Abstract
One of the most troublesome postharvest diseases of citrus fruits is sour rot, caused by Geotrichum citri-aurantii. Sour rot reduces the shelf life of the fruits leading to massive economic losses. This study investigated the potential for a combination of cinnamaldehyde and citral (CC; 1: 2, v/v) at reducing the incidence of sour rot postharvest and its possible effect on fruit quality. Our findings show that CC could totally inhibit germination of G. citri-aurantii spores, with the minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) both being 0.80 mL L−1. The combination (CC) acted against G. citri-aurantii by targeting the chitin content of the cell wall. Wax + CC (WCC; 1 × MFC) treatment also showed high efficiency in reducing the incidence of sour rot, which was 40% lower than in the control group by day 8 when all the fruits in the latter were rotten. Apart from vitamin c (Vc) content which was higher in the test group than in the control group, WCC treatment did not have any significant effect on the quality of the citrus fruits, the examined fruit quality parameters being weight loss rate, coloration index, firmness, pH, total soluble solid (TSS) content, Vc content, as well as solid acid ratio. These results indicate that the combination of cinnamaldehyde and citral (CC, 1: 2, v/v) can be used as a natural preservative to alleviate the progress of sour rot in citrus fruits postharvest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruit spoilage by fungi is a major concern during storage as it affects the fruit quality and shortens their shelf life (Liu et al. 2017; Suwanamornlert et al. 2018). Citrus fruits in particular are susceptible to several postharvest diseases which cause extensive losses. Among these diseases include sour rot caused by Geotrichum citri-aurantii, which greatly deteriorates the fruit quality, leading to massive economic losses in citrus fruit industry (Deng et al. 2018; Singh and Sharma 2018). Developing any potential control measures is thus a significant part in maintaining quality and extending the shelf-life of citrus fruits.
Currently, the management of sour rot postharvest is generally through use of synthetic chemicals or their mixtures (Li et al. 2019; Zhou et al. 2014). However, the effect of chemical fungicides to control sour rot is limited, and their prolonged use may lead to the development of fungicide-resistant pathogens (Bazioli et al. 2019; Mckay et al. 2012). Moreover, fungicide residues left on fruit surfaces may seriously affect the health of the consumers and pollute the environment (Chen et al. 2019; Liu et al. 2017). This has prompted efforts to develop non-hazardous and highly bioactive approaches to control sour rot disease in postharvest citrus fruits (Bazioli et al. 2019; Duan et al. 2018; Wuryatmo et al. 2014), among which include the use of plant essential oils.
Essential oils (EOs) are recognized by consumers as natural components of plants, making their application in the control of certain postharvest diseases of fruits and vegetables highly favored as they are reported to have broad fungicidal properties and are safer to both the consumers and to the environment than the synthetic fungicides (Boubaker et al. 2016; Shao et al. 2015). For example, cinnamaldehyde and citral are generally accepted as safe (GRAS) for human health and environment and have been registered by the Food and Drug Administration (FDA) as flavoring agents in foods (Wang et al. 2016, 2018a, b). Moreover, studies by Camele et al. (2012) and Manso et al. (2015) revealed that cinnamaldehyde and citral have strong inhibitory activities against postharvest Aspergillus, Fusarium, Penicillium and G. citri-aurantii in in vitro, being able to significantly reduce their postharvest diseases (Wu et al. 2017; Duan et al. 2018; Suwanamornlert et al. 2018; Tejeswini et al. 2014), thus can potentially be used in the control of sour rot. However, essential oils have low solubility in water and a very strong aroma which might be unpleasant to the consumers, hence limiting their application in postharvest fruit preservation (Ali et al. 2014; Fan et al. 2014). A combination of EOs and wax is preferred as it not only does not have a strong aroma but also effectively prevents the occurrence of postharvest disease, extending the shelf life of fruits while retaining and/or improving their natural appearance as well as improving the nutritional quality (Fan et al. 2014; Wu et al. 2017; Bazioli et al. 2019; Kong et al. 2019).
