Abstract
The production of kombucha involves the synthesis of a bacterial cellulose-based native film by a microbial consortium, typically regarded as a waste by-product in commercial kombucha manufacturing. In this study, films were successfully obtained using the microbial consortium of kombucha, combined with infusions of black tea, green tea, rosehip, coffee, and licorice. These films exhibited a flexible rubbery-like structure and demonstrated inherent biological activity. Comparative analysis revealed that the licorice-based films exhibited a regular and less porous structure, while the green and black tea-based films displayed a porous structure, resulting in higher water permeability and swelling. Remarkably, green tea-based films showcased notable antioxidant activity (DPPH: %74.22 ± 2.05, ABTS: %81.59 ± 2.39) and exhibited antimicrobial properties against E. coli, S. aureus, and B. cereus, owing to their high phenolic content (1.62 ± 0.04 μg GAE/g). The antimicrobial efficacy of green tea-based films surpassed that of the other films against pathogenic microorganisms. By enhancing their hydrophobic properties, these innovative films hold promising potential as cost-effective, active, and environmentally friendly materials for food packaging applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic-based environmental pollution has become a more serious problem than ever before, with mountains of waste plastic now piling up in most city landfills. The enormous masses of plastic garbage, created by eddy currents in the oceans, can even be seen from space (Lebreton et al. 2018). Macro-plastics on land and water are naturally broken down into microplastics by factors such as wind, air, sunlight, waves, currents, tides, salinity, and living organisms (Julienne et al. 2019). Unfortunately, these contaminants, which have indisputable adverse effects on life, can be found even in the world's most remote places, including the depths of the Mariana Trench (Jamieson et al. 2019). In recent times, the COVID-19 pandemic, and the psychological trauma it has created may have fueled the perception that reused or recycled packaging is not hygienic. As a result, the excessive use of hard-to-degrade disposable food packaging, which is among the leading causes of environmental pollution, has become a critical issue worldwide. However, replacing petroleum-based polymers with bio-based polymers like cellulose offers a robust solution to this problem.
Cellulose is a linear-chain organic compound with many glycosidic linked D-glucose units. Cellulose is known as the main component of cell walls of plants, algae, and oomycetes, the most abundant biopolymer on earth, and a major sustainable raw material (Kostag et al. 2019). The non-toxicity, biodegradability, and chemical stability of cellulose have captured the attention of numerous researchers working on eco-friendly food packaging. In many studies, the typical approach to fabricate packages is to use cellulose derivatives such as cellulose acetate, cellulose sulfate, cellulose nitrate, carboxymethyl cellulose, ethyl cellulose, and methyl cellulose. However, the process of derivatizing cellulose can lead to pollution from chemical residues and byproducts (Rose and Palkovits 2011). Furthermore, the techniques required to produce packaging materials from these cellulose derivatives can be energy-intensive and require advanced equipment and significant labor (Liu et al. 2021). Can a solution be found that allows both the raw material and the packaging material to be obtained simultaneously, without the negative impacts of derivatization and complex production techniques? One promising solution may be found in a by-product of a centuries-old beverage that has become increasingly popular in recent years.
Kombucha is a beverage made by brewing and sweetening various plant leaves, particularly black tea, and fermenting them with a Symbiotic Culture of Bacteria and Yeast (SCOBY) as the starter culture. The fermentation process, which involves a community of microorganisms, including Acetobacter from bacteria and Saccharomyces from yeast, is completed within 7–10 days (Jayabalan et al. 2014; Villarreal-Soto et al. 2018). The sugary solution that contains plant extracts is rich in polyphenols, organic acids, vitamins, minerals, and micronutrients, providing the necessary nutrients for the kombucha microbial community to thrive without requiring any additional nutrients (Emiljanowicz and Malinowska-Pańczyk 2020). The symbiotic relationship within the kombucha microbial consortium can be described as follows: yeasts break down sucrose into glucose and fructose, making them available to the bacteria. In response, the bacteria take part in nitrogen fixation and biofilm synthesis, which helps protect the community from possible contaminants (Villarreal-Soto et al. 2018). During the first few days of fermentation, the bacteria in the SCOBY form a floating layer that becomes visible, and it thickens as fermentation continues. Although this pellicle has various beneficial properties, it is typically discarded as a by-product at the end of fermentation since the purpose is to obtain the kombucha beverage.
The Kombucha pellicle is a type of bacterial cellulose which is structurally thinner and unbranched compared to plant cellulose, providing unique advantages such as resistance to aqueous environments and a larger surface area (Laavanya et al. 2021). Moreover, the pellicles can exhibit antioxidant and antimicrobial activity, depending on the plant used in fermentation (Ramírez Tapias et al. 2020). In addition to these benefits, the biocompatibility, eco-friendliness, and non-toxicity of Kombucha pellicles make them suitable for use in various fields, including textiles, medicine, and food packaging (Laavanya et al. 2021). Although there are limited studies characterizing Kombucha-based films in the literature (Ramírez Tapias et al. 2020, 2022), to our knowledge, the use of coffee, licorice, and rosehip in their production has not been reported yet.
