sci Nutr 17:1–17. https://doi.org/10.1080/10408398.2023.2200861
Cheng WN, Han SG (2020) Bovine mastitis: risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas J Anim Sci 33:1699–1713. https://doi.org/10.5713/ajas.20.0156
Cherifi T, Carrillo C, Lambert D et al (2018) Genomic characterization of Listeria monocytogenes isolates reveals that their persistence in a pig slaughterhouse is linked to the presence of benzalkonium chloride resistance genes. BMC Microbiol 18:1–13. https://doi.org/10.1186/s12866-018-1363-9
Chlebicz A, Śliżewska K (2018) Campylobacteriosis, Salmonellosis, Yersiniosis, and Listeriosis as zoonotic foodborne diseases: a review. Int J Environ Res Public Health 15:1–28. https://doi.org/10.3390/ijerph15050863
Clayton EM, Daly KM, Guinane CM et al (2014) Atypical Listeria innocua strains possess an intact LIPI-3. BMC Microbiol. https://doi.org/10.1186/1471-2180-14-58
Cruz CD, Pitman AR, Harrow SA, Fletcher GC (2014) Listeria monocytogenes associated with New Zealand seafood production and clinical cases: unique sequence types, truncated InlA, and attenuated invasiveness. Appl Environ Microbiol 80:1489–1497. https://doi.org/10.1128/AEM.03305-13
Desai AN, Anyoha A, Madoff LC, Lassmann B (2019) Changing epidemiology of Listeria monocytogenes outbreaks, sporadic cases, and recalls globally: a review of ProMED reports from 1996 to 2018. Int J Infect Dis 84:48–53. https://doi.org/10.1016/j.ijid.2019.04.021
Diether NE, Willing BP (2019) Microbial fermentation of dietary protein: an important factor in diet–microbe–host interaction. Microorganisms. https://doi.org/10.3390/microorganisms7010019
Dorey A, Marinho C, Piveteau P, O’Byrne C (2019) Role and regulation of the stress activated sigma factor sigma B (σ B ) in the saprophytic and host-associated life stages of Listeria monocytogenes, 1st edn. Elsevier Inc., Amsterdam
Fenlon DR, Wilson J (1989) The incidence of Listeria monocytogenes in raw milk from farm bulk tanks in North-East Scotland. J Appl Bacteriol 66:191–196. https://doi.org/10.1111/j.1365-2672.1989.tb02469.x
Garner D, Kathariou S (2016) Fresh produce-associated listeriosis outbreaks, sources of concern, teachable moments, and insights. J Food Prot 79:337–344. https://doi.org/10.4315/0362-028X.JFP-15-387
Gaul LK, Farag NH, Shim T et al (2013) Hospital-acquired listeriosis outbreak caused by contaminated diced celery-texas, 2010. Clin Infect Dis 56:20–26. https://doi.org/10.1093/cid/cis817
Górska-Warsewicz H, Rejman K, Laskowski W, Czeczotko M (2019) Milk and dairy products and their nutritional contribution to the average polish diet. Nutrients. https://doi.org/10.3390/nu11081771
Guariglia-Oropeza V, Orsi RH, Yu H et al (2014) Regulatory network features in Listeria monocytogenes-changing the way we talk. Front Cell Infect Microbiol 5:1–7. https://doi.org/10.3389/fcimb.2014.00014
Houlihan CF, Whitworth JAG (2019) Outbreak science: recent progress in the detection and response to outbreaks of infectious diseases. Clin Med J R Coll Physicians Lond 19:140–144. https://doi.org/10.7861/CLINMEDICINE.19-2-140
Jaishankar J, Srivastava P (2017) Molecular basis of stationary phase survival and applications. Front Microbiol 8:1–12. https://doi.org/10.3389/fmicb.2017.02000
Karatzas KAG, Suur L, O’Byrne CP (2012) Characterization of the intracellular glutamate decarboxylase system: analysis of its function, transcription, and role in the acid resistance of various strains of Listeria monocytogenes. Appl Environ Microbiol 78:3571–3579. https://doi.org/10.1128/AEM.00227-12
Kawacka I, Olejnik-Schmidt A, Schmidt M, Sip A (2020) Effectiveness of phage-based inhibition of Listeria monocytogenes in food products and food processing environments. Microorganisms 8:1–20. https://doi.org/10.3390/microorganisms8111764
Konosonoka IH, Jemeljanovs A, Osmane B et al (2012) Incidence of Listeria spp. in dairy cows feed and raw milk in Latvia. ISRN Vet Sci 2012:1–5. https://doi.org/10.5402/2012/435187
Kuenne C, Billion A, Mraheil MA et al (2013) Reassessment of the Listeria monocytogenes pan-genome reveals dynamic integration hotspots and mobile genetic elements as major components of the accessory genome. BMC Genomics 14:1–19. https://doi.org/10.1186/1471-2164-14-47
Lee S, Rakic-Martinez M, Graves LM et al (2013) Genetic determinants for cadmium and arsenic resistance among Listeria monocytogenes serotype 4B isolates from sporadic human listeriosis patients. Appl Environ Microbiol 79:2471–2476. https://doi.org/10.1128/AEM.03551-12