This study focused on (1) determining the antifungal effects of cinnamaldehyde, citral, and a combination of cinnamaldehyde and citral (CC) against mycelial growth and spores germination of G. citri-aurantii in vitro, (2) estimating the capacity of CC to control the occurrence of sour rot in vivo, and the effect of combination of wax + CC (WCC) on the quality of the citrus fruits, and (3) evaluating the effects of CC, and cinnamaldehyde and citral individually on the cell wall and cell membrane integrity of G. citri-aurantii.
Materials and methods
Pathogen
Geotrichum citri-aurantii used in this study was isolated from infected citrus fruits and identified by morphological and molecular biology methods. They were incubated on potato dextrose agar (PDA) at 28 ± 2 °C and the spore concentration adjusted to 5 × 106 spores mL−1 using a haemocytometer.
Fruit
Mature Satsuma mandarin fruits (Citrus unshiu Marc. cv. Miyagawa Wase) were harvested on 21th October, 2017, from a local orchard (latitude 27° 87’ N, longitude 112° 9’ E, altitude 44 m above sea level) near Xiangtan University, Xiangtan, China. Healthy fruits of uniform size and without scars were chosen for the experiments.
Chemicals
Cinnamaldehyde (99%) was obtained from Darui Fine Chemicals Co., Ltd, Shanghai, China, and citral (95%) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Cinnamaldehyde, citral and CC solutions were prepared by dissolving the requisite amount in Tween-80 (0.05%, v: v) and topped up to the final volume using distilled water. All the chemicals used were of analytic grade.
Effect of cinnamaldehyde, citral and CC on mycelial growth
This was tested in vitro by agar dilution method (Wu et al. 2017). Briefly, CC stock solution was added into sterilized PDA medium to generate final concentrations of 0, 0.20, 0.40, and 0.80 mL L−1. Similarly, final concentrations of cinnamaldehyde (0, 0.125, 0.25, 0.50, and 1.00 mL L−1) or citral (0, 0.125, 0.25, 0.50, and 1.00 mL L−1) were respectively added into PDA media. The mended media (20 mL) were poured into sterilized petri dishes (90 mm diameter). A 6-mm diameter disc of inoculum was cut from the edge of an actively growing culture on PDA plates with a sterile cork borer, and placed at the center of each new petri dish. Each treatment was performed in triplicate and the culture plates incubated at 28 ± 2 °C for 2 days. The lowest concentration that completely inhibited the growth of the pathogen after the 2 d of incubation was considered as the MIC. The lowest concentration that prevented 99.5% growth of the pathogen after 4 d of incubation was regarded as the MFC.
Effect of cinnamaldehyde, citral and CC on spore germination
For the potato dextrose broth (PDB) incubation method, cinnamaldehyde (0.27 mL L−1), citral (0.53 mL L−1) and CC (0.80 mL L−1) were separately added into PDB, each with a final concentration of 106 spores mL−1. The mixtures were each incubated for 3, 6 and 9 h in triplicate at 28 ± 2 °C, in a shaking incubator at 160 r min−1. Spores in PDB without essential oils served as the control group. The spore germination at 3, 6 and 9 h of incubation was monitored under the microscope (Wu et al. 2017), and the percentage germination of spores calculated according by the formula;
Effect of CC on the incidence of sour rot
This was determined in vivo as previously described (Duan et al. 2018; Fan et al. 2014) but with minor modifications. All fresh citrus fruits were surface-sterilized by immersing in sodium hypochlorite solution (2%, v/v) for 2 min, then washed with distilled water, wounded (depth of 3 mm and width of 3 mm) with a sterile needle, inoculated with 20 μL of G. citri-aurantii spore suspension (105 spores mL−1), and left to air-dry. After being inoculated with fungi, the fruits were sprayed with wax amended with CC at 0 × and 1 × MFC. Fruits with wax and pathogen inoculation were used as a control. The inoculated fruits were kept in sealed incubators at 25 ± 2 °C to ensure high relative humidity (85–90% relative humidity). Twenty Satsuma mandarin fruits constituted a single replicate, and each treatment was performed in triplicate. The incidence rate of disease (measured by counting the number of green mold-infected wounds) was calculated as follows:
Fruit quality parameters
Fruits were randomly chosen from each of the test and control groups (similar to those described in Sect. 2.6) after storage at an interval of 2 d. Physiological fruit quality indicators, including weight loss rate, coloration index, firmness, pH, total soluble solid content (TSS), titratable acidity (TA), and vitamin C (Vc) content were tested. The methods used were included in previous papers (Fan et al. 2014; Wilkerson et al. 2013).
