Abstract
Listeria monocytogenes is a food-borne bacterium that causes listeriosis upon the ingestion of contaminated food. Traditional methods to detect L. monocytogenes require pre-enrichment broths to increase its concentration. To improve the screening of contaminated food and prevent listeriosis outbreaks, rapid, specific and sensitive assays are needed to detect L. monocytogenes. This study developed a prototype lateral flow immunochromatographic assay (LFIA) employing antibodies against L. monocytogenes Internalin A (InlA) and Internalin B (InlB) proteins, that are involved in non-phagocytic cell invasion. The following antibodies were used to capture L. monocytogenes antigenic targets: mouse anti-Internalin A monoclonal antibody (MAb-2D12) conjugated to colloidal gold nanoparticles and a mouse anti-Internalin B polyclonal antibody. This test was able to detect pure L. monocytogenes from culture with a limit of detection (LOD) ranging from 5.9 × 103 to 1.5 × 104 CFU/mL. In milk artificially contaminated with L. monocytogenes, the LOD was 1 × 105 CFU/mL. This prototype test discriminated L. monocytogenes from other bacterial species (Listeria innocua, Enterobacter cloacae, Bacillus cereus). Results indicate that this LFIA developed using antibodies against L. monocytogenes InlA and InlB proteins is a sensitive and specific tool that can be potentially useful to rapidly detect L. monocytogenes in contaminated food.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Listeria monocytogenes is a Gram-positive invasive food-borne bacterial pathogen that upon ingestion of highly contaminated food (up to ~ 109 bacteria) by healthy adults causes listeriosis, a mild to severe gastroenteritis characterized by diarrhea, abdominal cramps and flu-like symptoms (Schlech 2019). However in children, elderly, immunocompromised individuals and pregnant women, even low levels of food contamination (~ 102–104 bacteria) can be potentially lethal (Mclauchlin 1990). Following the ingestion of contaminated food, L. monocytogenes cross the intestinal barrier, spread into the bloodstream through the lymph nodes and disseminate to target tissues, such as the liver and the spleen manifesting a plethora of effects on the cells due to the activity of potent virulence factors (Drolia and Bhunia 2019). In immunocompromised individuals, L. monocytogenes can cross the blood–brain barrier or the fetoplacental barrier and cause potentially fatal meningitis, sepsis, premature birth or abortion. Despite its low incidence, the mortality rate of listeriosis ranges from 20 to 30% (Schlech 2019).
Listeria genus comprises 26 species and two of them, L. monocytogenes and Listeria ivanovii are pathogenic to humans and ruminants, respectively (Carlin et al. 2021). L. ivanovii has also been described as an opportunistic pathogen associated with gastroenteritis and bacteremia in humans (Snapir et al. 2006). L. innocua represents the most prevalent bacteria in listeria-contaminated foods and this poses a difficulty for the specific capture and detection of the pathogenic listeria (Oravcová et al. 2008).
Traditional methods for L. monocytogenes detection in food include long time bacterial culture with selective pre-enrichment steps (Bhunia 2018). Sensitive and specific molecular methods based on the detection of bacterial nucleic acid such as the polymerase chain reaction (PCR) have been proposed, but they are still considered expensive and complex for routine use (Wu 2019). In contrast, lateral flow immunochromatographic assay (LFIA) represents a simple, affordable, user- and field-friendly diagnostic tool that can provide quick visual results for L. monocytogenes detection (Cho et al. 2015; Li et al. 2017; Liu et al. 2017). Commercially available rapid tests for L. monocytogenes have low detection capacity, are time consuming and costly due to prior culture required to increase the amount of bacteria to detectable levels (Ueda et al. 2013).
