Abstract
Huge amount of waste is generated by the pineapple processing industries which raises concerns regarding its safe disposal into the environment. This ever-increasing problem of waste management can be solved by the valorization of pineapple by-products to high-value compounds. The extraction of proteolytic enzyme, bromelain from pineapple rind using green techniques can help to overcome the drawbacks associated with conventional methods. In the present study, the extraction of bromelain from pineapple rind using microwave assisted technique resulted in considerable amount of proteolytic activity (127.8 U/mL) and protein content (2.55 mg/mL). The optimized extraction conditions were found as 200 W microwave power, 1:5 solid/ liquid ratio and after treatment time of 10 min. Highest specific activity (512 U/mg) of bromelain was obtained after using gel filtration chromatography. FTIR result confirmed the presence of functional groups in bromelain, whereas, XRD analysis indicated the semi-crystalline nature of bromelain. The results indicated MAE as an effective green technique for the extraction of bromelain from pineapple rind. The proteolytic action of the extracted bromelain makes it a suitable functional ingredient for its applications in bakery, dairy, and seafood processing industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pineapple is a perennial herb known as Ananas comosus and belongs to the botanical family Bromeliaceae. After citrus, bananas and mangoes, the most important tropical fruit produced globally is pineapple in terms of its production and yield. The leading producers of pineapple are Thailand, Brazil, Costa Rica, China, Indonesia, Philippines, India, Nigeria, Colombia, Mexico (FAOSTAT 2020). Four varieties of pineapple are available around the world including Abacaxi, Smooth Cayenne, Queen, and Red Spanish (Huang et al. 2021). Pineapple has been utilized in fruit processing industries as a main ingredient in fruit juice concentrates, jellies, squash, jams, essence, and pickles. Besides having an acceptable taste and flavor, pineapple is rich source of essential nutrients, vitamins, minerals, dietary fiber, and, antioxidants. The rich composition of pineapple in nutritive substances provides various health benefits like prevention of colon cancer, immunomodulatory, anti-inflammatory, and antimicrobial activity.
Pineapple fruit is composed of 30% pulp, where, 70% of it is discarded in the form of peel, crown, core, trimmings, and residue (Nakthong et al. 2017). Therefore, a large amount of waste is generated by the pineapple processing industries which is often discarded into the environment without any further treatment. This may lead to serious environmental hazards due to emission of greenhouse gases during decomposition. This instigated for the valorization of pineapple by-products for its utilization as a source of proteins, and other bioactive compounds including a valuable proteolytic enzyme, bromelain. The bioactive compounds have been gaining importance for the development and commercialization of nutraceuticals and innovative functional food products, especially in the post-pandemic era (Galanakis 2023). The food ingredients and active compounds have been popularly known for health-promoting actions such as anti-inflammatory, antimicrobial, and antiviral effects (Galanakis et al. 2020).
Since pineapple is considered as the popular source of the protease enzyme, bromelain, it is known to be of the most important tropical fruit (Ketnawa et al. 2010). Bromelain is found in the rind, leaves, stem, crown, tubes, and bark of pineapple fruit (Mohan et al., 2016). Extensive applications of bromelain have been reported in pharmaceutical, food, and detergent industries. Some of the important areas of utilization of bromelain in the food industries include meat tenderization, baking, brewing, and, protein hydrolysates production (Ketnawa et al. 2012). Along with this, action of bromelain results in gentle peeling of skin, therefore, it has been used as an active ingredient in cosmetic products (Ramli et al., 2017).
The adopted extraction and purification strategy for bromelain should be cheap, high yielding and speedy (Gupta et al. 2004). In previous studies, the conventional approach for bromelain extraction involved cell disruption technique using homogenization and crushing of pineapple rind followed by filtration/centrifugation to obtain crude bromelain extract (Colletti et al. 2021). This process is time consuming, has high chances of presence of impurities, and results in lower extraction yield. Moreover, conventional isolation and purification techniques are considered obsolete due to their low purification potential (Soares et al. 2012). These problems can be circumvented with the use of emerging green technologies like microwave-assisted extraction (MAE) (Yan et al. 2020). MAE is an innovative technology which involves the use of microwave energy causing molecular motion by ionic conduction and dipole rotation (da Rosa et al. 2019). MAE leads to increase in temperature and pressure which causes changes in cell structure and increased penetration of solvent into the sample matrix.