Effects of cinnamaldehyde, citral and CC on the cell wall integrity of G. citri-aurantii
The distribution of chitin in the cell wall of G. citri-aurantii was analyzed by calcofluor white (Sigma, St. Louis, MO, USA) staining coupled with fluorescence microscopy (Nikon ECLIPSE TS100, Japan) (OuYang et al. 2019). Cinnamaldehyde (0.27 mL L−1), citral (0.53 mL L−1) and CC (0.80 mL L−1) were added into PDB with a final concentration of 106 spores mL−1. The mixtures were incubated for 3, 6 and 9 h in triplicate at 28 ± 2 °C in a shaking incubator at 160 r min−1, then centrifuged at 4000 g min−1 for 10 min. The collected spores were stained with 10 μL of calcofluor white (CFW) stain and 10 μL KOH (10%), following the manufacturer's instructions. Fungal cultures in PDB without essential oils were used as a control, and the samples were observed under a fluorescence microscope.
Effects of cinnamaldehyde, citral and CC on plasma membrane integrity
Membrane integrity (MI) was examined by the method of Wu et al. (2017). Cinnamaldehyde (0.27 mL L−1), citral (0.53 mL L−1) and CC (0.80 mL L−1) were added into PDB with a final concentration of 106 spores mL−1. The mixtures were incubated for 3, 6 and 9 h in triplicate at 28 ± 2 °C in a shaking incubator at 160 r min−1, then centrifuged at 4000 g min−1 for 10 min, washed with distilled water and suspended in PBS (0.05 mol L−1, pH = 7.0). The suspension was then stained with propidium iodide (PI) (0.01 g L−1) in a 37 °C water bath for 5 min and washed twice using PBS. Fungal culture in PDB without EOs was used as a control. The number of spores in bright-field constituted the ‘total number of spores’. The stained spores that could be observed under the fluorescent microscope (Nikon ECLIPSE TS100, Japan) were counted and MI calculated according to the formula:
Statistical analysis
Each assay was performed in triplicate, and the data analyzed using SPSS 19.0 Software. Significance in differences between mean values of the data sets were determined using Duncan's Multiple Range test (P < 0.05) following one-way ANOVA.
Results
Mycelial growth in vitro
The mycelial growth of G. citri-aurantii in vitro considerably decreased with increase in concentrations of cinnamaldehyde, citral and CC (Table 1). The MIC values of cinnamaldehyde, citral and CC were equal to their MFC values, which were estimated to be 0.50, 1.0 and 0.80 mL L−1 respectively. Basing on the ratio of the MIC values of cinnamaldehyde and citral (0.5: 1.0), a combination ratio of 1: 2 was chosen for the formation of CC. When the CC treatment completely inhibited the growth of G. citri-aurantii, the concentrations of cinnamaldehyde and citral in the mixture were only 0.27 and 0.53 mL L−1, respectively.
Inhibition of spore germination
The effects of cinnamaldehyde, citral and CC on the germination of G. citri-aurantii spores is shown in Fig. 1. After 3 h of incubation, all spores in the three treatment groups had not germinated, while those in the control group had germinated with a rate of 12.58 ± 1.72% (P < 0.05). 6 h after incubation, the germination rates in the cinnamaldehyde, citral and CC groups were 0.00 ± 0.00, 6.67 ± 1.36, and 0.00 ± 0.00%, respectively, which were significantly lower than that of the control group (49.82 ± 6.06%, P < 0.05). By 9 h of incubation, the germination rate in the control group increased to 90.47 ± 2.34%, while those of the test groups treated with cinnamaldehyde and citral individually were only 5.66 ± 7.81 and 10.81 ± 1.82%, respectively. Spores in the CC group had not germinated at all even after the 9 h incubation period.