Manuel CS, Van SA, Wiedmann M et al (2015) Prevalence and distribution of Listeria monocytogenes inlA alleles prone to phase variation and inlA alleles with premature stop codon mutations among human, food, animal, and environmental isolates. Appl Environ Microbiol 81:8339–8345. https://doi.org/10.1128/AEM.02752-15
Marquis H, Goldfine H, Portnoy DA (1997) Proteolytic pathways of activation and degradation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J Cell Biol 137:1381–1392. https://doi.org/10.1083/jcb.137.6.1381
Mateus T, Silva J, Maia RL, Teixeira P (2013) Listeriosis during pregnancy: a public health concern. ISRN Obstet Gynecol 2013:1–6. https://doi.org/10.1155/2013/851712
Maury MM, Tsai YH, Charlier C et al (2016) Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat Genet 48:308–313. https://doi.org/10.1038/ng.3501
Maury MM, Bracq-Dieye H, Huang L et al (2019) Hypervirulent Listeria monocytogenes clones’ adaption to mammalian gut accounts for their association with dairy products. Nat Commun. https://doi.org/10.1038/s41467-019-10380-0
Mazaheri T, Cervantes-Huamán BRH, Bermúdez-Capdevila M et al (2021) Listeria monocytogenes biofilms in the food industry: is the current hygiene program sufficient to combat the persistence of the pathogen? Microorganisms 9:1–19. https://doi.org/10.3390/microorganisms9010181
Mehlhorn H (2016) Food-borne disease burden epidemiology reference group. Encycl Parasitol. https://doi.org/10.1007/978-3-662-43978-4_3884
Miro E, Rossen JWA, Chlebowicz MA et al (2020) Core/whole genome multilocus sequence typing and core genome SNP-based typing of OXA-48-producing Klebsiella pneumoniae clinical isolates from Spain. Front Microbiol. https://doi.org/10.3389/fmicb.2019.02961
Møretrø T, Schirmer BCT, Heir E et al (2017) Tolerance to quaternary ammonium compound disinfectants may enhance growth of Listeria monocytogenes in the food industry. Int J Food Microbiol 241:215–224. https://doi.org/10.1016/j.ijfoodmicro.2016.10.025
Moura SM, Destro MT, Franco BDGM (1993) Incidence of Listeria species in raw and pasteurized milk produced in São Paulo, Brazil. Int J Food Microbiol 19:229–237. https://doi.org/10.1016/0168-1605(93)90080-Z
Moura A, Tourdjman M, Leclercq A et al (2017) Real-time whole-genome sequencing for surveillance of Listeria monocytogenes, France. Emerg Infect Dis 23:1462–1470. https://doi.org/10.3201/eid2309.170336
Muhterem-Uyar M, Ciolacu L, Wagner KH et al (2018) New aspects on Listeria monocytogenes ST5-ECVI predominance in a heavily contaminated cheese processing environment. Front Microbiol 9:1–14. https://doi.org/10.3389/fmicb.2018.00064
Müller A, Rychli K, Zaiser A et al (2014) The Listeria monocytogenes transposon Tn6188 provides increased tolerance to various quaternary ammonium compounds and ethidium bromide. FEMS Microbiol Lett 361:166–173. https://doi.org/10.1111/1574-6968.12626
Munir MT, Federighi M (2020) Control of foodborne biological hazards by ionizing radiations. Foods 9:1–23. https://doi.org/10.3390/foods9070878
Osek J, Lachtara B, Wieczorek K (2022) Listeria monocytogenes—how this pathogen survives in food-production environments? Front Microbiol 13:1–21. https://doi.org/10.3389/fmicb.2022.866462
Palaiodimou L, Fanning S, Fox EM (2021) Genomic insights into persistence of Listeria species in the food processing environment. J Appl Microbiol 131:2082–2094. https://doi.org/10.1111/jam.15089
Panebianco F, Rubiola S, Di Ciccio PA (2022) The use of ozone as an eco-friendly strategy against microbial biofilm in dairy manufacturing plants: a review. Microorganisms. https://doi.org/10.3390/microorganisms10010162
Parida SK, Mohapatra T (2016) Whole genome sequencing. Princ Pract Plant Genomics 3:120–174. https://doi.org/10.1201/9781439845523-6
Partridge SR, Kwong SM, Firth N, Jensen SO (2018) Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev 31:1–61. https://doi.org/10.1128/CMR.00088-17
Perera MN, Abuladze T, Li M et al (2015) Bacteriophage cocktail significantly reduces or eliminates Listeria monocytogenes contamination on lettuce, apples, cheese, smoked salmon and frozen foods. Food Microbiol 52:42–48. https://doi.org/10.1016/j.fm.2015.06.006
Petrišič N, Kozorog M, Aden S et al (2021) The molecular mechanisms of listeriolysin O-induced lipid membrane damage. Biochim Biophys Acta Biomembr. https://doi.org/10.1016/j.bbamem.2021.183604