The present study was carried out using popular commercial variety of pineapple i.e., ‘Kew, which is mainly grown (70%) around the world (TFNET News Compilation, 2016). Extraction of crude bromelain from pineapple rind was done using MAE technique. Further, optimization, purification (using dialysis and gel filtration chromatography method) as well as structural characterization was also carried out.
Materials and methods
Materials
The pineapple rind was procured from the fruit processors in local markets of Longowal, Punjab, India. The pineapple rind was then dried using cabinet dryer (40 ± 1 °C) for 6 h and grounded to make fine powder. The pineapple rind powder was stored in the refrigerator (4 °C) till further analysis. All the chemicals and enzymes used in this experimental work were of analytical grade.
Proximate analysis
The proximate analysis of pineapple rind powder was carried out to analyze moisture, protein, fat, crude fiber, ash content following the standard protocol (AOAC 2007). Kjeldahl method was used for determination of nitrogen content and multiplied with factor 6.25 for conversion to protein. The available carbohydrates were determined by subtraction of percentage sum of other proximate constituents from 100.
Microwave assisted extraction of crude bromelain
Extraction using distilled water
Preparation of crude bromelain extract from pineapple rind powder was carried out using microwave assisted extraction method (2450 MHz, Samsung Model MS23F301TAK/TL, Malaysia) and distilled water as the solvent (Ketnawa et al. 2012). The oven was modified for condensation of vapours produced in the sample during extraction (Kashyap et al. 2022). The pineapple rind powder (5 g) was added to distilled water (50 mL) and microwave treatment was given to it. The mixture was then centrifuged (5000 g, 10 min), filtered, and finally the supernatant was stored at 20° C.
Extraction using ethanol
Preparation of crude bromelain extract from pineapple rind powder was carried out using microwave assisted extraction with ethanol as solvent (Huang et al. 2021). The pineapple rind powder (5 g) was added to ethanol (50 mL) and microwave treatment was given to it. The mixture was then centrifuged (5000 g, 10 min), filtered, and finally the supernatant was stored at 20° C.
Extraction using acetone
Preparation of crude bromelain extract from pineapple rind powder was carried out using microwave assisted extraction with acetone as solvent (Huang et al. 2021). The pineapple rind powder (5 g) was added to acetone (50 mL) and microwave treatment was given to it. The mixture was then centrifuged (5000 g, 10 min), filtered, and finally the supernatant was stored at 20° C.
Extraction using phosphate buffer
Preparation of crude bromelain extract from pineapple rind powder was carried out using microwave assisted extraction with phosphate buffer as solvent (Gul et al., 2021). The pineapple rind powder (5 g) was added to acetone (50 mL) and microwave treatment was given to it. The mixture was then centrifuged (5000 g, 10 min), filtered, and finally the supernatant was stored at 20° C.
Extraction using NaOH and HCl solution
Preparation of crude bromelain extract from pineapple rind powder was carried out using microwave assisted extraction with different NaOH and HCl solutions (0.6 M) as solvent (Wen et al. 2021). The pineapple rind powder (5 g) was added to NaOH (0.6 M, 50 mL) and HCl solutions (0.6 M, 50 mL) and microwave treatment was given to it. The mixture was then centrifuged (5000 g, 10 min), filtered, and finally the supernatant was stored at 20o C.
Based on the preliminary trials, effect of solid to liquid ratio (1:3–1:10 g/mL), microwave power (100–300 W), and treatment time (5–10 min) was observed on the proteolytic activity and protein content of crude bromelain extracted from pineapple rind. The process optimization was carried out using response surface methodology.
Purification of crude bromelain extract
Ammonium sulphate precipitation
Initial purification of crude bromelain was carried out using ammonium sulphate precipitation method (Gautam et al. 2010). The process involved, pinch by pinch addition of ammonium sulphate salt (6.6 g) to crude extract (15 mL) with continuous stirring (45 min) on a magnetic stirrer and kept overnight at 4 °C. It was then centrifuged (10000 g, 10 min) and the collected pellet was re-dissolved in phosphate buffer (0.01 M, 10 mL) which was utilized for subsequent analysis.
Dialysis
Crude bromelain extract after initial purification by ammonium sulphate precipitation was subjected to dialysis for further purification (Gautam et al. 2010). The crude extract was dissolved in tris HCl buffer (10 mL, 10 mM) and placed in a dialysis bag. The bag was suspended in beaker (500 mL) containing phosphate buffer-NaCl solution (100 mM), the setup was kept in refrigerator and the solution was changed after every 6 h until there is no precipitation. The dialysis process was continued for almost 48 h and the obtained extract was stored for further analysis.