Effect of CC treatment on the incidence of sour rot decay
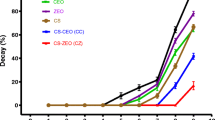
WCC (1 × MFC) effectively reduced sour rot decay of citrus fruits (Fig. 2a), and alleviated the disease progression in inoculated citrus fruits treated with WCC (Fig. 2b). Fruits in the control group began to decay 3 d post inoculation, with 7 ± 3% decay rate, at which time, the CC treated fruits were not infected at all. Following 8 d of inoculation, the citrus fruits in the control group were all rotten and covered by a visible white mold layer, while the fruits in the 1 × MFC WCC treatments had incurred a sour rot rate of only 60 ± 0%, with merely watery lesions visible.
a Decay incidence in inoculated fruit treated with WCC (0 × and 1 × MFC) during storage at 25 ± 2 °C for 5 d and 85%-90% relative humidity; b Disease progression in inoculated citrus fruit treated with CC (0 × and 1 × MFC) during storage at 25 ± 2 °C and 85%-90% RH. The data presented are the means of pooled data. Error bars indicate the SDs of the means (n = 3)
Effects of CC treatment on fruit quality parameters
WCC (1 × MFC) treatment did not significantly affect the quality of citrus fruits (Table 2). The weight loss rate, coloration index, firmness, pH, TSS content, as well as solid acid ratio were not influenced by the WCC treatments after storage at 25 ± 2 °C for 6 d. However, the Vc content in WCC treated fruits was about 39.33% and 21.54% higher than in WCK-treated fruits after 2 and 6 days, respectively.
Effects of cinnamaldehyde, citral and CC on the cell wall integrity
As shown in Fig. 3, cinnamaldehyde and CC treatments significantly affected the distribution of chitin on the cell walls of G. citri-aurantii spores. The cell walls of spores in the control group and citral treated group showed a normal deposition of chitin. The spores in the cinnamaldehyde and CC treated groups markedly showed weaker fluorescence than the other treatments after 3 h incubation. Similarly, the blue fluorescence of cells in the CC treated group was weaker than the corresponding single treatments and the control group as observed at 6 and 9 h incubation.
Effects of cinnamaldehyde, citral and CC on the plasma membrane integrity
The MI values of G. citri-aurantii spores treated with citral or CC greatly decreased with increase in incubation time, as opposed to the relatively high levels observed in the control group and cinnamaldehyde treated group (100.00 ± 0.00%, P < 0.05) (Fig. 4). By 3 h of incubation, the MI values of all groups were 100.00 ± 0.00%, but as the exposure time increased to 9 h, treatment with CC resulted in a decreased MI value (33.38 ± 1.59%) compared to the corresponding single treatments of cinnamaldehyde (100.00 ± 0.00%) and citral (55.71 ± 5.15%), with the control group retaining an MI value of 100.00 ± 0.00%.
The effects of cinnamaldehyde, citronellal and CC on the plasma membrane of G. citri-aurantii spores, a stands for the percentage of plasma membrane integrity; b stands for the PI staining results of bright field and fluorescent images. The data presented are the means of pooled data. Error bars indicate the SDs of the means (n = 3)
Discussion
Cinnamaldehyde and citral have been reported to strongly inhibit growth of a wide range of fungal species (Utama et al. 2002; Duan et al. 2018; Gao et al. 2018; Wu et al. 2017). For example, at a concentration below 0.2%, cinnamaldehyde can completely inhibit growth of Aspergillus, Fusarium, Penicillium and Rhizopus in vivo (Manso et al. 2015; Tejeswini et al. 2014), and citral exhibit significant antifungal efficiency against postharvest Botrytis cinerea, P. expansum, P. digitatum, and P. italicum (Camele et al. 2012; OuYang et al. 2019; Wang et al. 2018a, b). In this study, cinnamaldehyde and citral individually exhibited very good fungicidal actions against G. citri-aurantii with MFC values of 0.50 and 1.00 mL L−1 respectively. In addition, cinnamaldehyde and citral treatments significantly reduced the germination rate of G. citri-aurantii spores, an observation that is consistent with findings by Thavong et al. (2011) and Wang et al. (2018b).