The aim of this study was to produce films using kombucha with different plant infusions through the in-situ method and to explore their potential as food packaging. Therefore, native bacterial cellulose films were produced using kombucha culture and plant infusions of coffee, licorice, rosehip, black tea, and green tea. Then, the films produced by in-situ method were characterized for their morphological, mechanical, and bioactivity properties.
Materials and methods
Materials
Black tea (Dogus, Turkey), green tea (Dogadan, Turkey), rosehip (Mest, Turkey), licorice (Ramco, Turkey), coffee bean (Kahve dunyasi, %100 Arabica, Turkey) and sugar were purchased from suppliers in Yozgat province. Kombucha starter culture was provided from Yozgat Bozok University, Bogazliyan Vocational School (Yozgat, Turkey). Ten percent of this culture was subcultured periodically (once in 21 days) with black tea (4 g/L) and sugar (90 g/L). The production conditions of kombucha used as a starter were the same as the fermentation conditions required to produce films, which are detailed in Sect. "Kombucha fermentation and film preparation". All chemicals used in the study were purchased from Merck, except when otherwise stated.
Kombucha fermentation and film preparation
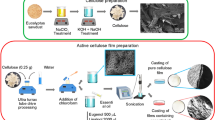
Kombucha was produced according to the method described by Ramírez Tapias et al. (2022) with minor modifications. Fig S1 shows the flowchart of the production of kombucha and films. To prepare the sugar solution, 90 g of sucrose was dissolved in 1 L of boiling water. Meanwhile, 4 g of each plant type was separately weighed and placed in a steel brewing strainer. Coffee beans were ground to a French-press size, while other plants were used as they were. The herbs were infused in the sugar solution for 20 min, after which the brew strainer was removed, and the solution was cooled to 25 °C. Then, it was inoculated with 10% starter kombucha liquid culture and incubated in filtered glass jars for 21 days under dark room conditions at 25 ± 2 °C. After 21 days, the film floating on the liquid was removed from the jar and washed with distilled water until the pH of the rinse water was 7.0 ± 0.2. The biomass was then kept in an oven at 105 °C for 2 h for microbial inactivation. Next, it was cast at 40 °C for 12 h to convert the soft-thick hydrogel pellicle into a flexible film. The films produced by in-situ method were stored in a polyethylene zip bag at + 4 °C for a period not exceeding one month until the analyses. The schematic illustrations of the production of films are shown in Fig. 1.
Characterization of native films
Physical characteristics and appearance
The thickness of the films was determined using a digital caliper. For measuring the amount of production, the pellicle formed at the end of the fermentation was dried at 105 °C for 10 h and then weighed and expressed as g/L. Native films were photographed using a Canon EOS 600d digital camera and a lightbox. The areas where only films are seen in digital photographs were selected as regions of interest (ROI) with Photoshop 2020 software. Mean L* (represents darkness to lightness), a* (represents greenness to redness) and b* (represents blueness to yellowness) values were obtained from histograms of ROIs.
Scanning electron microscopy
A Field Emission Scanning Electron Microscope (FE-SEM) branded Zeiss ULTRA PLUS (Carl Zeiss Microscopy GmbH, Jena, Germany) was used to investigate the morphologies of the native films.
Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR) spectra of the native films and infusions before fermentation were collected using on Bruker ALPHA FTIR Spectrometer (Bruker Optic GmbH, Ettlingen, Germany). A total of 24 scans were recorded in the range of 400–4000 cm−1.
Thermogravimetric analysis
Thermogravimetric analysis (TGA) was performed to examine the thermal degradation behavior of the films using a thermogravimetric analyzer (STA 7300, Hitachi, Japan). The samples were heated from room temperature to 550 °C at a rate of 10 °C/min and a nitrogen gas flow rate of 2 mL/min.
Water contact angle
The water contact angle (WCA) was measured using an automatic optical tension meter system (Attension Theta Optical Tensiometer, Biolin Scientific, Sweden). To measure the WCA, a 5 µL water droplet was placed on the sample surface, which was positioned on a flat horizontal stage and illuminated by a light-emitting diode (LED) light. The droplet's shadow was projected onto a camera with a resolution of 1280 × 1024 pixels. The droplet image was then analyzed automatically using One Attension software (Biolin Scientific, Sweden), which employs a base detection system to determine the water contact angle.