The advances in the understanding of L. monocytogenes biology including the steps involved in host cell invasion revealed new potential targets for immunodiagnosis such as the internalin A (InlA) and InlB, two members of a family of 25 proteins known as internalins, which bind to eukaryotic cell membrane receptors (Radoshevich and Cossart 2018). L. monocytogenes can be uptaken by phagocytic cells or internalized into non-phagocytic cells, which is considered one of its hallmarks. In non-phagocytic cells, such as goblet cells, InlA binds the E-cadherin while in trophoblasts, InlB binds the hepatocyte growth factor receptor, inducing bacterial uptake through receptor-mediated endocytosis (Drolia and Bhunia 2019). InlA and InlB proteins have been shown to be immunogenic representing important targets for antibody recognition (Banada and Bhunia 2008; Drolia and Bhunia 2019). The production of anti-InlA monoclonal antibodies (MAb) and of anti-InlB polyclonal antibodies (PAb) and their application for the detection of L. monocytogenes in food samples has been reported (Banada and Bhunia 2008; Tully et al. 2008; Mendonça et al. 2012; Lathrop et al. 2014). InlA and InlB in the Listeria genus have highly immunogenic and non-conserved amino acid sequences (Bierne and Cossart 2007).
The goal of this study was to develop a prototype lateral flow diagnostic tool for the detection of L. monocytogenes based on the use of a combination of antibodies against InlA and InlB proteins. As a proof of concept, the performance of this prototype was evaluated in pure culture of L. monocytogenes and in artificially contaminated milk samples. The specificity of this test was also evaluated.
Materials and methods
Antibodies and bacteria
The prototype LFIA employed a mouse anti-internalin A monoclonal antibody (MAb-2D12) previously described (Mendonça et al. 2012) and a mouse anti-internalin B polyclonal antibodies (PAb-InlB), which were kindly provided by the Laboratory of Applied Immunology (Federal University of Pelotas, Pelotas, RS, Brazil). The strains of L. monocytogenes (ATCC 7644 and 19,117), Listeria innocua (CLIP 12,612), Enterobacter cloacae (ATCC 13,047) and Bacillus cereus (ATCC 11,778) were provided by the Laboratory of Food Microbiology (Federal University of Pelotas, Pelotas, RS, Brazil).
Bacterial cultures
In order to obtain bacterial activation prior to use, L. monocytogenes and L. innocua strains were cultivated at 37 ºC for 16- 18 h in tryptic soy broth (TSB, 22,092, Sigma-Aldrich, St. Louis, USA) supplemented with 0.6% (w/v) yeast extract (Y1,625, Sigma-Aldrich). The strains of E. cloacae and B. cereus were cultivated in brain heart infusion broth (BHI, 53,286, Sigma-Aldrich) at 37 °C for 16- 18 h. The bacterial cultures were diluted ten-fold (ratio factor 1:5), and each dilution was plated onto TSB agar plates to count colony forming units (CFU). The optical density (OD600) of each dilution was recorded (Metter Toledo, Columbus, USA) and the OD values were plotted on graphs to generate standard curves and linear regression equations from bacteria growth (Fig. 1).