The combination of Cinnamaldehyde and citral (CC, v/v, 1:2) inhibited the growth of G. citri-aurantii with MFC of 0.80 mL L−1, the concentrations of cinnamaldehyde and citral in the mixture being 0.27 and 0.53 mL L−1, respectively. These values are almost half the MICs of each of the individual components, implying that within the combination, very low concentrations of each essential oil component is required to realize a significantly great inhibitory effect, hence pointing towards a kind of cooperative mechanism to their antifungal activity. Similarly, CC treatment totally inhibited germination of G. citri-aurantii spores during the 9 h incubation period yet separate treatments with cinnamaldehyde and citral during the same duration of time resulted in growth of some spores with rates of 5.66 ± 7.81% and 10.81 ± 1.82% respectively. These results imply that the combination of cinnamaldehyde and citral (CC) was highly effective at inhibiting overall growth of G. citri-aurantii than the individual components, an observation consistent with those reported by Hossain et al. (2016), Stević (2014), Sukatta et al. (2008), Suwanamornlert et al. (2018) and Zhou et al. (2019) that the combination of two or more individual components of essential oils might have higher antifungal potential than the individual components acting alone. In fact, Li et al. (2019) too alluded to the fact that the antimicrobial activities of fungicides can be enhanced by combining them with other antimicrobial agents or a variety of them.
A recent study by Ji et al. (2019) reported that the increase in antifungal activity of combinations of EOs was due to the cumulative effect of the mechanisms of the individual EOs in the mixture. Cinnamaldehyde is known to exert its antifungal activity by disrupting the cell wall (OuYang et al. 2019; Shreaz et al. 2016), while citral inhibits fungal growth by disrupting the cell membrane (Tao et al. 2014; Zhou et al. 2014). Basing on the report by Ji et al. (2019), we hypothesized that the effect of CC against G. citri-aurantii was relate to the destruction of both the cell wall and the cell membrane. Our results showed that CC treatment significantly weakened the blue fluorescence of G. citri-aurantii spores in the test samples than in the control group and the individual treatment groups, suggesting that CC damaged the cell wall integrity by reducing the chitin content. However, the cell membrane integrity of the spores in CC treatment did not lower compared to those of the control and the individual treatment groups, suggesting that the inhibitory mechanism of CC was not associated with damage of the cell membrane. On the contrary, while examining the antifungal mechanism of a combination of cinnamaldehyde (45 mg L−1) and citral (70 mg L−1) against P. expansum, Wang et al. (2018a, 2018b) found that the drug combination targeted the membrane structure but not the cell wall structure. This discrepancy may be caused by the difference in combination ratios of the individual EO components and the difference in strains used for the study. According to our current results, the inhibitory effect of CC evidently proceeded through destruction of cell wall integrity, with citral probably enhancing the ability of cinnamaldehyde to damage the cell wall. This suggests that the antifungal mechanism of a combination of EOs is not a simple superposition of individual mechanisms but rather an interaction between the two components resulting into a stronger, single mechanism, that might be very significant in reducing or eliminating the incidence of occurrence of resistant fungal pathogens.
From our current results, CC effectively inhibited G. citri-aurantii not only in vitro, but also in vivo. 1 × MFC of CC proved highly effective at inhibiting the occurrence of sour rot in vivo, its effectiveness at controlling the incidence of sour rot being significantly higher than that of cinnamaldehyde alone. Similarly, the incidence of fruit decay in 0.80 mL L−1 CC treatment was 60%, at which time, all the fruits in the control group had decayed. This incidence rate is less than the 80% fruit decay rate for the 0.50 mL L−1 cinnamaldehyde treatments reported in our previous study (Wu et al., 2017). This confirms that combining cinnamaldehyde and citral enhances their in vivo antifungal ability against citrus fruit pathogens, which may be related to their good antifungal abilities plus their ability to induce defense responses in citrus fruits (Gao et al. 2018, Wang et al. 2016 and Fan et al. 2014). This results also show that a low concentration of the constituent essential oils is required in the combination (CC) to realize significant inhibitory effect which solves the concerns by Tejeswini et al. (2014) and Catherine et al. (2012) that a higher concentration of the antimicrobial compound was needed both in vivo than in vitro to realize significant inhibitory effect.
