Abstract
Sedimentation of particles in cocoa drink is a technological challenge for the food industry. This study investigates the effect of different stabilizers (alginate, xanthan gum or carrageenan) on the suspension stability of cinnamon-cocoa drink made from 2 types of cocoa powder (natural or alkalized). Rheological and microstructural properties determination was used to examine the stabilization effect mechanism. The cocoa powder characteristic was investigated to study the correlation between cocoa powder properties and suspension stability. The results showed that xanthan gum is the most effective stabilizer to prevent particle sedimentation of the cinnamon-cocoa drink. Xanthan gum formed a network entrapping the particles. It increased the viscosity from 2.47 to 70.44 mPa s at a shear rate of 10/s. The drink formulated with alkalized cocoa powder has a better stability than that formulated with natural cocoa powder. However, at the concentration of 0.1% (w/v), xanthan gum could prevent sedimentation regardless the type of cocoa powder. The addition of xanthan gum up to 0.1% (w/v) had no significant effect on pH and antioxidant properties of the cinnamon-chocolate drink with a minor change in the lightness (L*) parameter. As such, the value of L*, pH, phenolic content and antioxidant activity of the cinnamon-cocoa drinks remained stable at around 22.5 ± 0.9, 7.2 ± 0.1, 0.31 ± 0.5 mg epicatechin equivalent /ml and 0.44 ± 0.3 mg tannic acid equivalent /ml, respectively. This study can be useful for the food industry to define a novel strategy to produce “ready-to-drink” cocoa-based beverage with prolonged suspension stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cocoa (Theobroma cacao L.), the main ingredient of chocolate and cocoa drink, is one of the most important agricultural products of Indonesia. This country has approximately 1.5 million hectares of cocoa plantations with a total production of 220.000 tons in 2019 (Food and Agriculture Organisation 2020). Beside conventional chocolate products, in this country many industries produce chocolates and cocoa drinks enriched with herbs and spices, including cinnamon (Cinnamomum Sp.). It can be understood as cinnamon, especially the species of C. burmannii Blume, is native to Indonesia and endemic in many regions in this country (Menggala et al. 2019). According to Food and Agriculture Organisation (2020), Indonesia produced approximately 90,000 tons of cinnamon per year in the last decade. As reported by Hardjohutomo (1967) in the publication of first Indonesia national cookbook namely Mustika Rasa, cinnamon has been widely used in various ethnic foods and cuisines in Indonesia since the ancient time.
Cinnamon has a basic function as flavoring agent (Ribeiro-Santos et al. 2017; Muhammad and Dewettinck 2017; Muhammad et al. 2020a, b). Nevertheless, cinnamon enrichment in chocolates and cocoa drinks significantly improved their phenolic content and antioxidant activity (Ilmi et al. 2017; Muhammad et al.2019, 2020a, b). Catechin, epicatechin, procyanidin B2, quercitrin, 3, 4-dihydroxybenzaldehyde, protocatechuic acid and cinnamic acid were detected in cinnamon extract (Muhammad et al. 2021). Our previous study even showed a potential synergistic effect between antioxidant compounds in cocoa and cinnamon, offering an opportunity to create a healthier product (Muhammad et al. 2017).
Considering the potential market for cocoa drink, development of ‘ready-to-drink’ cocoa beverage product is still being conducted by the food industry (Toker et al. 2012; Rivas et al. 2018). In the development of “ready-to-drink” cocoa beverage, cocoa particle sedimentation is a technological challenge to be resolved by the food industry as it affects the product’s appearance, and accordingly decreases the consumer acceptance on the product. Hydrocolloids, such as xanthan gum, λ-carrageenan and alginate, are often used as stabilizing agents in foods, including beverage products (Dogan et al. 2013; Toker et al. 2013; Manuhara et al. 2016; Holkar et al. 2019). The addition of hydrocolloids is hypothesized to be able to improve the stability of cinnamon-cocoa drink.
Aside the influence of stabilizing agents, alkalization of cocoa powder also impacts significantly on the stability of cocoa particle suspension. Alkalization is usually carried out to modify the color of cocoa powder to be darker. Nevertheless, it can also improve the dispersibility of cocoa powder in water (Oracz et al. 2015). This study therefore aims to investigate the effect of stabilizer and the type of cocoa powder as well as their interrelation on the suspension stability of cinnamon-enriched cocoa drink.
Materials and methods
Characterization of cocoa powder
Two types of cocoa powder, namely natural cocoa powder (Cocoa Processing Co. Ltd., Ghana) and alkalized cocoa powder (Delhaize-Bio, Delhaize Le Lion/De Leeuw, Belgium) were used as the cocoa powder sample. The cocoa powder was characterized in terms of solubility, wettability, fat content, pH, color properties and particle size.