Gel filtration chromatography
Gel filtration chromatography was used for the purification of bromelain with Sephadex G-75 resin (Costa et al. 2014). The resin was packed into a glass column (200 mm long, 17.5 mm in diameter) and one and a half volume of column was equilibrated with acetate buffer. The resin was left in contact with the sample for one hour after application. Following an elution with acetate buffer (1 M, pH 4.5) at a flow rate of 0.7 mL/min, the eluent was collected and once no more protein was eluted, the column was washed using acetate buffer (5 × 10–3 M, pH 4.5). After purification, the enzyme was kept frozen at -20 °C.
Determination of bromelain activity
The determination of bromelain activity was done using casein as substrate (Mala et al. 2021). The solution containing enzyme (500 µL) was mixed with casein (0.65% (w/v), 2.5 mL) in potassium phosphate buffer (0.05 M, pH 7.5) and then kept in a water bath (37 °C, 10 min). The reaction was stopped when trichloroacetic acid (2.5 mL, 110 mM) was added. This was followed by centrifugation (5000 g, 10 min) after 20 min and the obtained supernatant was then mixed with sodium carbonate solution (2.5 mL, 500 mM) and folin ciocalteau phenol reagent (500 µL, 20%, v/v). Incubation was done at room temperature for 30 min and the absorbance was red at 660 nm using UV–vis spectrophotometer (DR 5000, HACH Germany).
The proteolytic activity (units/mL) was calculated by using following formula:
Specific activity and purification fold
Specific activity of bromelain was determined as the ratio of the total volumetric activity and protein concentration in the sample (Ye et al., 2021). The result of specific activity was expressed as (U/mg) activity units per mg of protein (Coelho et al. 2013).
Purification fold of enzyme was calculated as ratio of specific activity after purification to the initial specific activity.
Experimental design and data analysis
The effect of various process variables (solid/liquid ratio 1:3–1:10, microwave power 100–300 W, and time 5–15 min) on proteolytic activity and protein content of bromelain extracted from pineapple rind was determined by Response Surface Methodology (RSM) using Box-Behnken design (BBD) (Table 1). Design Expert 11.0.7.1 software (Statease Inc., Minneapolis, USA) was used for data analysis and study of regression equation coefficients. Verification of model was done by determination of R2. Validation of model was done using analysis of variance (ANOVA) and the model’s fit value was determined by statistical testing of each term with F-values of p ≤ 0.05.
Structural characterization of purified bromelain extract
Fourier transform infrared analysis
Fourier transform infrared (FTIR) analysis was carried out for identification of the specific components of the bromelain based on their functional groups. A diffuse reflectance accessory was used to carry out spectral analysis for all samples in an FTIR instrument (Perken Elmer Spectrum, RX-I, USA) for detection of characteristic chemical functional groups. The spectrum was then recorded with absorbance mode (4 cm−1 resolution) in the range of 600–4000 cm−1 (Nor et al. 2015).
X-ray diffraction analysis
Diffraction pattern of bromelain was determined using X-ray diffraction (XRD) analysis using PAN-analytic-X’pert PRO MRD (Almelo, Netherlands). At 40 kV and 25 mA tube current, measurements were done using Cu line as radiation source. Diffraction angle ranging from 3.0 to 45.0 o2θ was used for scanning of the samples at the scanning rate of 2° min−1 (Sharma and Sharma, 2018).
Results and discussion
The proximate analysis of pineapple rind as well as extraction of bromelain from pineapple rind was carried out using microwave assisted extraction. The process optimization as well as purification and structural elucidation of the extracted bromelain was also performed which has been discussed in this section.
Proximate analysis of pineapple rind
The results of proximate analysis of pineapple rind indicated moisture and carbohydrate content of 86.66 ± 0.07% and 18.65 ± 0.22%, respectively. The other proximate components included protein (3.98 ± 0.015%), ash (8.62 ± 0.33%), fat (0.98 ± 0.03%) and crude fiber (12.98 ± 0.16%). The proximate analysis of pineapple rind obtained in the current study was comparable to the earlier reported value of pineapple rind (Upadhyay et al. 2010). The fat, ash and fiber content of pineapple rind found in the current study was higher than the fat (0.63 ± 0.03%), ash (6.80 ± 0.28%) and crude fiber (13.96 ± 0.14%) content reported in previous studies on pineapple rind (Aruna 2019).