Apart from the Vc content which was higher in the test samples than in the control group, CC treatment had very minor effects on fruit quality parameters. Previous studies have similarly reported that cinnamaldehyde and citral reduced postharvest decay in fruits without exacerbating any fruit quality parameters (Duan et al. 2018; Fan et al. 2014; Gao et al. 2018; Wang et al. 2016). The observed increase in Vc content could actual be of nutritional advantage to the consumers and a testimony to reports that EOs do improve the nutritional quality of fruits (Fan et al. 2014; Wu et al. 2017; Bazioli et al. 2019; Kong et al. 2019). The combination of cinnamaldehyde and citral is therefore a potentially promising botanical fungicide to suppress G. citri-aurantii growth in the control of sour rot in citrus fruits.
In conclusion, the combination of cinnamaldehyde and citral (1:2, v/v) act by reducing the chitin content to destroy the integrity of fungal cell wall thus effectively inhibiting the growth of G. citri-aurantii both in vitro and in vivo. Very low concentrations of the individual components are required to form the combination, and a 1 × MFC WCC treatment which effectively inhibits the fungal growth, does not impair the fruit quality indicating that it should be considered for use in the postharvest management of sour rot in citrus fruits.
References
Ali A, Chow WL, Zahid N, Ong MK (2014) Efficacy of propolis and cinnamon oil coating in controlling post-harvest anthracnose and quality of chilli (Capsicum annuum L.) during cold storage. Food Bioproc Technol 7(9):2742–2748. https://doi.org/10.1007/s11947-013-1237-y
Bazioli JM, Belinato JR, Costa JH, Akiyama DY, Pontes JGD, Kupper KC, Augusto F, de Carvalho JE, Fill TP (2019) Biological control of citrus postharvest phytopathogens. Toxins 11(8):460. https://doi.org/10.3390/toxins11080460
Boubaker H, Karim H, El Hamdaoui A, Msanda F, Leach D, Bombarda I, Vanloot P, Abbad A, Boudyach EH, Ait Ben Aoumara A (2016) Chemical characterization and antifungal activities of four Thymus species essential oils against postharvest fungal pathogens of citrus. Ind Crop Prod 86:95–101. https://doi.org/10.1016/j.indcrop.2016.03.036
Camele I, Altieri L, De Martino L, De Feo V, Mancini E, Rana GL (2012) In vitro control of post-harvest fruit rot fungi by some plant essential oil components. Int J Mol Sci 13(2):2290–2300. https://doi.org/10.3390/ijms13022290
Catherine AA, Deepika H, Negi PS (2012) Antibacterial activity of eugenol and peppermint oil in model food systems. J Essent Oil Res 24(5):481–486. https://doi.org/10.1080/10412905.2012.703513
Chen JY, Shen YT, Chen CY, Wan CP (2019) Inhibition of key citrus postharvest fungal strains by plant extracts in vitro and in vivo: A review. Plants -Basel 8(2):26. https://doi.org/10.3390/plants8020026
Deng B, Wang WH, Deng LL, Yao SX, Ming J, Zeng KF (2018) Comparative RNA-seq analysis of citrus fruit in response to infection with three major postharvest fungi. Postharvest Biol Technol 146:134–146. https://doi.org/10.1016/j.postharvbio.2018.08.012
Duan XF, OuYang QL, Tao NG (2018) Effect of applying cinnamaldehyde incorporated in wax on green mould decay in citrus fruits. J Sci Food Agr 98(2):527–533. https://doi.org/10.1002/jsfa.8490
Fan F, Tao NG, Jia L, He XL (2014) Use of citral incorporated in postharvest wax of citrus fruit as a botanical fungicide against Penicillium digitatum. Postharvest Biol Technol 90:52–55. https://doi.org/10.1016/j.postharvbio.2013.12.005