Swelling index
The bacterial cellulose films derived from kombucha were cut into square dimensions measuring 5 cm2, and their swelling index (SI) was measured. In the initial step, a piece of sample was subjected to drying in an oven for 24 h at 70 °C. The weight of the dried sample was then measured and recorded as the initial dry weight (W1). Subsequently, the dried sample was immersed in 20 mL of deionized water and stirred for 24 h at 30 °C. After removal from the water, the weight of the swollen sample was recorded as the wet weight (W2). The swelling index (SI) was calculated using Eq. 1:
Water vapor transmission rate
The water vapor transmission rate (WVTR) of the native films was quantitatively measured using the methods described in Zhang et al. (2016)
Mechanical characteristics
The mechanical properties of the films were measured using the Shimadzu Universal Testing Machine (AGS-1kNXD, Shimadzu, Tokyo, Japan) equipped with a 1 kN load cell. The test speed was set to 1 mm/min. Maximum strength (MF) (N), elongation (E) (%), and tensile strength (TS) (MPa) values were calculated based on the average of ten measurements.
Total phenolic content and antioxidant capacity of native films
The total phenolic content (TPC) was determined using the Folin-Ciocalteu reagent based on a reduction reaction (Dogan et al. 2022). For this analysis, a film sample weighing 50 mg was homogenized in 6 mL of distilled water. The homogenate was then subjected to centrifugation at 4100 rpm for 10 min. From the resulting supernatant, 0.3 mL was taken and mixed with 2.5 mL of Folin-Ciocalteu reagent (10% v/v). To this mixture, 2 mL of a 7.5% sodium carbonate solution (w/v) was added. The reaction mixture was incubated at 50 °C for 5 min, followed by measuring the absorbance at 760 nm. To obtain the standard curve, gallic acid solutions ranging from 0 to 1000 ppm were used. The total phenolic content was expressed as micrograms of gallic acid equivalents per gram of film (μg GAE/g film).
In vitro antioxidant activities of the films were determined by 2,2 diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis-3ethylbenzothiazoline-6-sulfonic acid (ABTS) scavenging methods. In the DPPH analysis, 5 mL of methanolic (25 ppm) DPPH solution and 50 mg of film were mixed. After 30 min of incubation at room conditions in a dark place, absorbance was measured at 515 nm. using a spectrophotometer (Optizen 3220UV, Mecasys Co., Ltd., Korea). In ABTS analysis, aqueous ABTS (7 mmol/L) and potassium persulfate (2.45 mmol/L) solutions were mixed and incubated in a dark place at room ambient. The mixture was then diluted with phosphate buffer saline (pH 7.6) until the absorbance at 734 nm reached 0.7 ± 0.02. 5 mL diluted solution was mixed with 50 mg sample. After incubation for 6 min, absorbance was measured at 734 nm by using a spectrophotometer (Optizen 3220UV, Mecasys Co., Ltd., Korea). Blanks for both DPPH and ABTS were prepared using the same amount of ethanol instead of the sample. The results of both analyzes were expressed as % (Doğan et al. 2022a).
In-vitro antimicrobial activity of native films
The antimicrobial activities of the films were determined against gram-positive bacteria Staphylococcus aureus ATCC 29213 (S. aureus) and Bacillus cereus NCIMB 7464 (B. cereus), gram-negative bacteria Escherichia coli ATCC 35218 (E. coli), and fungal pathogen Candida albicans (C. albicans) ATCC 90028. The antimicrobial activity of the samples was assessed using the agar-based disc diffusion method. To prepare the microorganisms, stock cultures were transferred to 5 mL Mueller Hinton Broth for each microorganism and incubated at 35 °C until they reached 108 CFU/mL based on OD600 absorbance. Next, 100 µL of the diluted bacterial load was inoculated onto Mueller Hinton Agar and spread over the entire petri dish with a sterile swab stick, then allowed to absorb for 5 min. On the other hand, 6 mm diameter film discs cut with a cork borer were sterilized under UV light for 30 min in a laminar flow cabinet. The film discs were then placed on the agar, and the petri dishes were incubated for 24 h at 35 °C. The zone diameters formed around the discs were measured with a caliper and recorded (Doğan et al. 2022b).
Statistical analysis
The results were analyzed using one-way analysis of variance (ANOVA) and expressed as mean ± standard deviation. The normality of the data was checked using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Homogeneity of variance was assessed by considering the distributions of lack of symmetry (skewness) and pointiness (kurtosis). The significance of differences (p < 0.05) between the data was determined using the IBM-SPSS 22.0 statistical package (SPSS Inc., Chicago, IL, USA), and group means were compared using the Duncan multiple comparison test. All analyses were performed in triplicate under the same conditions.
Result and discussion
Visual properties, productivity, and thickness of films
The appearance, color values, and some physical properties of the films produced with different plant infusions are shown in Fig. 2. After 21 days of fermentation, Black tea was found to be the most productive, followed by rosehip, green tea, coffee, and licorice, in that order. This difference in productivity may be attributed to the varying micronutrient compositions of each herbal infusion, which may have either stimulated or inhibited microbial growth during fermentation. The development of the kombucha consortium critically affects the productivity of the bacterial cellulose produced. The sucrose used by the microorganisms as the primary carbon source was the same in all samples. However, each herbal infusion's micronutrients, such as nitrogenous components, polyphenols, and vitamins, differ. While some of these components may positively affect the microbial proliferation required for pellicle formation, some of them may have an antimicrobial effect and slow down the fermentation. For example, theobromine and L-theanine (Gaggìa et al. 2018) in black tea have stimulant effects on microorganisms. However, the trigonelline, protocatechuic acid (Rawangkan et al. 2022) that coffee has, and the glycyrrhizin and licochalcone (Wang et al. 2015) that licorice has have strong antimicrobial effects.