Effect of microwave assisted technique on the extraction of bromelain from pineapple rind
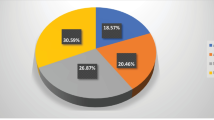
Extraction of bromelain from pineapple rind was carried out using microwave assisted technique which has been presented in Fig. 1. Extraction of bromelain from pineapple rind using microwave assisted extraction indicated the highest proteolytic activity (98.89 U/mL) and protein content (2.35 mg/mL) obtained using ethanol as the solvent. It was followed by HCl, distilled water, phosphate buffer and acetone and NaOH. In the previous studies on microwave assisted extraction of crude bromelain, the highest proteolytic activity and protein content was found to be 92.78 U/mL and 2.55 mg/mL, respectively (Mala et al. 2021). Based on the preliminary trials, process optimization of microwave assisted extraction of bromelain from pineapple rind was done using response surface methodology. The different extraction parameters including solid/ liquid ratio (1:3 to 1:10), microwave power (100 to 300 W), and time (5 to 15 min) were evaluated to study their effect on proteolytic activity and protein content of bromelain. BBD was chosen to study the interactive effect of different process variables.
Extraction of crude bromelain from pineapple rind for the determination of proteolytic activity by using microwave assisted extraction
MAE technique was used to extract crude bromelain from pineapple rind by varying process parameters (solid/liquid ratio (1:3 to 1:10), microwave power (100 to 300 W), and treatment time (5 to 15 min)) for the determination of proteolytic activity. The interactive effects of extraction parameters involved in the microwave assisted extraction of bromelain from pineapple rind on the proteolytic activity were presented by response surface plots (Fig. 2). A significant effect (p ≤ 0.05) of the extraction parameters (solid/liquid ratio, microwave power, and treatment time) was observed on the proteolytic activity of bromelain extracted from pineapple rind (Table 2a).
Interactive effect of solid/ liquid ratio and time on extraction of crude bromelain from pineapple rind has been depicted in Fig. 2a. The interactive effect of time and solid/liquid ratio indicated that with an increase in treatment time from 5 to 10 min and solid/ liquid ratio from 1:3 to 1:5, an increase in proteolytic activity was observed (127.8 U/mL), however, with further increase in time (15 min) and solid/liquid ratio (1:10), proteolytic activity was found to decrease (72.19 U/mL). This may be due to degradation of thermolabile compounds on exposure to microwave irradiation for longer time duration which resulted in reduced proteolytic activity (Doulabi et al. 2020).
Interactive effect of power and solid/ liquid ratio on extraction of crude bromelain from pineapple rind has been depicted in Fig. 2b. The interactive effect of solid/liquid ratio and power indicated that with increase in microwave power from 100 to 200 W, and solid/ liquid ratio from 1:3 to 1:5, an increase in proteolytic activity (127.8 U/mL) was observed, however, with further increase in power (300 W) and solid/liquid ratio (1:10), proteolytic activity was found to decrease (49.46 U/mL). This might be as the solid particles caused an uneven absorption of microwave radiation which led to lower proteolytic activity (Behere et al. 2020).
Interactive effect of power and time on extraction of crude bromelain from pineapple rind has been depicted in Fig. 2c. The interactive effect of treatment time and power indicated that with an increase in power from 100 to 200 W and treatment time from 5 to 10 min, an increase in proteolytic activity (127.8 U/mL) was observed, however, with further increase in power (300 W) and time (15 min), proteolytic activity (70.99 U/mL) was found to decrease. This may be due to degradation of the enzyme on prolonged exposure to microwave irradiation (Mandal and Mandal 2010).
Extraction of crude bromelain from pineapple rind for the determination of protein content by using microwave assisted extraction
MAE technique was used for the extraction of crude bromelain from pineapple rind by varying process parameters (solid/liquid ratio (1:3 to 1:10), microwave power (100 to 180 W), and treatment time (5 to 15 min)) for the determination of protein content. The interactive effects of extraction parameters involved in the microwave assisted extraction of bromelain from pineapple rind on the protein content were presented by response surface plots (Fig. 3). A significant effect (p ≤ 0.05) of the extraction parameters (solid/liquid ratio, microwave power, and time) was observed on the protein content of bromelain extracted from pineapple rind (Table 3).