Gao Y, Kan CN, Chen M, Chen CY, Chen YH, Fu YQ, Wan CP, Chen JY (2018) Effects of chitosan-based coatings enriched with cinnamaldehyde on Mandarin fruit cv. Ponkan during Room-Temperature Storage Coatings 8(10):372. https://doi.org/10.3390/coatings8100372
Hossain F, Follett P, Vu Dang K, Harich M, Salmieri S, Lacroix M (2016) Evidence for synergistic activity of plant-derived essential oils against fungal pathogens of food. Food Microbiol 53:24–30. https://doi.org/10.1016/j.fm.2015.08.006
Ji H, Kim H, Beuchat LR, Ryua J (2019) Synergistic antimicrobial activities of essential oil vapours against Penicillium corylophilum on a laboratory medium and beef jerky. Int J Food Microbiol 291:104–110. https://doi.org/10.1016/j.ijfoodmicro.2018.11.023
Kong J, Zhang Y, Ju J, Xie YF, Guo YH, Cheng YL, Qian H, Quek SY, Yao WR (2019) Antifungal effects of thymol and salicylic acid on cell membrane and mitochondria of Rhizopus stolonifer and their application in postharvest preservation of tomatoes. Food Chem 285:380–388. https://doi.org/10.1016/j.foodchem.2019.01.099
Li L, Tang X, OuYang QL, Tao NG (2019) Combination of sodium dehydroacetate and sodium silicate reduces sour rot of citrus fruit. Postharvest Biol Technol 151:19–25. https://doi.org/10.1016/j.postharvbio.2019.01.006
Liu K, Zhou X, Fu M (2017) Inhibiting effects of epsilon-poly-lysine (ε-PL) on Pencillium digitatum and its involved mechanism. Postharvest Biol Technol 123:94–101. https://doi.org/10.1016/j.postharvbio.2016.08.015
Manso S, Becerril R, Nerin C, Gomez-Lus R (2015) Influence of pH and temperature variations on vapor phase action of an antifungal food packaging against five mold strains. Food Control 47:20–26. https://doi.org/10.1016/j.foodcont.2014.06.014
Mckay AH, Forster H, Adaskaveg JE (2012) Efficacy and application strategies for propiconazole as a new postharvest fungicide for managing sour rot and green mold of citrus fruit. Plant Dis 96(2):235–242. https://doi.org/10.1094/PDIS-06-11-0525
OuYang QL, Duan XF, Li L, Tao NG (2019) Cinnamaldehyde exerts its antifungal activity by disrupting the cell wall integrity of Geotrichum citri-aurantii. Front Microbiol 10:55. https://doi.org/10.3389/fmicb.2019.00055
Shao XF, Cao B, Xu F, Xie SH, Yu DD, Wang HF (2015) Effect of postharvest application of chitosan combined with clove oil against citrus green mold. Postharvest Biol Technol 99:37–43. https://doi.org/10.1016/j.postharvbio.2014.07.014
Shreaz S, Wani WA, Behbehani JM, Raja V, Irshad M, Karched M, Ali I, Siddiqi WA, Hun LT (2016) Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 2016:116–131. https://doi.org/10.1016/j.fitote.2016.05.016
Singh D, Sharma RR (2018) Postharvest diseases of fruits and vegetables and their management. Postharvest Disinfection of Fruits and Vegetables. Academic Press, Cambridge, pp 1–52. https://doi.org/10.1016/B978-0-12-812698-1.00001-7
Stević T (2014) Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind Crop Prod 55(55):116–122. https://doi.org/10.1016/j.indcrop.2014.02.011
Sukatta U, Haruthaithanasan V, Chantarapanont W, Dilokkunanant U, Suppakul P (2008) Antifungal activity of clove and cinnamon oil and their synergistic against postharvest decay of grape in vitro. Kasetsart J Nat Sci 42(5):169–174
Suwanamornlert P, Sangchote S, Chinsirikul W, Sane A, Chonhenchob V (2018) Antifungal activity of plant-derived compounds and their synergism against major postharvest pathogens of longan fruit in vitro. Int J Food Microbiol 271:8–14. https://doi.org/10.1016/j.ijfoodmicro.2018.02.009
Tao NG, OuYang QL, Jia L (2014) Citral inhibits mycelial growth of Penicillium italicum by a membrane damage mechanism. Food Control 41:116–121. https://doi.org/10.1016/j.foodcont.2014.01.010