It appears that the color values of the films produced in this study were primarily influenced by the type of herbal infusion used. The films obtained from rosehip were found to have a reddish color (a* > 35) due to the presence of anthocyanins, while the films were also somewhat yellowish (b* > 13), which could be attributed to the color compounds in the plants as well as Maillard reactions during fermentation and heat treatment. The lightness values of the films were relatively high (L* > 46), which could be due to the transparency of the films and lack of opacity. It is interesting to note that the yellowness values of the films in this study were similar to those observed in a previous study that used kombucha to produce films, where the melanoidins formed through Maillard reactions were responsible for the yellow color of the films (Ramírez Tapias et al. 2022).
Microstructural examination of films
The micromorphological structures of the film surfaces are shown in Fig. 3, revealing that the native films had four distinct microstructures. The films of green and black teas had a microporous structure that was similar to each other, which could make them suitable for packaging certain food products where aeration is important. The rosehip bacterial cellulose had a hybrid 3D form with a film/nanofiber structure, and non-uniform coagulated structures were also observed on its surface. In the coffee film, bacterial structures in the form of bacilli were found, which can be attributed to acetobacteria synthesizing bacterial cellulose. Partial cracks were also found on this film. The licorice film, on the other hand, had a flat and smooth two-dimensional structure. Apart from the plant infusions, heat treatment during the conditioning process of the films also contributed to the differentiation of bacterial cellulose structures. The film thickness and density also had an impact on how heat affected the films. The porous, flat, or 3D structures of the films make them flexible in terms of their potential applications.
FTIR spectroscopy of films and herbal infusions
FTIR spectra of herbal infusions (dark colored) and native films (light colored) are visualized as integrated in Fig. 4a. The first impression on the spectrum was that, as expected, bacterial celluloses exhibited much more bands than herbal infusions. The spectral profiles formed by these bands indicated the presence of various groups of bacterial cellulose: I-3600–3000 cm−1 (Sharma and Bhardwaj 2019) (water and simple carbohydrates, representing stretching vibrations of hydroxyl group), II- 2900–2800 cm-1 (Cerrutti et al. 2016) (functional cellulose, representing stretching of C–H), III- 1700–1550 cm-1 (Cerrutti et al. 2016) (water molecules, representing bending of O–H), IV- Multiple peaks between 1490 and 1200 cm-1 (Fuller et al. 2018)(impurities) and, V − 850–1200 cm-1 (Cerrutti et al. 2016; Sharma and Bhardwaj 2019) (macro carbohydrate molecules, representing stretching C–O–C).
The FTIR spectra in Fig. 4a also revealed some differences between the spectra of the herbal infusions. The herbal infusion spectra showed absorption bands characteristic of their specific chemical composition. For example, the spectrum of black tea showed a peak at 1652 cm−1 which corresponds to the stretching vibration of the carbonyl group in the theophylline molecule. The spectrum of rosehip showed a peak at 1737 cm−1 which is due to the carbonyl group of esters. The spectrum of coffee showed a peak at 1620 cm−1 which is attributed to the stretching vibration of the carbonyl group in quinic acid. The licorice infusion spectrum showed a peak at 1599 cm-1 which corresponds to the stretching vibration of C = C in flavonoids. The presence of bacterial cellulose in the native films was confirmed by the absorption band at 1060 cm-1 in the V band, which was not observed in the spectra of the herbal infusions. These differences in spectral features provide additional evidence of the unique chemical composition of bacterial cellulose and the potential of herbal infusions to affect its properties.
Thermal degradation of films
TGA measurements were conducted to determine the thermal characteristics of films produced using different herbal infusions, as shown in Fig. 4b. A relatively low weight loss was observed, which can be attributed to the evaporation of adsorbed water from room temperature to 105 °C. The major degradation process of cellulose glycosidic units involves depolymerization, dehydration, and decomposition, which usually takes place in the 200–400 °C range (Vanderfleet et al. 2019). Secondary weight losses at this stage were faster in coffee, licorice, and rosehip samples compared to black tea and green tea samples. In fact, black tea and green tea samples exhibited typical bacterial cellulose glycosidic degradation behavior, starting around 300 °C (Cerrutti et al. 2016; Ramírez Tapias et al. 2020, 2022). It has been reported that the clarification process improves the thermal stability of bacterial cellulose films made with kombucha (Amarasekara et al. 2020). The faster degradation of coffee, licorice, and rosehip samples may be due to their morphological structures, which may make it difficult to remove residues during the washing process. Finally, the mass decreases after 350 °C due to the oxidation and volatilization of char, which was similar for all samples (Vanderfleet et al. 2019).