The protocol of Dogan et al. (2016) was used as the reference of the solubility and wettability analyses. The solubility test was performed by adding 4 g of the cocoa powder into 40 ml of water (80 °C). The mixture was constantly stirred (30 min) and then centrifuged (Sigma 4K15, Sartorius AG, Waldbronn, Germany) at 13,102 g. Afterwards, 15 g of the supernatant was put in an evaporation dish and dried at 120 °C until a stable weight was reached.
In the wettability test, 40 ml of water at the temperature of 80 °C was prepared in a cylindrical container with diameter of 5 cm. Each cocoa powder (4 g) was poured into the container. The wettability was determined by the time required for all cocoa powder to become wet and disappear from the surface of the water. Result was expressed as wetting time (s).
Fat content analysis was determined by Soxhlet extraction method. Cocoa powder (4 g) was boiled for 15 min in a solvent containing distilled water (45 ml) and HCl (25%, 55 ml) and then filtered using a filter paper. After that, the filter paper containing the sample was dried followed by a Soxhlet extraction using petroleum ether (200 ml) for 4 h. A vacuum rotary evaporator (Laborota 4000 Heidolph, Germany) was used to remove the solvent and collect the fat.
The pH of the cocoa powder was measured using a pH meter (Schott Instruments, LAB 850). Prior to the measurement, the cocoa powder (6.5 g) was mixed with water (180 ml) at the temperature of 80 °C. The pH determination was conducted at 20 °C. Colour measurement in terms of the L*, a*, b* values at the Specular Component Excluded (SCE) mode was conducted using a colorimeter (Minolta Model CM-2500D Spectrophotometer, Konica Minolta Sensing, Tokyo, Japan). The L*, a* and b* values were then used to determine the level of the Chroma (C*) and the °Hue of the sample using Eqs. 1 and 2, respectively. The particle size of the cocoa powder in terms of D (4, 3) was determined as a suspension in water using a Malvern Mastersizer 3000 (Malvern Instrument Ltd, UK).
Preparation of cinnamon-cocoa drink
The cocoa drink was prepared by mixing 6.5 g of cocoa powder, 12 g of sugar (Fijne Kristalsuiker 365, Delhaize Le Lion/De Leeuw, Belgium) and 2 g of vegetable creamer (AH Koffiecreamer, Albert Heijn B.V., Netherlands) and 0.1 g of cinnamon powder (CV. Orizho Yogyakarta, Indonesia) in 100 ml of water at 80 °C. Separately, three solutions of stabilizers, namely alginate (Type 500–1000 cPs, Harke Food, Germany), λ-carrageenan (Satiagum MM 30, Cargill France SAS, France) and xanthan gum (Satiaxane CX 931, Cargill France SAS, France) at a level of 0.18 g in 80 ml of water (50 °C) were prepared. The stabilizer solutions were then mixed with the cocoa drink for 2 min with continuous stirring and thus, cocoa drink with a total volume of 180 ml was reached. Thus, the stabilizer was at the concentration 0.1% (w/v). The stability was observed after 24 h by visual assessment. The best stabilizer was then selected based on the sedimentation index parameter. The sample with lower sedimentation index has a better suspension stability. To obtain a better understanding of the effect of the stabilizer on the suspension stability of the cocoa particle in cocoa drink, a gradual increase of the selected stabilizer from 0 (control) to 0.1% (w/v) was applied.
Characterisation of cinnamon-cocoa drink
The formulated cocoa drink was characterized in terms of pH and colour as described in Sect. 2.1. As cocoa and cinnamon are well-known sources of polyphenols exhibiting antioxidant activity, the total phenolic content and the antioxidant activity of the formulated cocoa drink were analysed using Folin–Ciocalteu and phosphomolybdenum methods, respectively, as described in Muhammad et al. (2017).
The analysis using Folin–Ciocalteu method was started by incubation of a mixture of sample (200 µl), Folin–Ciocalteu reagent (200 µl) and distilled water for 6 min. Afterwards 2.5 ml of Na2CO3 solution (7% (w/v)) and 2.1 ml of distilled water were added to the mixture. After incubation for 90 min, the absorbance at 760 nm was measured using UV–visible spectrophotometry (Varian Cary 50 Bio, Agilent Technology). The total phenolic content was estimated using a standard plot of epicatechin equivalent.
In the phosphomolybdenum method, an antioxidant reagent consisting of sulphuric acid (0.6 M), sodium phosphate (28 mM) and ammonium molybdate (4 mM) was firstly prepared. The sample (0.5 ml) was mixed with 4.5 ml of the antioxidant reagent, and then placed in a water bath (95 °C, 90 min). The absorbance was measured at 695 nm. A standard plot of tannic acid was used to calculate the antioxidant activity of the sample.