Interactive effect of time and solid/ liquid ratio on extraction of crude bromelain from pineapple rind has been depicted in Fig. 3a. The interactive effect of solid/ liquid ratio and time indicated that with an increase in time from 5 to 10 min and solid/ liquid ratio from 1:3 to 1:5, an increase in protein content was observed (2.55 mg/mL), however, with further increase in time (15 min) and solid/ liquid ratio (1:10), protein content was found to decrease (1.84 mg/ml). This may be due to degradation in protein caused by prolonged microwave irradiation time and absorption of microwave radiation by the solid particles which lead to lower protein recovery (Mala et al. 2021; Behere et al. 2020).
Interactive effect of power and solid/ liquid ratio on extraction of crude bromelain from pineapple rind has been depicted in Fig. 3b. The interactive effect of solid/ liquid ratio and power indicated that with an increase in power from 100 to 200 W and solid/ liquid ratio from 1:3 to 1:5, an increase in protein content (2.55 mg/ mL) was observed, however, with further increase in solid/liquid ratio (1:10) and power (300 W), protein content was found to decrease (1.77 mg/mL). This might be due to excessive swelling of the plant matrix caused by large amount of solvent, which absorbs more microwave energy and generate excessive heat. The additional heat may cause thermal degradation of extracted solutes (Behere et al., 2020).
Interactive effect of power and time on extraction of crude bromelain extract from pineapple rind has been depicted in Fig. 3c). The interactive effect of time and power indicated that with an increase in power from 100 to 200 W and treatment time from 5 to 10 min, an increase in protein content (2.55 mg/ mL) was observed, however, with further increase in power (300 W) and time (15 min), protein content (1.95 mg/ mL) was found to decrease, which may be due to the degradation of protein at high microwave power. Similar effect of microwave power was observed in the previous studies on paprika where a decrease in the final protein content was observed at higher microwave power (Kiss et al., 2000). The results of the optimization of different process variables (temperature, treatment time, and liquid to solid ratio) on the extraction of bromelain using microwave assisted extraction revealed that the highest proteolytic activity (127.8 U/ml) and protein content (2.55 mg/mL) of bromelain was obtained with 1:5 solid/liquid ratio, microwave power of 200 W after microwave irradiation time of 10 min. The present study involves lower solvent consumption (1:5) and lesser irradiation time (10 min) which makes the process more economical, whereas, higher value of solid to liquid ratio (1:20) and irradiation time (40 min) was observed in the previous reported studies on extraction of protein from pineapple peels (Bansod et al. 2023).
Purification of crude bromelain extracted from pineapple rind
Ammonium sulphate precipitation
Purification of crude bromelain extracted from pineapple rind using ammonium sulphate precipitation resulted in proteolytic activity of 127.8 U/mL. Proteolytic activity of bromelain obtained in present study using ammonium sulphate (50% saturation) was found to be slightly higher than the previously reported value of proteolytic activity (120.05 U/mL) obtained using ammonium sulphate (60% saturation) precipitation method (Gul et al., 2021). This might be due to the difference in saturation range of ammonium sulphate used in the present study. Purification of enzyme using ammonium sulphate precipitation is a cost-effective procedure as it may help reduce the number of steps in purification of biomolecules (Soares et al. 2011).
Dialysis
Purification of crude bromelain extracted from pineapple rind using dialysis resulted in proteolytic activity of 150.29 U/mL. The proteolytic activity of bromelain obtained after purification using dialysis was higher as compared to that of crude bromelain activity. This might be as the structural integrity of the purified bromelain was maintained by this purification as well as due to the presence of other proteins in the purified enzyme extract (Bhagavathy et al., 2019).
Gel filtration chromatography
Gel filtration chromatography was carried out for the purification of crude bromelain using Sephadex G-75 resin. Purification of crude bromelain extracted from pineapple rind using gel filtration chromatography resulted in proteolytic activity of 36.01 U/mL and specific activity of 512 U/mg with purification-fold of 10.26. Value of specific activity (512 U/mg) reported in the present study was lower than the previously reported specific activity (729.16 U/mg) of bromelain where Sephadex G-50 was used as resin in gel filtration chromatography performed in combination with ion-exchange chromatography (Ilyas et al., 2018).
Structural characterization of bromelain extract obtained from pineapple rind
Structural characterization of purified bromelain extract obtained from pineapple rind using microwave assisted extraction was carried out using Fourier transform infrared spectroscopy and X-ray diffraction analysis which has been discussed in this section (Fig. 4).