Water contact angle of films
In addition to their surface morphology, intramolecular hydrogen bonds of bacterial celluloses are one of the most important reasons for their hydrophilic character (Shao et al. 2016). WCA values of Kombucha based films and water drop profiles on the film are shown in Fig. 4c. As seen, the bacterial celluloses we obtained in our study were highly hydrophilic. Surface roughness is a factor that can increase hydrophilicity. However, WCA values of black tea, green tea and rosehip samples were lower than coffee and licorice samples. The porous structure of these samples may have caused rapid absorption of the water droplet and ultimately reduced contact angles. In contrast, the non-porous surface of the licorice sample contributed to its relatively hydrophobicity. The findings revealed that kombucha-based films should be treated with applications such as a secondary surface coating or the addition of additives that can weaken hydrogen bonds for food applications with high water activity.
Mechanical properties of films
The mechanical properties of Kombucha-based native films were evaluated by analyzing their uniaxial vertical stress behaviors at 25 degrees and 50% relative humidity conditions, and the resulting MF (N), E (%) and TS (MPa) values are presented in Fig. 5.a. These values provide information on the flexibility and strength of the films, which are key factors for food packaging applications. The data obtained from the experiments indicated that the films were relatively ductile and elastic, apart from the Licorice sample. Previous studies have reported that bacterial cellulose films are typically brittle and break suddenly when they reach the maximum stress value with the application of load (Retegi et al. 2010; Ramírez Tapias et al. 2022). Therefore, the relatively high MF and TS values measured in the Licorice sample (15.71 ± 1.21 N and 8.76 ± 0.93 MPa, respectively) were surprising. However, the elongation of this sample was approximately 2.5 times lower than that of the green tea sample, which had the highest elongation. It is known that the mechanical properties of bacterial cellulose films are greatly influenced by both the synthesis and post-treatment conditions. During the fermentation process, acid and carbohydrate components are formed in different amounts and compositions from the herbal infusions, which can affect the mechanical properties of the films (Ramírez Tapias et al. 2022). Moreover, post-treatments or conditioning processes, such as purification, dehydration, or casting, can also significantly impact the mechanical characteristics of the films. Thus, it is likely that these factors contributed to the observed variation in the mechanical properties of the films. In summary, the mechanical properties of the Kombucha-based films were evaluated using uniaxial vertical stress tests, and the data obtained indicate that the films are relatively ductile and elastic, except for the Licorice sample. The observed variation in the mechanical properties of the films is likely due to the synthesis and post-treatment conditions, as well as the herbal infusions used.
Swelling index of films
Understanding the swelling behavior of bacterial cellulose films produced with different plant infusions is crucial for their potential applications. In Fig. 5.b, the SI values of the films are presented. The films' swelling properties are influenced by factors such as pore density, three-dimensional structure, and surface porosity. Among the samples tested, the Licorice sample displayed a unique characteristic. It had the lowest SI due to its smooth and less porous surface. The reduced porosity limited liquid ingress and restricted the film's expansion, resulting in minimal swelling. This suggests that Licorice-derived bacterial cellulose films could be suitable for applications requiring low swelling behavior. In contrast, the black tea and green tea samples exhibited higher SI values, indicating their greater moisture absorption and retention capacity. These samples had porous surface structures that facilitated liquid penetration, leading to increased swelling. The presence of bioactive compounds or structural components in the tea infusions may have contributed to the films' porous nature and influenced their swelling behavior. Additionally, the coffee and rosehip samples showed significant increases in SI. Their fibrous structures provided a larger surface area for liquid interaction, promoting higher absorption and swelling. This suggests that cellulose films derived from coffee and rosehip could be promising for applications requiring controlled release of active compounds or enhanced moisture absorption.
Water vapor transmission rate of films
The water vapor transmission rate (WVTR) plays a crucial role in evaluating the performance of active packaging materials utilized for food storage applications. It determines the extent of moisture exchange between the packaged goods and the surrounding environment, making it a critical parameter to consider. A low WVTR indicates effective blocking of moisture transfer.
In this study, WVTR values of bacterial cellulose-based films were examined, and it was found that they varied depending on the herbal infusions used during film preparation (Fig. 5a). The porosity and regularity of the films were identified as influential factors affecting water vapor permeability. Specifically, the licorice sample, characterized by a more regular and less porous structure, exhibited limited water vapor permeability. Conversely, the green tea and black tea samples, known for their porous structures, displayed higher WVTR values. Packaging materials with high water vapor permeability, such as those observed in the green tea and black tea samples, can be advantageous in maintaining the freshness of perishable food items, including fresh fruits/vegetables and meat, under appropriate storage conditions. Additionally, rosehip and coffee samples, which possessed a relatively less porous but semi-fibrous structure, also exhibited notable water vapor permeability.