Suspension stability test of the cinnamon-cocoa drink
The suspension stability of the cocoa drink was periodically assessed by visual assessment, and then the sedimentation index (SI) was calculated using Eq. 3 as described in the study of Rivas et al. (2018), where V is the total sample volume and Vs is the volume of the drink that the cocoa particles have settled-down (indicated by a relatively clear area).
Microstructural observation of cinnamon-cocoa drink
A JEOL JSM 7100F SEM equipped with a PP3010T Cryo-SEM Preparation System (Oxford Instruments, Oxfordshire, UK) was used to observe the microstructural properties of the cocoa drink. In short, a small drop of the formulated cocoa drink was placed on the cryo-specimen holder, and then cryo-fixed in slush nitrogen (− 210 °C). The sample was then transferred to the cryo-unit in the frozen state, followed by consecutive steps of fracturing, sublimation (20 min, − 70 °C), sputter-coating with platinum. Finally, the sample was transferred into the microscope and observed at − 140 °C.
Rheological measurement of cinnamon-cocoa drink
The rheological measurement of the formulated cocoa drink, in terms of apparent viscosity, was carried out using an AR2000-ex rheometer (TA Instruments, New Castle, USA) following the study of Muhammad et al. (2019). Briefly, 20 g of cocoa drink was analysed using a concentric DIN cylinder (cylinder: 42.00 mm; rotor outer radius: 14.00 mm; stator inner radius: 15.00 mm) at 20 °C and at different level of shear rate (from 10 to 50 s−1).
Statistical analysis
Data analysis was performed by IBM SPSS Statistic 26 Software (IBM, New York, U.S.). The Independent Samples T-test was used to test the statistical differences between 2 samples (cinnamon-cocoa drink prepared by natural and alkalized cocoa powder). The differences were considered significant at 0.95 confidence level. Pearson product-moment correlation test was performed to determine the correlation coefficient between stabilizer concentration and the viscosity of the cinnamon-cocoa drink.
Results and discussion
Characteristics of the cocoa powder
The characteristics of cocoa powder samples are shown in Table 1. In the making of cocoa drink, solubility is an important parameter to study as the soluble solid of cocoa powder affect taste perception of the cocoa drink (Benković et al. 2013). It was recorded that the solubility of the natural and alkalized cocoa powder was 21.6 and 24.3%, respectively. Alkalized cocoa powder had a longer wetting time than natural cocoa powder. The wetting time of the natural and alkalized cocoa powder was measured at the level of 82 ± 12 and 347 ± 34 s, respectively. This result is reasonable since alkalization leads to an increased wetting time (Selamat et al. 1998).
The wettability of cocoa powder can also be influenced by its fat content. The hydrophilic character of the continuous phase prevents the fast dissolution of the cocoa powder which has a hydrophobic character due to the presence of cocoa butter on the powder surface. According to Fäldt and Bergenståhl (1996), the more hydrophobic the surface of a particle is, the longer it takes to disperse in the water. In this case, the phase angle between powder surface and penetrating water increases which leads to a decreasing wettability. As well-stressed by Amer et al. (2017), the interfacial dynamics of particle-water interactions are strongly influenced by the hydrophobic properties of the particle. Alkalization can be very useful to increase the wettability because base catalysed hydrolysis reactions during alkalization increase chemical sorption sites for water molecules in cocoa particle. Thus, the improvement in wetting kinetics by pH modification can be attributed to the chemical modification in the particle. The modification resulted in a more hydrophilic particle with lower phase angle, and thus increased the wettability. Remarkably, in this study, the wettability was inversely correlated with the fat content of the cocoa powder. As presented in Table 1, the fat content of the natural cocoa powder was 8.2% while that of alkalized cocoa powder was 2.6%. Hence, we concluded that the alkalization treatment has a stronger influence than the fat content on the wetting time of cocoa powder.
As expected, the pH value of natural cocoa powder was lower than the alkalized cocoa powder. During alkalization, an alkaline solution increases the pH value of cocoa powder aiming to remove astringent flavour and to neutralize acidity. Alkalization not only affects the pH of the cocoa powder, but also improve the colour of the cocoa powder. An increase in pH leads to darker cocoa products as the alkaline pH allows the polyphenol oxidase to catalyse oxidation and polymerization of polyphenols (Oracz et al. 2015). As shown in the L* and Chroma values, a darker colour was observed in the alkalized cocoa powder. The °Hue value, defined as the subjective perception of colour, showed a range between 0° (representing red colour) and 90° (representing yellow colour). The natural cocoa powder had a high portion of yellow as indicated by a Hue angle of 59.3° ± 0.5. The alkalized cocoa powder with a Hue angle of 19.6 ± 0.5 was close to 0° meaning that the sample with a high portion of red.