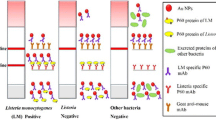
Methodology employed for the development of the prototype lateral flow immunochromatography assay (LFIA) for the detection of Listeria monocytogenes by using a combination of antibodies: anti-Internalin A (MAb-2D12) conjugated to colloidal gold nanoparticles and anti-Internalin B (PAb anti-InlB). Pure cultures of L. monocytogenes and artificially contaminated milk (2nd and 3rd schemes) were used as test samples while cultures of Listeria innocua, Bacillus cereus and Enterobacter cloacae (1st scheme) were used as specificity controls (1st scheme). Bacterial cultures were plated onto TSB agar plates and colony forming units (CFU) were counted (1st scheme). The optical density (OD600) of each dilution was recorded, and values were plotted on standard curves and linear regression equations were based on the concentrations (CFU/mL) and respective ODs. Next, L. monocytogenes was cultivated in TSB-YE and eight different dilutions (2nd scheme) were tested with the prototype LFIA. Lastly, ten different dilutions of L. monocytogenes were artificially inoculated into pasteurized milk samples (3rd scheme) and these samples were tested in lateral flow prototypes. TSB-YE: Tryptic Soy Broth with Yeast Extract; BHI: Brain Heart Infusion; OD: optical density; T: test line; C: control line; CFU: colony formed units
Preparation of the L. monocytogenes Internalin lateral flow immunochromatographic assay (LM Internalin LFIA) prototype
As depicted in Fig. 1, the LFIA prototype developed in this study was composed of a sample pad, a conjugated pad containing 33 µg/mL of MAb-2D12 conjugated to colloidal gold nanoparticles (AuNP, 520,918, Sigma-Aldrich), a nitrocellulose (NC) membrane containing the test and the control lines and an absorbent pad. As previously described, the MAb-2D12 is an IgG2a antibody, highly specific to the L. monocytogenes Internalin A epitope that recognizes all L. monocytogenes serotypes (Mendonça et al. 2012). The conjugation of gold nanoparticles (AuNP) to the MAb-2D12 was performed as described elsewhere (Snowden and Hommel 1991; Gussenhoven et al. 1997). Anti-InlB polyclonal antibodies at 1 mg/mL and protein A were dropped on the NC membrane (Test/T and Control/C lines, respectively). A XYZ Airjet Dispenser System from BioDot Inc (Irvine, CA, USA) was used to deposit the capture agent in NC cards and to dispense the conjugate in the fiber-fleece. Lastly, the assembled card was cut into strips (5 mm width) using CM4000 BioDot Paper Cutter (Irvine, CA, USA) and stored at 4 °C until the strips were built into a proper plastic housing.
Determination of the limit of detection (LOD) of the LM Internalin LFIA prototype test
To determine the LOD of the test, pure culture of L. monocytogenes (TSB medium, 37 °C for 16–18 h) was used in different concentrations that were calculated by linear regression equation: 5.9 × 103, 2.96 × 104, 1.48 × 105, 7.42 × 105, 3.71 × 106, 1.85 × 107, 9.28 × 107, 4.64 × 108, 1 × 109 CFU/mL. The L. monocytogenes dilutions were prepared in TSB medium and PBS pH 7.2, and 35 µL of each dilution was tested with the LFIA prototype. Test results were visually read after 15 min and the test was considered valid when the control line was clearly visible. The tests were considered positive whenever a distinct purple staining of the test line was observed. To assess the prototype reproducibility, a second test batch and a second curve were prepared and tested by an independent researcher (data not shown) using a different lot and the respective concentrations of L. monocytogenes culture, prepared as previously described. To assess the test specificity, L. innocua, E. cloacae and B. cereus strains were cultivated in BHI at 37 °C for 24 h and different dilutions were tested as described for L. monocytogenes strain.
Preparation of L. monocytogenes contaminated sample food and detection
A food sample, of artificially contaminated pasteurized milk, purchased from a local market was tested with the prototype. For this, L. monocytogenes at 5.0 × 109 CFU/mL (TSB medium) was artificially inoculated into 50 mL of the milk sample and homogenized for 1 min. Nine serial dilutions of the artificially contaminated milk were prepared and each milk dilution was used as an individual sample for testing.
Results
Evaluation of the performance of the LM Internalin Prototype LFIA to detect L. monocytogenes and of its sensitivity
The prototype LM Internalin LFIA was evaluated for its capacity to detect different concentrations of L. monocytogenes obtained from pure cultures. For the quantitative determination of L. monocytogenes in culture samples, the linear relationship between the optical density (OD600) and the log concentrations was developed which ranged from 1 × 101 to 1 × 1010 (linear regression equation y = 4.754 × 109 x – 2.570 × 108; R2 = 0.9975) (Fig. 2 and Fig. S1). When the two batches of cultures were tested, the LOD of the LM Internalin LFIA prototype was 5.9 × 103 CFU/mL (Fig. 3a) and 1.5 × 104 CFU/mL (Fig. 3b) of L. monocytogenes, respectively.