Fourier transform infrared spectroscopy
FTIR spectroscopy of control and purified bromelain extract obtained from pineapple rind using microwave assisted extraction has been depicted in Fig. 4a-a’. Characteristic peaks of bromelain were observed at 3271.15 cm−1 (N–H stretching vibration of secondary amide), 2927.90 cm−1 (aliphatic C-H stretching), 1416.78 cm−1 (C = C stretching), and 2928.01 cm−1 (methylene asymmetric C-H stretching) 1644.23 cm−1 (C = C stretching), and 923.75 cm−1 (third overtone C-H stretching). The wavelength in the range 3338- 3380 cm−1 spectrum showed the presence of N–H stretching vibrations. The bands near 1179–1149 cm−1, 1255– 1290 cm−1, and, 1517–1587 cm−1, were assigned to C-N stretching vibration frequencies (Sharma and Sharma, 2018). Similar peaks of bromelain were reported in previous studies (Nor et al. 2015; Sharma and Sharma 2018; Soares et al. 2012).
X-ray diffraction analysis
X-ray diffraction (XRD) analysis of purified bromelain extract obtained from pineapple rind using microwave assisted extraction has been presented in Fig. 4b. The bromelain extract showed the presence of sharp peaks between 10–23° 2θ and 5–25° 2θ, respectively. The diffused peaks displayed by diffractogram indicated the amorphous behaviour of bromelain. Intense peaks were observed at 20°, 35° and 40° 2θ, whereas low intensity peaks were observed at 5° and 10° 2θ. The results indicated that bromelain had semi-crystalline nature with intense peaks at 18.72°, 19.54°, 20.34° and 21.04° 2θ and low intensity peaks observed at 12.47°, 16.18° and 23.82° 2θ (Sharma and Sharma, 2018).
Conclusion
Microwave assisted extraction has been found as an effective green technique for the extraction of bromelain from pineapple rind with potential to be utilized at industrial level. The optimized extraction conditions which resulted in highest proteolytic activity (127.8 U/mL) and protein content (2.55 mg/mL) of bromelain extract were 1:5 solid/liquid ratio, 200 W microwave power and treatment time of 10 min. Purification after gel filtration chromatography resulted in highest specific activity (512 U/mg). FTIR analysis confirmed the presence of functional groups in bromelain, whereas, XRD analysis depicted semi-crystalline nature of extracted bromelain. The results indicated pineapple rind as a chief source of proteolytic enzyme, bromelain, and protein. The employment of MAE technique for the extraction of bromelain can be an effective valorization strategy for value addition of pineapple waste while maintaining sustainability of the process. The extracted bromelain finds applications in several food industries for meat tenderization, making of protein hydrolysate, cheese, and fish sauce production.
Abbreviations
- MAE:
-
Microwave assisted extraction
- RSM:
-
Response surface methodology
- BBD:
-
Box-Behnken design
- SEM:
-
Scanning electron microscopy
- XRD:
-
X-ray diffraction
- FTIR:
-
Fourier transform infrared spectroscopy
References
AOAC (2007) Officials’ methods of analysis of AOAC international (18th ed.). Gaithersburg.
Aruna TE (2019) Production of value-added product from pineapple peels using solid state fermentation. Innov Food Sci Emerg Technol 57:102193. https://doi.org/10.1016/j.ifset.2019.102193
Bansod SP, Parikh JK, Sarangi PK (2023) Pineapple peel waste valorization for extraction of bio-active compounds and protein: microwave assisted method and Box Behnken design optimization. Environ Res 221:115237. https://doi.org/10.1016/j.envres.2023.115237
Barekat S, Soltanizadeh N (2017) Improvement of meat tenderness by simultaneous application of high-intensity ultrasonic radiation and papain treatment. Innov Food Sci Emerg Technol 39:223–229. https://doi.org/10.1016/j.ifset.2016.12.009
Behere M, Patil SS, Rathod VK (2020) Rapid extraction of watermelon seed proteins using microwave and its functional properties. Prep Biochem Biotechnol 51(3):252–259. https://doi.org/10.1080/10826068.2020.1808792
Bhagavathy S, Pushya K, Gayathridevi R, Jeniffer J (2019) Purification, characterization and application of bromelain from Ananas comosus. J Appl Adv Res 4(5):133–140. https://doi.org/10.21839/jaar.2019.v4i5.304.