Total phenolic content and antioxidant capacity of films
The TPC values for each native film are presented in Table 1. The results revealed significant variations in TPCs among the different plant-based native films, indicating the influence of plant ingredients on the phenolic composition. The native film derived from licorice exhibited a relatively lower TPC, while the black tea sample demonstrated a moderate TPC. The green tea sample displayed a higher TPC, suggesting the presence of substantial phenolic compounds inherent to the tea leaves. The native film derived from coffee exhibited a moderate TPC, while the rosehip sample showed a significant TPC, indicating a rich phenolic composition specific to rosehip. These findings highlight the role of plant ingredients in shaping the TPC values of native films, emphasizing their potential as sources of diverse phenolic compounds with antioxidant properties.
One of the key elements that can be expected from an active packaging material is its ability to protect food against oxidation related to its antioxidant capacity. Antioxidant capacities of kombucha based films determined by DPPH and ABTS methods are shown in Table 1. In our study, green tea and rosehip samples attracted attention with their high antioxidant capacity. Green tea contains a significant amount of polyphenolic compounds, especially epigallocatechin-3-gallate and epicatechin-3-gallate (Namal Senanayake 2013). Rosehip, on the other hand, is rich in ascorbic, citric, and malic acids, as well as carotenoids such as lycopene, b-cryptoxanthin and rubixanthin (Adamczak et al. 2012). The above-mentioned components of green tea and rosehip contributed to the antioxidant activities of the films. In addition, during kombucha fermentation, microorganisms can hydrolyze polyphenols to smaller molecules that show more bioactive properties (Jayabalan et al. 2014). Moreover, although the films were washed until the pH values were neutralized in this study, synthase acids of acetic acid bacteria, incredibly dominant microorganisms in the kombucha microbial consortium, can be encapsulated with tightly woven cellulose fibers, which is a factor that may contribute to antioxidant activity. In our previous study (Doğan et al. 2022a), the antioxidant capacity of cross-linked PVP-based nanofibers containing different ratios of lavender essential oil was performed under the same conditions as the current study. In the aforementioned study, the sample containing the maximum bioactive agent inhibited DPPH by 54.54% and ABTS by 68.06%. The results obtained in the current study showed that bacterial cellulose films produced from green tea and rosehip without the addition of an external bioactive agent by the in-situ method were superior to centrifugal spun coating materials in terms of antioxidant characteristics. Also, Ramírez Tapias et al. (2022) reported in their study that the highest antioxidant activity was detected in kombucha-based films infused with yerba mate, which was consistent with ours, and that the films could show bioactivity without an external active agent. These self-bioactive films have the potential to protect food from oxidative spoilage in various applications such as meat, seafood, fresh-cut fruits and vegetables, nuts, and fatty dairy products.
Antimicrobial activity of films
Food packaging systems with antimicrobial properties can damage the cell walls or metabolism of foodborne pathogens and spoilage microorganisms. This prevents the proliferation of microorganisms, allowing food to stay fresh longer. In addition, antimicrobial food packaging can help prevent foodborne illness. None of the in-situ produced films exhibited antifungal activity against C. albicans. C. albicans can form a biofilm to increase its resistance to antimicrobial agents. Biofilm, a kind of defense mechanism, is the functioning of a matrix in which microorganisms bind to each other. This matrix can increase the resistance of microorganisms to antimicrobial agents by different mechanisms and reduces the cidal pressure on the microorganism in extreme conditions. For example, microorganisms on the biofilm can inhibit the penetration of antifungal products. In addition, cells on the biofilm are exposed to less oxygen, which may reduce the effectiveness of antimicrobial agents on microorganisms (Fan et al. 2022). The antimicrobial effects of kombucha-based bacterial cellulose films against S. aureus, E. coli and B. cereus are given in Table 1. All films obtained from the different herbal infusions were biologically active. The antimicrobial effects of the films produced by the in-situ method can be attributed to the organic acids and phenolic compounds trapped in the cellulosic matrix. Moreover, lactic acid strains in the kombucha consortium can produce bacteriocins and petites, which are potential antimicrobial components (Jayabalan et al. 2014). The antimicrobial activity of the samples against all three bacteria was in the form of green tea > rosehip > black tea > coffee > licorice. The films showed higher antimicrobial activity against S. aureus and B. cereus. This was probably due to the lipopolysaccharide outer membrane of gram-negative E. coli, which gave it antimicrobial resistance against the films. The results revealed that S. aureus was the most sensitive species to the films. Antimicrobial packaging systems aim to reduce the microbial load of contaminated food to safe limits. This is usually achieved by embedding the antimicrobial agent into the polymer matrix that forms the skeleton of the packaging material (Cooksey 2005). Bacterial celluloses were widely considered microbiologically inactive and were often used as antimicrobial agent carriers (Wei et al. 2011; El-Gendi et al. 2022). However, bacterial cellulose obtained in the presented study showed a direct antimicrobial effect since it was synthesized by plant infusions and the fermentation of phytochemicals related to them. Conjointly, the antimicrobial effect results revealed that kombucha-based films could be a promising material in antimicrobial packaging systems.