Correlation between optical density of L. monocytogenes cultures and bacterial concentration. L. monocytogenes grown in tryptic soy broth were serially diluted and plated to determine CFU. A graph correlating the ODs and CFU/mL was plotted and the linear regression equation was determined. CFU: colony forming units
LM internalin LFIA and the detection of L. monocytogenes in artificially contaminated milk
Commercially available pasteurized milk contaminated in the laboratory with different concentrations of L. monocytogenes, obtained from cultures, were tested with the LM Internalin LFIA. L. monocytogenes concentrations in milk samples ranged from 5 × 104 CFU/mL to 4 × 1010 CFU/mL and as depicted in Fig. 4L. monocytogenes in milk was detected in the range of 1 × 105 to 4 × 1010 CFU/mL concentrations.
Evaluation of the limit of detection (LOD) of the LM Internalin LFIA prototype for L. monocytogenes in artificially contaminated pasteurized milk. The following concentrations were used: 4 × 1010, 9 × 109, 1 × 109, 3 × 108, 7 × 107, 1 × 107, 3 × 106, 6 × 105, 1 × 105, 5 × 104 CFU/mL)\; CFU: colony forming units
Evaluation of the specificity of the LM Internalin LFIA prototype
L. innocua, E. cloacae and B. cereus strains at concentrations of 5 × 109 CFU/mL tested negative with the LM Internalin LFIA (Fig. 5).
Discussion
This study presents the results of a sensitive and specific prototype rapid lateral flow immunoassay for L monocytogenes detection (LM Internalin LFIA) which was developed based on the use of anti-Internalin A and B antibodies. Highly specific and sensitive antibodies produced against immunogenic virulence/adhesion factors of L. monocytogenes, such as the Internalin A and B, are considered key reagents for the development of immunodetection tools (Li et al. 2017). L. monocytogenes is a ubiquitous opportunistic pathogen found in food-processing environments and food products (Bhunia 2018) representing both a constant threat to the food industry and also a challenge to detect and eliminate this pathogen from final products and from the processing environments (Phraephaisarn et al. 2017). The advantage of a qualitative rapid test for L. monocytogenes detection, as the one described here in, lies on the fact that immunochromatography represents the simplest and quickest method for pathogen detection in a sample requiring around 15 min for final results (Liu et al. 2017; Wu 2019). Also, the use of colloidal gold nanoparticles as a detection label, allows the easy monitoring of the color reaction which can be scored by naked eye, without the use of any costly equipment. Although outbreaks are relatively rare, consumption of L. monocytogenes tainted food products can cause overwhelming health and economic consequences (Hoffmann et al. 2014). Therefore, a high-throughput, rapid, specific and sensitive method to detect L. monocytogenes is always highly needed to guarantee the safety and quality of food, especially meat and dairy products. Considering the features of the LM Internalin LFIA reported in this study, it represents a potential point-of-care (POC) diagnostic tool for L. monocytogenes detection.
Another potential advantage of the use of a sensitive, rapid lateral flow tests is the direct detection without the need of previous conventional culture methods, that although simple, of low cost and reliable, may take 5– 7 days for its detection and confirmation in food products (Bhunia 20142018). The prototype LM Internalin LFIA developed using a combination of Internalin A and B antibodies was able to directly detect L. monocytogenes both from culture and in artificially contaminated milk with good sensitivity. Although other food products were not tested, it is possible that it may be able to identify the pathogen in processed food products without the need of previous culture. Usually, due to the low number of contaminating bacteria in food samples, pre-enrichment cultures are needed to expand bacterial growth to detectable numbers, however, besides the long time needed, during the culture time, the suspected sample can be contaminated by sample residues and by other bacteria (Ueda et al. 2013; Bhunia 2014; Li et al. 2017). An ideal diagnostic tool for the detection of L. monocytogenes and the prevention of listeriosis outbreaks should be simple, specific, sensitive, affordable and provide rapid results after testing as the lateral flow test provides.