Coelho DF, Silveira E, Pessoa Junior A, Tambourgi EB (2013) Bromelain purification through unconventional aqueous two-phase system (PEG/ammonium sulphate). Bioprocess Biosyst Eng 36(2):185–192. https://doi.org/10.1007/s00449-012-0774-5
Colletti A, Li S, Marengo M, Adinolfi S, Cravotto G (2021) Recent advances and insights into bromelain processing, pharmacokinetics and therapeutic uses. Appl Sci 11(18):8428. https://doi.org/10.3390/app11188428
Costa HB, Fernandes PM, Romão W, Ventura JA (2014) A new procedure based on column chromatography to purify bromelain by ion exchange plus gel filtration chromatographies. Ind Crops Prod 59:163–168. https://doi.org/10.1016/j.indcrop.2014.04.042
Csiktusnadi Kiss GA, Forgacs E, Cserhati T, Mota T, Morais H, Ramos A (2000) Optimisation of the microwave-assisted extraction of pigments from paprika (Capsicum annuum L.) Powders. Journal of Chromatography A. 2000, 889, 41–49. DOI: 10.1016/ S0021–9673(00)00440–4. https://doi.org/10.1016/s0021-9673(00)00440-4.
da Rosa GS, Vanga SK, Gariepy Y, & Raghavan V. (2019). Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innovative Food Science and Emerging Technologies, 58. https://doi.org/10.1016/j.ifset.2019.102234
Doulabi M, Golmakani MT, Ansari S (2020) Evaluation and optimization of microwave-assisted extraction of bioactive compounds from eggplant peel by- product. J Food Process Preserv 44(11):e14853. https://doi.org/10.1111/jfpp.14853
FAOSTAT. (2020). Production of pineapples: Top 10 producers. FAO: Rome, Italy. http://www.fao.org/faostat/en/#data/QC/visualize.
Galanakis CM (2023) The “Vertigo” of the food sector within the triangle of climate change, the post-pandemic world, and the Russian-Ukrainian War. Foods 12(4):721. https://doi.org/10.3390/foods12040721
Galanakis CM, Aldawoud TM, Rizou M, Rowan NJ, Ibrahim SA (2020) Food ingredients and active compounds against the coronavirus disease (COVID-19) pandemic: a comprehensive review. Foods 9(11):1701. https://doi.org/10.3390/foods9111701
Gautam SS, Mishra SK, Dash V, Goyal AK, Rath G. (2010). Comparative study of extraction, purification and estimation of bromelain from stem and fruit of pineapple plant. Thai Journal of Pharmaceutical Sciences, 34(2).
Gul A, Siddiqui M, Arain H, Khan S, Khan H, & Ishrat U (2021) Extraction, partial purification and characterization of bromelain from pineapple (Ananas Comosus) crown, core and peel waste. Brazilian Archives of Biology and Technology, 64. https://doi.org/10.1590/1678-4324-2021200639.
Gupta R, Gupta N, Rathi P (2004) Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol 64:763–781. https://doi.org/10.1007/s00253-004-1568-8
Huang CW, Lin IJ, Liu YM, Mau JL (2021) Composition, enzyme, and antioxidant activities of pineapple. Int J Food Prop 24(1):1244–1251. https://doi.org/10.1080/10942912.2021.1958840
Ilyas NM, Setiasih S, Hudiyono S (2018). Inhibition Study of EDTA and PCMB on purified bromelain activity from pineapple core [Ananas comosus (L.) Merr.] using ion exchange chromatography column and antiplatelet activity test. https://doi.org/10.5220/0008358601300139.
Kashyap P, Riar CS, Jindal N (2022) Effect of extraction methods and simulated in vitro gastrointestinal digestion on phenolic compound profile, bio-accessibility, and antioxidant activity of Meghalayan cherry (Prunus nepalensis) pomace extracts. LWT 153:112570. https://doi.org/10.1016/j.lwt.2021.112570
Ketnawa S, Chaiwut P, Rawdkuen S (2012) Pineapple wastes: a potential source for bromelain extraction. Food Bioprod Process 90(3):385–391. https://doi.org/10.1016/j.fbp.2011.12.006
Ketnawa S, Rawdkuen S, Chaiwut P (2010) Two phase partitioning and collagen hydrolysis of bromelain from pineapple peel Nang Lae cultivar. Biochem Eng J 52(2–3):205–211. https://doi.org/10.1016/j.bej.2010.08.012
Mala T, Sadiq MB, Anal AK (2021) Comparative extraction of bromelain and bioactive peptides from pineapple byproducts by ultrasonic-and microwave-assisted extractions. J Food Process Eng 44(6):e13709. https://doi.org/10.1111/jfpe.13709
Mandal V, Mandal SC (2010) Design and performance evaluation of a microwave based low carbon yielding extraction technique for naturally occurring bioactive triterpenoid: Oleanolic acid. Biochem Eng J 50(1–2):63–70. https://doi.org/10.1016/j.bej.2010.03.005
Mohan R, Sivakumar V, Rangasamy T, Muralidharan C (2016) Optimisation of bromelain enzyme extraction from pineapple (Ananas comosus) and application in process industry. Am J Biochem Biotechnol 12(3):188-195. https://doi.org/10.3844/ajbbsp.2016.188.195.