Conclusion
Bacterial cellulose films were synthesized from kombucha cultures infused with different herbs, including green tea and rosehip, and were found to have strong antioxidant and antimicrobial properties. Using the pellicle as a raw material for food packaging not only offers a sustainable and environmentally friendly solution, but also adds value to the kombucha production process by reducing waste. This approach to developing innovative and sustainable food packaging solutions could help reduce the negative impact of plastic-based packaging on the environment. However, the hydrophilic nature of the kombucha-based cellulose may limit its direct use for certain applications. Therefore, future studies could investigate blending the kombucha-based cellulose with different polymers or coating it with natural hydrophobic materials for specialized uses. Overall, these findings suggest that kombucha-based bacterial cellulose films have potential as a sustainable and effective alternative to traditional plastic-based food packaging materials.
Data availability
Even though adequate data has been given in the form of tables and figures, however, all authors declare that if more data required then the data will be provided on request basis.
Abbreviations
- 3D:
-
Three-dimensional
- ABTS:
-
2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- ANOVA:
-
Analysis of variance
- ATCC:
-
American type culture collection
- CFU:
-
Colony forming units
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- E:
-
Elongation
- FE-SEM:
-
Field emission scanning electron microscope
- FTIR:
-
Fourier transform infrared
- MF:
-
Maximum force
- NCIMB:
-
The national collection of industrial, food and marine bacteria
- OD:
-
Optical density
- ROI:
-
Region of interest
- SCOBY:
-
Symbiotic culture of bacteria and yeast
- TGA:
-
Thermogravimetric analysis
- TS:
-
Tensile strength
- TPC:
-
Total phenolic content
- UV:
-
Ultraviolet
- WCA:
-
Water contact angle
References
Adamczak A, Buchwald W, Zieliński J, Mielcarek S (2012) Flavonoid and organic acid content in rose hips (Rosa L., Sect. Caninae Dc. Em. Christ.). Acta Biol Cracov Bot 54:105–112. https://doi.org/10.2478/v10182-012-0012-0
Amarasekara AS, Wang D, Grady TL (2020) A comparison of kombucha SCOBY bacterial cellulose purification methods. SN Appl Sci 2:1–7. https://doi.org/10.1007/S42452-020-1982-2/FIGURES/4
Cerrutti P, Roldán P, García RM et al (2016) Production of bacterial nanocellulose from wine industry residues: Importance of fermentation time on pellicle characteristics. J Appl Polym Sci 133:43109. https://doi.org/10.1002/APP.43109
Cooksey K (2005) Effectiveness of antimicrobial food packaging materials. Food Addit Contam 22:980–987. https://doi.org/10.1080/02652030500246164
Dogan C, Celik S, Dogan N (2022) Changes of quality characteristics of functional fruit yogurts fortified with husk extracts of various nuts during cold storage. J Microbiol Biotechnol Food Sci 12:e5830–e5830. https://doi.org/10.55251/jmbfs.5830
Doğan C, Doğan N, Gungor M et al (2022a) Novel active food packaging based on centrifugally spun nanofibers containing lavender essential oil: Rapid fabrication, characterization, and application to preserve of minced lamb meat. Food Packag Shelf Life 34:100942. https://doi.org/10.1016/j.fpsl.2022.100942
Doğan N, Doğan C, Eticha AK et al (2022b) Centrifugally spun micro-nanofibers based on lemon peel oil/gelatin as novel edible active food packaging: fabrication, characterization, and application to prevent foodborne pathogens E. coli and S. aureus in cheese. Food Control 139:109081. https://doi.org/10.1016/j.foodcont.2022.109081
El-Gendi H, Salama A, El-Fakharany EM, Saleh AK (2022) Optimization of bacterial cellulose production from prickly pear peels and its ex situ impregnation with fruit byproducts for antimicrobial and strawberry packaging applications. Carbohydr Polym. https://doi.org/10.1016/J.CARBPOL.2022.120383
Emiljanowicz KE, Malinowska-Pańczyk E (2020) Kombucha from alternative raw materials–The review. Crit Rev Food Sci Nutr 60:3185–3194. https://doi.org/10.1080/10408398.2019.1679714
Fan F, Liu Y, Liu Y et al (2022) Candida albicans biofilms: antifungal resistance, immune evasion, and emerging therapeutic strategies. Int J Antimicrob Agents 60:106673. https://doi.org/10.1016/j.ijantimicag.2022.106673
Fuller ME, Andaya C, McClay K (2018) Evaluation of ATR-FTIR for analysis of bacterial cellulose impurities. J Microbiol Methods 144:145–151. https://doi.org/10.1016/J.MIMET.2017.10.017