We consider that the use of highly specific and sensitive anti-Internalin antibodies employed in this LFIA was crucial for achieving good sensitivity, especially the use of the monoclonal antibody Mab-2D12 which was conjugated to colloidal gold nanoparticles and employed as a label signal. The anti-InlA MAb-2D12 was previously described to be specific for L. monocytogenes and L. ivanovii and when used in immunomagnetic separation assay provided highly specific capture efficiency for both bacteria enabling their detection at low levels from buffer or food using fiber-optic sensor (Mendonça et al. 2012). In immunodiagnostic tests, antibodies have been employed as affinity ligands to separate and concentrate the target analyte from sample matrices when coupled to paramagnetic beads (Banada and Bhunia 2008; Mendonça et al. 2012; Bhunia 2014) or as recognition or reporter molecules (Tully et al. 2008; Dwivedi and Jaykus 2011). Different immunologic detection methods including ELISA (Mendonça et al. 2012; Lv et al. 2019), colloidal gold immunochromatography (Ueda et al. 2013; Cho et al. 2015; Liu et al. 2017; Wu 2019) and immunomagnetic separation method (Uusitalo et al. 2016) have been described for the detection of pathogens and toxins. However, one the greatest pitfalls of immunologic methods to detect L. monocytogenes is their low sensitivity characterized by a high bacteria concentration for their LOD. The prototype LM Internalin LFIA presented in this study showed good sensitivity in two batches of bacterial cultures (5.9 × 103 and 1.5 × 104 CFU/mL) and from these concentrations, the color signals produced by gold nanoparticles at the test line on the nitrocellulose immunostrip increased. The LOD of commercially available lateral flow rapid tests (Dupont Qualicon, Neogen Corp., and Oxoid Ltd) ranged from 105 to 106 cells/mL (Ueda et al. 2013). Other studies have reported tests with lower sensitivity for L. monocytogenes detection: Ueda et al. (2013) (LOD 6.9 × 106 CFU/mL), Li et al. (2017) (LOD 4.0 × 105 CFU/mL). A previous study reported that the use of immunomagnetic beads as bacterial pre-treatment increased the LOD by one log (4.0 × 104 CFU/mL) however, this increase in sensitivity required an additional step of pre-enrichment, making it more complex and costly (Li et al. 2017). Therefore, the good sensitivity of the LM Internalin LFIA without pre-enrichment step increases the chances of its applicability in the field as a POC.
When the LM Internalin LFIA was performed with artificially contaminated milk, the obtained LOD (1 × 105 CFU/mL) was lower than the sensitivity of a previously described lateral flow enzyme concentration assay using artificially contaminated milk samples (LOD of 1 × 102 CFU/mL) (Cho and Irudayaraj 2013). However, this test required a special equipment because of the additional step for bacterial separation and concentration by magnetic nanoparticles. A fluorescent lateral flow using immunomagnetic separation of bacteria in artificially contaminated milk reported a LOD of 1 × 104 CFU/mL (Li et al. 2017), nevertheless with the drawback of additional time and cost. The sensitivity of the tests mentioned above was improved by combining other non-immunological techniques which added complex reading steps, while the LOD of our prototype defined by visual readings was established by the use of highly specific antibodies against InlA and InlB proteins that are present on the bacterial surface (Radoshevich and Cossart 2018; Drolia and Bhunia 2019).