Pistor S, Chakraborty T, Niebuhr K et al (1994) The ActA protein of Listeria monocytogenes acts as a nucleator inducing reorganization of the actin cytoskeleton. EMBO J 13:758–763. https://doi.org/10.1002/j.1460-2075.1994.tb06318.x
Połaska M, Sokołowska B (2019) Review bacteriophages—a new hope or a huge problem in the food industry. AIMS Microbiol 5:324–347. https://doi.org/10.3934/microbiol.2019.4.324
Pouillot R, Klontz KC, Chen Y et al (2016) Infectious dose of Listeria monocytogenes in outbreak linked to ice cream, United States, 2015. Emerg Infect Dis 22:2113–2119. https://doi.org/10.3201/eid2212.160165
Ricci A, Allende A, Bolton D et al (2018) Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. https://doi.org/10.2903/j.efsa.2018.5134
Rodríguez-Díaz JM, Calahorrano-Moreno MB, Ordoñez-Bailon JJ et al (2022) Contaminants in the cow’s milk we consume? pasteurization and other technologies in the elimination of contaminants. F100Research 11:1–34. https://doi.org/10.12688/f1000research.108779.1
Sayevand HR, Bakhtiary F, Pointner A et al (2018) Bacterial diversity in traditional doogh in comparison to industrial doogh. Curr Microbiol 75:386–393. https://doi.org/10.1007/s00284-017-1392-x
Seyoum ET, Woldetsadik DA, Mekonen TK et al (2015) Prevalence of Listeria monocytogenes in raw bovine milk and milk products from central highlands of Ethiopia. J Infect Dev Ctries 9:1204–1209. https://doi.org/10.3855/jidc.6211
Shamloo E, Hosseini H, Moghadam AZ et al (2019) Importance of Listeria monocytogenes in food safety: a review of its prevalence, detection, and antibiotic resistance. Iran J Vet Res 20:241–254
Sibanda T, Buys EM (2022) Listeria monocytogenes pathogenesis: the role of stress adaptation. Microorganisms. https://doi.org/10.3390/microorganisms10081522
Taylor AJ, Stasiewicz MJ (2019) Persistent and sporadic Listeria monocytogenes strains do not differ when growing at 37 °c, in planktonic state, under different food associated stresses or energy sources. BMC Microbiol 19:1–13. https://doi.org/10.1186/s12866-019-1631-3
Te Giffel MC, Zwietering MH (1999) Validation of predictive models describing the growth of Listeria monocytogenes. Int J Food Microbioal 46:135–149. https://doi.org/10.1016/S0168-1605(98)00189-5
Unrath N, McCabe E, Macori G, Fanning S (2021) Application of whole genome sequencing to aid in deciphering the persistence potential of Listeria monocytogenes in food production environments. Microorganisms. https://doi.org/10.3390/microorganisms9091856
Wechsler H, Levine S, Idelson RK et al (1983) The physician’s role in health promotion-a survey of primary-care practitioners. N Engl J Med 308(2):97–100. https://doi.org/10.1056/NEJM198301133080211
Wiktorczyk-Kapischke N, Wałecka-Zacharska E, Skowron K et al (2022) Comparison of selected phenotypic features of persistent and sporadic strains of Listeria monocytogenes sampled from fish processing plants. Foods. https://doi.org/10.3390/foods11101492
Wiktorczyk-Kapischke N, Skowron K, Wałecka-Zacharska E et al (2023) Assessment of the influence of selected stress factors on the growth and survival of Listeria monocytogenes. BMC Microbiol 23:1–21. https://doi.org/10.1186/s12866-023-02766-4
Won S, Lee J, Kim J et al (2020) Comparative whole cell proteomics of Listeria monocytogenes at different growth temperatures. J Microbiol Biotechnol 30:259–270. https://doi.org/10.4014/jmb.1911.11027
Funding
No funding was received for the said study.
Author information
Authors and Affiliations
Contributions
RA: conceptualization, design of the study, writing, review, and editing. VK: writing, review, and editing. AJJ: conceptualization, design of the study, writing, review, and editing.
Corresponding author
Ethics declarations
Conflicts of interest
Ahire JJ was employed by Dr. Reddy’s Laboratories Limited. Dr. Reddy’s Laboratories had no direct and indirect role in the study design/analysis/writing/publication of this review article. Other authors have no conflict of interest to declare.
Consent for publication
The authors give their consent for the publication of the manuscript, which includes figures within the text to be published in the Journal of Food Science and Technology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rohilla, A., Kumar, V. & Ahire, J.J. Unveiling the persistent threat: recent insights into Listeria monocytogenes adaptation, biofilm formation, and pathogenicity in foodborne infections. J Food Sci Technol 61, 1428–1438 (2024). https://doi.org/10.1007/s13197-023-05918-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05918-6