Nakthong N, Wongsagonsup R, Amornsakchai T (2017) Characteristics and potential utilizations of starch from pineapple stem waste. Ind Crops Prod 105:74–82. https://doi.org/10.1016/j.indcrop.2017.04.048
Nor MZM, Ramchandran L, Duke M, Vasiljevic T (2015) Characteristic properties of crude pineapple waste extract for bromelain purification by membrane processing. J Food Sci Technol 52(11):7103–7112. https://doi.org/10.1007/s13197-015-1812-5
Ramli ANM, Aznan TNT, Illias RM (2017) Bromelain: from production to commercialisation. J Sci Food Agric 97(5):1386–1395. https://doi.org/10.1002/jsfa.8122
Sharma M, Sharma R (2018) Implications of designing a bromelain loaded enteric nanoformulation on its stability and anti-inflammatory potential upon oral administration. RSC Adv 8(5):2541–2551. https://doi.org/10.1039/C7RA13555F
Soares P, Coelho D, Mazzola P, Silveira E, Carneiro-da-Cunha MG, Pessoa A, Tambourgi E (2011) Studies on bromelain precipitation by ethanol, poly (ethylene glycol) and ammonium sulphate. Tenth Int Conf Chem Process Eng. https://doi.org/10.3303/CET1124164
Soares PA, Vaz AF, Correia MT, Pessoa A Jr, Carneiro-da-Cunha MG (2012) Purification of bromelain from pineapple wastes by ethanol precipitation. Sep Purif Technol 98:389–395. https://doi.org/10.1016/j.seppur.2012.06.042
TFNET News Compilation, (May 10, 2016), Tropical fruit Information. International Tropical Fruits Network. https://www.itfnet.org/v1/2016/05/pineapple-common-varieties/
Upadhyay A, Lama JP, Tawata S (2010) Utilization of pineapple waste: a review. Journal of Food Science and Technology Nepal 6:10–18. https://doi.org/10.3126/jfstn.v6i0.8255
Wen L, Álvarez C, Zhang Z, Poojary MM, Lund MN, Sun DW, Tiwari BK (2021) Optimisation and characterisation of protein extraction from coffee silverskin assisted by ultrasound or microwave techniques. Biomass Conversion and Biorefinery 11(5):1575–1585. https://doi.org/10.1007/s13399-020-00712-2
Yan Z, Zhang H, Dzah CS, Zhang J, Diao C, Ma H, Duan Y (2020) Subcritical water extraction, identification, antioxidant and antiproliferative activity of polyphenols from lotus seedpod. Sep Purif Technol 236:116217
Acknowledgements
Authors would like to acknowledge the financial support provided by ASEAN India Science & Technology Development Fund (AISTDF), by Science & Engineering Research Board, Department of Science & Technology (SERB-DST) under Grant No. IMRC/AISTDF/CRD/2019/000141.
Funding
The research project was funded by ASEAN India Science & Technology Development Fund (AISTDF), Science & Engineering Research Board- Department of Science & Technology (SERB-DST) under Grant No. IMRC/AISTDF/CRD/2019/000141.
Author information
Authors and Affiliations
Contributions
Ritika Kaushal: Formal analysis, Brahmeet Kaur: Investigation, Methodology, Writing—original draft, Parmjit S. Panesar: Conceptualization, Writing—review & editing, Supervision, Project administration, Anil K. Anal: Conceptualization, Supervision, Son Chu-Ky: Supervision, Guidance.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaushal, R., Kaur, B., Panesar, P.S. et al. Valorization of pineapple rind for bromelain extraction using microwave assisted technique: optimization, purification, and structural characterization. J Food Sci Technol 61, 551–562 (2024). https://doi.org/10.1007/s13197-023-05863-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05863-4