Another important feature of a diagnostic tool refers to its specificity. No cross reaction was observed when pure culture of B. cereus, E. cloacae and L. innocua at concentration of 5 × 109 CFU/mL were tested indicating that the LM Internalin LFIA can discriminate L. monocytogenes from other Listeria species and from other non-Listeria gastroenteric bacteria. The absence of cross-reaction with L. innocua is also an advantage of the prototype described, as L. innocua is the most frequently found bacteria in Listeria-contaminated food, representing a difficulty for the specific capture and detection of the pathogenic Listeria (Oravcová et al. 2008). Also, food products tainted with L. monocytogenes may often be contaminated with other Listeria spp. both pathogenic and non-pathogenic as well as other background microbiota and, as L monocytogenes grows slowly, other bacteria may outcompete, so lower concentrations of L. monocytogenes may be expected and lead to false-negative results (Gnanou Besse et al. 2010; O’Connor et al. 2010). As our prototype did not require pre-enrichment step, the possibility of false negative results due to overgrowth of other species is reduced. As previously mentioned, the Mab used in the LM Internalin LFIA is specific for both human pathogenic bacteria, L. monocytogenes and L. ivanovii (Mendonça et al. 2012), therefore although not tested, this prototype has also the potential to detect the pathogenic L. ivanovii that is an opportunistic pathogen that is associated with gastroenteritis and bacteremia in humans (Snapir et al. 2006).
Although promising, we acknowledge that this study has limitations such as the need to test if the LM Internalin LFIA can detect L. monocytogenes in other types of food besides milk. However, a previous study demonstrated that the MAb-2D12 was used as a capture agent and reporter antibody in a fiber-optic sensor test that was able to detect L. monocytogenes in hot dogs and soft cheese (Mendonça et al. 2012). It is known that the recovery of low numbers of pathogens from complex food matrices can interfere with their rapid and sensitive detection (Bhunia 2008). Moreover, the use of polyclonal antibodies limits the detection of low populations of L. monocytogenes because antigen recognition is less specific, and antibodies can cross-react with the food matrix (Bhunia 2008). There is no availability of anti-InlB monoclonal antibodies on the market, limiting our current capacity to improve the LOD of our prototype. We also acknowledge that the capacity of the LM Internalin LFIA to discriminate other pathogens should be tested.
Conclusions
We have developed a sensitive and specific rapid lateral flow immunochromatographic assay to detect L. monocytogenes based on the use of anti-Internalin A monoclonal antibodies and anti-Internalin B polyclonal antibodies. Our prototype test detected L. monocytogenes from cultures and in artificially contaminated milk with good sensitivity compared to commercially available tests. No cross reaction was observed with the non-pathogenic L. innocua and other gastro-enteric bacteria (B. cereus, E. cloacae). Preliminary data on the sensitivity and specificity of this prototype test, which provides visual results within 15 min, indicate its potential use as a point-of-care test, for the rapid detection of L. monocytogenes in contaminated food or industry environment.
Data Availability
Not Applicable.
Code Availability
Not Applicable.
Abbreviations
- AuNP:
-
Colloidal Gold Nanoparticle
- BHI:
-
Brain Heart Infusion
- InlA:
-
Internalin A
- InlB:
-
Internalin B
- LFIA:
-
Lateral Flow Immunochromatographic Assay
- LM:
-
Listeria Monocytogenes
- LOD:
-
Limit of Detection
- MAb:
-
Monoclonal Antibody
- NC:
-
Nitrocellulose Membrane
- OD:
-
Optical Density
- PAb:
-
Polyclonal Antibody
- TSB:
-
Tryptic Soy Broth