Gaggìa F, Baffoni L, Galiano M et al (2018) Kombucha beverage from green, black and rooibos teas: a comparative study looking at microbiology chemistry and antioxidant activity. Nutrients 2019 11:1. https://doi.org/10.3390/NU11010001
Jamieson AJ, Brooks LSR, Reid WDK et al (2019) Microplastics and synthetic particles ingested by deep-sea amphipods in six of the deepest marine ecosystems on Earth. R Soc Open Sci. https://doi.org/10.1098/rsos.180667
Jayabalan R, Malbaša R, Lončar ES et al (2014) A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr Rev Food Sci Food Saf 13:538–550. https://doi.org/10.1111/1541-4337.12073
Julienne F, Delorme N, Lagarde F (2019) From macroplastics to microplastics: role of water in the fragmentation of polyethylene. Chemosphere 236:124409. https://doi.org/10.1016/j.chemosphere.2019.124409
Kostag M, Gericke M, Heinze T, El Seoud OA (2019) Twenty-five years of cellulose chemistry: innovations in the dissolution of the biopolymer and its transformation into esters and ethers. Cellulose 26:139–184. https://doi.org/10.1007/s10570-018-2198-0
Laavanya D, Shirkole S, Balasubramanian P (2021) Current challenges, applications and future perspectives of SCOBY cellulose of Kombucha fermentation. J Clean Prod 295:126454. https://doi.org/10.1016/j.jclepro.2021.126454
Lebreton L, Slat B, Ferrari F et al (2018) Evidence that the Great Pacific garbage patch is rapidly accumulating plastic. Sci Rep 8:1–15. https://doi.org/10.1038/s41598-018-22939-w
Liu Y, Ahmed S, Sameen DE et al (2021) A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci Technol 112:532–546. https://doi.org/10.1016/j.tifs.2021.04.016
NamlSenanayake SPJ (2013) Green tea extract: Chemistry, antioxidant properties and food applications - a review. J Funct Foods 5(4):1529–1541
RamírezTapias YA, Peltzer MA, Delgado JF, Salvay AG (2020) Kombucha tea by-product as source of novel materials: formulation and characterization of films. Food Bioprocess Technol 13:1166–1180. https://doi.org/10.1007/s11947-020-02471-4
RamírezTapias YA, Monte MV, Peltzer MA, Salvay AG (2022) Bacterial cellulose films production by Kombucha symbiotic community cultured on different herbal infusions. Food Chem 372:131346. https://doi.org/10.1016/j.foodchem.2021.131346
Rawangkan A, Siriphap A, Yosboonruang A et al (2022) Potential antimicrobial properties of coffee beans and coffee by-products against drug-resistant vibrio cholerae. Front Nutr 9:710. https://doi.org/10.3389/FNUT.2022.865684/BIBTEX
Retegi A, Gabilondo N, Peña C et al (2010) Bacterial cellulose films with controlled microstructure-mechanical property relationships. Cellulose 17:661–669. https://doi.org/10.1007/S10570-009-9389-7/FIGURES/6
Rose M, Palkovits R (2011) Cellulose-based sustainable polymers: state of the art and future trends. Macromol Rapid Commun 32:1299–1311. https://doi.org/10.1002/marc.201100230
Shao W, Wang S, Liu H et al (2016) Preparation of bacterial cellulose/graphene nanosheets composite films with enhanced mechanical performances. Carbohyd Polym 138:166–171. https://doi.org/10.1016/J.CARBPOL.2015.11.033
Sharma C, Bhardwaj NK (2019) Biotransformation of fermented black tea into bacterial nanocellulose via symbiotic interplay of microorganisms. Int J Biol Macromol 132:166–177. https://doi.org/10.1016/J.IJBIOMAC.2019.03.202
Vanderfleet OM, Reid MS, Bras J et al (2019) Insight into thermal stability of cellulose nanocrystals from new hydrolysis methods with acid blends. Cellulose 26:507–528. https://doi.org/10.1007/s10570-018-2175-7
Villarreal-Soto SA, Beaufort S, Bouajila J et al (2018) Understanding Kombucha tea fermentation: a review. J Food Sci 83:580–588. https://doi.org/10.1111/1750-3841.14068
Wang L, Yang R, Yuan B et al (2015) The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharmaceut Sinica B 5:310. https://doi.org/10.1016/J.APSB.2015.05.005
Wei B, Yang G, Hong F (2011) Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohyd Polym 84:533–538. https://doi.org/10.1016/j.carbpol.2010.12.017
Zhang P, Zhao Y, Shi Q (2016) Characterization of a novel edible film based on gum ghatti: Effect of plasticizer type and concentration. Carbohyd Polym 153:345–355. https://doi.org/10.1016/j.carbpol.2016.07.082
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
ND designed, analyzed, drafted and revised the work of this article. The author has read and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Doğan, N. Native bacterial cellulose films based on kombucha pellicle as a potential active food packaging. J Food Sci Technol 60, 2893–2904 (2023). https://doi.org/10.1007/s13197-023-05808-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05808-x