Tejeswini MG, Sowmya HV, Swarnalatha SP, Negi PS (2014) Antifungal activity of essential oils and their combinations in in vitro and in vivo conditions. Arch Phytopathol Plant Prot 47(5):564–570. https://doi.org/10.1080/03235408.2013.814235
Thavong P, Archbold DD, Pankasemsuk T, Koslanund R (2011) Hexanal vapours suppress spore germination, mycelial growth, and fungal-derived cell wall degrading enzymes of postharvest pathogens of longan fruit. Chiang Mai J Sci 38:139–150
Utama IMS, Wills RBH, Ben-yehoshua S, Kuek C (2002) In vitro efficacy of plant volatiles for inhibiting the growth of fruit and vegetable decay microorganisms. J Agr Food Chem 50(22):6371–6377. https://doi.org/10.1021/jf020484d
Wang Y, Shan T, Yuan Y, Yue T (2016) Overall quality properties of kiwifruit treated by cinnamaldehyde and citral: microbial, antioxidant capacity during cold storage. J Food Sci 81(12):H3043–H3051. https://doi.org/10.1111/1750-3841.13536
Wang Y, Feng KW, Yang HH, Zhang ZW, Yuan YH, Yue TL (2018a) Effect of cinnamaldehyde and citral combination on transcriptional profile, growth, oxidative damage and patulin biosynthesis of Penicillium expansum. Front Microbiol 9:597. https://doi.org/10.3389/fmicb.2018.00597
Wang Y, Feng KW, Yang HH, Yuan YH, Yue TL (2018b) Antifungal mechanism of cinnamaldehyde and citral combination against Penicillium expansum based on FT-IR fingerprint, plasma membrane, oxidative stress and volatile profile. RSC Adv 8(11):5806–5815. https://doi.org/10.1039/c7ra12191a
Wilkerson ED, Anthon GE, Barrett DM, Sayaion GFG, Santos AM, Rodriguez-Saona LE (2013) Rapid assessment of quality parameters in processing tomatoes using hand-held and benchtop infrared spectrometers and multivariate analysis. J Agr Food Chem 61(9):2088–2095. https://doi.org/10.1021/jf304968f
Wu YL, Duan XF, Jing GX, Ta NG (2017) Cinnamaldehyde inhibits the mycelial growth of Geotrichum citri-aurantii and induces defense responses against sour rot in citrus fruit. Postharvest Biol Technol 129:23–28. https://doi.org/10.1016/j.postharvbio.2017.03.004
Wuryatmo E, Able AJ, Ford CM, Scott ES (2014) Effect of volatile citral on the development of blue mould, green mould and sour rot on navel orange. Australas Plant Path 43:403–411. https://doi.org/10.1007/s13313-014-0281-z
Zhou H, Tao NG, Jia L (2014) Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control 37:277–283. https://doi.org/10.1016/j.foodcont.2013.09.057
Zhou L, Zhang Z, Wei M, Xie YJ, He S, Shi HA, Lin ZF (2019) Evaluation of the antifungal activity of individual and combined monoterpenes against Rhizopus stolonifer and Absidia coerulea. Environ Sci Pollut Research 26(8):7804–7809. https://doi.org/10.1007/s11356-019-04278-z
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 31772364), Collaborative Innovation Center of New Chemical Technologies for Environmental Benignity and Efficient Resource Utilization, the Scientific Research Fund of Hunan Provincial Education Department (No. 19A476), the Natural Science Foundation of Hunan Province (No. 2020JJ5536) and the Scientific Research Foundation for Doctors of Xiangtan University (No. 19QDZ12).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
OuYang, Q., Reymick, O.O. & Tao, N. A combination of cinnamaldehyde and citral greatly alleviates postharvest occurrence of sour rot in citrus fruits without compromising the fruit quality. J Food Sci Technol 59, 2776–2783 (2022). https://doi.org/10.1007/s13197-021-05300-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-021-05300-4