References
Banada PP, Bhunia AK (2008) Antibodies and immunoassays for detection of bacterial pathogens. Princ bact detect biosensors recognit recept microsystems. Springer, London, pp 567–602
Bhunia AK (2008) Biosensors and bio-based methods for the separation and detection of foodborne pathogens. Adv Food Nutr Res 54:1–44. https://doi.org/10.1016/S1043-4526(07)00001-0
Bhunia AK (2014) One day to one hour: How quickly can foodborne pathogens be detected? Future Microbiol 9:935–946. https://doi.org/10.2217/fmb.14.61
Bhunia AK (2018) Foodborne microbial pathogens. Springer, New York
Bierne H, Cossart P (2007) Listeria monocytogenes Surface Proteins: from genome predictions to function. Microbiol Mol Biol Rev 71:377–397. https://doi.org/10.1128/mmbr.00039-06
Carlin CR, Liao J, Weller D et al (2021) Listeria cossartiae sp Nov, listeria immobilis sp nov, listeria portnoyi sp nov and listeria rustica sp nov, isolated from agricultural water and natural environments. Int J Syst Evol Microbiol. https://doi.org/10.1099/ijsem.0.004795
Cho I, Irudayaraj J (2013) Lateral-flow enzyme immunoconcentration for rapid detection of listeria monocytogenes. Anal Bioanal Chem 405:3313–3319. https://doi.org/10.1007/s00216-013-6742-3
Cho IH, Bhunia A, Irudayaraj J (2015) Rapid pathogen detection by lateral-flow immunochromatographic assay with gold nanoparticle-assisted enzyme signal amplification. Int J Food Microbiol 206:60–66. https://doi.org/10.1016/j.ijfoodmicro.2015.04.032
Drolia R, Bhunia AK (2019) Crossing the intestinal barrier via listeria adhesion protein and Internalin A. Trends Microbiol 27:408–425. https://doi.org/10.1016/j.tim.2018.12.007
Dwivedi HP, Jaykus LA (2011) Detection of pathogens in foods: The current state-of-the-art and future directions. Crit Rev Microbiol 37:40–63. https://doi.org/10.3109/1040841X.2010.506430
Gnanou Besse N, Barre L, Buhariwalla C et al (2010) The overgrowth of Listeria monocytogenes by other Listeria spp. in food samples undergoing enrichment cultivation has a nutritional basis. Int J Food Microbiol 136:345–351. https://doi.org/10.1016/j.ijfoodmicro.2009.10.025
Gussenhoven GC, Van Der Hoorn MAWG, Goris MGA et al (1997) LEPTO dipstick, a dipstick assay for detection of Leptospira-specific immunoglobulin M antibodies in human sera. J Clin Microbiol 35:92–97
Hoffmann S, Batz MB, Junior Morris G (2014) Annual Cost of Illness and Quality-Adjusted Life Year Losses in the United States Due to 14 Foodborne Pathogens. J Food Prot 75:1292–1302. https://doi.org/10.4315/0362-028X
Lathrop AA, Bailey TW, Kim K-P, Bhunia AK (2014) Pathogen-specific antigen target for production of antibodies produced by comparative genomics. Antib Technol J 4:13–22. https://doi.org/10.2147/anti.s54848
Li Q, Zhang S, Cai Y et al (2017) Rapid detection of Listeria monocytogenes using fluorescence immunochromatographic assay combined with immunomagnetic separation technique. Int J Food Sci Technol 52:1559–1566. https://doi.org/10.1111/ijfs.13428
Liu H, Du X, Zang Y-X et al (2017) SERS-based lateral flow strip biosensor for simultaneous detection of listeria monocytogenes and salmonella enterica serotype enteritidis. J Agric Food Chem 65:10290–10299. https://doi.org/10.1021/acs.jafc.7b03957
Lv X, Huang Y, Liu D et al (2019) Multicolor and ultrasensitive ELISA based on fluorescence hybrid chain reaction for simultaneous detection of pathogens. J Agric Food Chem 67:9390–9398. https://doi.org/10.1021/acs.jafc.9b03414
Mclauchlin J (1990) Human listeriosis in Britain, 1967–85, a summary of 722 cases: 1. Listeriosis during pregnancy and in the newborn. Epidemiol Infect 104:181–189. https://doi.org/10.1017/S0950268800059343
Mendonça M, Conrad NL, Conceição FR et al (2012) Highly specific fiber optic immunosensor coupled with immunomagnetic separation for detection of low levels of Listeria monocytogenes and L ivanovii. BMC Microbiol. https://doi.org/10.1186/1471-2180-12-275
O’Connor L, O’Leary M, Leonard N et al (2010) The characterization of Listeria spp. isolated from food products and the food-processing environment. Lett Appl Microbiol 51:490–498. https://doi.org/10.1111/j.1472-765X.2010.02928.x
Oravcová K, Trnčíková T, Kuchta T, Kaclíková E (2008) Limitation in the detection of Listeria monocytogenes in food in the presence of competing Listeria innocua. J Appl Microbiol 104:429–437. https://doi.org/10.1111/j.1365-2672.2007.03554.x
Phraephaisarn C, Khumthong R, Takahashi H et al (2017) A novel biomarker for detection of Listeria species in food processing factory. Food Control 73:1032–1038. https://doi.org/10.1016/j.foodcont.2016.10.001
Radoshevich L, Cossart P (2018) Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol 16:32–46. https://doi.org/10.1038/nrmicro.2017.126
Schlech WF (2019) Epidemiology and clinical manifestations of listeria monocytogenes infection. Gram-postive pathogens. ASM press, Washington, pp 793–802
Snapir YM, Vaisbein E, Nassar F (2006) Low virulence but potentially fatal outcome-Listeria ivanovii. Eur J Intern Med 17:286–287. https://doi.org/10.1016/j.ejim.2005.12.006
Snowden K, Hommel M (1991) Antigen detection immunoassay using dipsticks and colloidal dyes. J Lmmunological Methods 140:57–65. https://doi.org/10.1016/0022-1759(91)90126-z
Tully E, Higson SP, O’Kennedy R (2008) The development of a “labeless” immunosensor for the detection of Listeria monocytogenes cell surface protein, Internalin B. Biosens Bioelectron 23:906–912. https://doi.org/10.1016/j.bios.2007.09.011
Ueda S, Iwase M, Kuwabara Y (2013) Evaluation of Immunochromatography for the rapid and specific identification of listeria monocytogenes from food. Biocontrol Sci 18:157–161. https://doi.org/10.4265/bio.18.157
Uusitalo S, Kögler M, Välimaa AL et al (2016) Detection of: Listeria innocua on roll-to-roll produced SERS substrates with gold nanoparticles. RSC Adv 6:62981–62989. https://doi.org/10.1039/c6ra08313g
Wu Z (2019) Simultaneous detection of listeria monocytogenes and salmonella typhimurium by a SERS-based lateral flow immunochromatographic assay. Food Anal Methods 12:1086–1091. https://doi.org/10.1007/s12161-019-01444-4
Acknowledgements
Students’ fellowships were granted by: the Brazilian National Research Council (grant # 133188/2016-7 2016-2018), the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES grant # 88882.385457/2007-01 2019-2023) and the Alliances Program for Education and Training (Scholarships Brazil—PAEC OAS-GCUB). AK is a research fellow from the Brazilian Research Council/CNPq (“Bolsista de Produtividade em Pesquisa, Grant # 314366/2020-2). MMAS is PVN-II research fellow from FAPEAM/Amazonas (PECTI-AM/SAÚDE Program, Grant #004/2020) and a research fellow from the Brazilian Research Council/CNPq (“Bolsista de Produtividade em Pesquisa, Grant # 311986-2019-6).
Funding
Not Applicable.
Author information
Authors and Affiliations
Contributions
Conception and design: RSM, ESF, MM, FRC, MMAS, SBS; Acquisition of data: RSM, ESF, AV, DRS, ANM, TGS; Analysis and interpretation of data: LLL, ESF, RSM, AK, MMAS, SBS; Drafting the manuscript: LLL, ESF, SBS, MMAS; Revising the manuscript critically: LLL, RSM, AK, MM, FRC, MMAS, SBS; Approval of the final manuscript: LLL, ESF, MM, ANM, AV, DRS, TGS, RSM, MMAS, FRC, AK, SBS.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Ethics approval
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lopes-Luz, L., Silva-Filho, E., Mendonça, M. et al. Combined antibodies against internalins A and B proteins have potential application in immunoassay for detection of Listeria monocytogenes. J Food Sci Technol 60, 123–131 (2023). https://doi.org/10.1007/s13197-022-05597-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-022-05597-9