In the purpose of making high quality cocoa drink, knowing the particle size of cocoa powder is important as it can be useful to predict the suspension stability as well as have significant effect in the mouth feel as bigger particle size tends to result in a gritty perception (Muhammad et al. 2019). The particle size of the cocoa powder was measured as a suspension in water in terms of D (4, 3) (the volume–weighed mean). It was shown that the volume–weighed mean of the natural and alkalized cocoa powder was 28.5 ± 0.7 µm and 24.1 ± 0.6 µm, respectively. Thus, we consider that the both types of cocoa powder have a comparable particle size.
Characteristics of cinnamon-cocoa drink
Two types of cocoa drinks were formulated using different types of cocoa powder to investigate the correlation between the characteristics of the raw material and the quality of the end-product. As shown in Table 2, the cinnamon-cocoa drink formulated with natural cocoa powder has a higher lightness value with a lower pH value compared to the drink with alkalized cocoa powder. This phenomenon can be understood since the colour properties of the raw materials were also different as shown in Table 1. It clearly indicates that the quality of the cinnamon-cocoa drink was directly proportional with the characteristics of the cocoa powder.
As cocoa and cinnamon are well-known as valuable sources of phenolic compounds, it is interesting to study the total phenolic content and the antioxidant activity of the cinnamon-cocoa drink. According to Jaeger et al. (2009), polyphenol content in a beverage product is beneficial for marketing since exposing the potential health effects of polyphenol-rich beverages may enhance their appeal to consumers. The results show that per ml of cinnamon-cocoa drink contained total phenolic content up to 0.36 mg epicatechin equivalent and possessed antioxidant activity up to 0.46 mg tannic acid equivalent, depending on the type of the cocoa powder used for the formulation.
The common theoretical recognition of alkalization of cocoa states that the alkalized cocoa powder has a lower phenolic content and antioxidant activity than the natural cocoa powder. This is because alkalization induces oxidation of polyphenols resulting in the reduction of phenols. During alkalization, polymerization occurs, resulting in the decrease in antioxidant activity (Oracz et al. 2015). In this current research however, the alkalized and the natural cocoa powder are not comparable since the samples were acquired from different sources. The variety, the growing region and the processing conditions of cocoa significantly affect their phenolic content (Oracz et al. 2015). Nevertheless, the data obtained in this part of study can be useful for evaluating the effect of stabilizer on the phenolic content and antioxidant activity in the next part of the study.
Improvement of cinnamon-cocoa drink suspension stability
In the selection step, xanthan gum was found as the most effective stabilizer to retard sedimentation of particles in the cinnamon-cocoa drink (Fig. 1). It was indicated by the hazy appearance with light brown colour in the entire part of the sample. In the sample without stabilizer, the particles of the drink have fully settled within 24 h after preparation with a sedimentation index of 90. Even though alginate and λ-carrageenan have been widely used as stabilizing agents in many products, the suspension of the cinnamon-cocoa drink formulated with these hydrocolloids was also unstable.
The difference in the functionality of the hydrocolloids to stabilize the particles in the cinnamon-cocoa drink may be due to their structure’s variance. Alginate consists of 2 monomeric units: β-(1 → 4) linked d-mannuronic acid (M) residues and α-(1 → 4)-linked l-guluronic acid (G) residues (Ching et al. 2017). This structure allows alginate to form ionic gel. However, the alginate gel formation is highly dependent on the alginate affinity to cation as the gelation process can occur by cooperative binding of divalent cations and the G-block regions of the polymer. The gel formation of alginate was not effective to slow down the cocoa particle sedimentation. As such, the apparent viscosity of alginate and xanthan gum solution at the concentration of 0.1% (w/v) were around 45 and 78 mPas, respectively, when observed at a shear rate of of 10/s. It has been widely accepted that the sedimentation velocity of particles in suspension follows the Stokes’ Law where the sedimentation velocity of particles is slower at a higher viscosity.
Lamda-carrageenan consists of long linear chains of d-galactose and d-anhydrogalactose with three sulphate group per disaccharide without 3,6-anhydro-d-galactopyranosyl units (Liu et al. 2015). This type of carrageenan has a higher linear charge density and solubility than ι-carrageenan and κ-carrageenan. Lamda-carrageenan is commonly used as a strong thickener in many food systems. However, the thickening ability of λ-carrageenan was lower than xanthan gum at the similar concentration, and thus at the level of 0.1%, λ-carrageenan was not sufficient to stabilize cocoa particle suspension. At a shear rate of 10/s, the apparent viscosity of λ-carrageenan at the concentration of 0.1% (w/v) was around 16 mPa.s which was lower than that of xanthan gum.
To further study the performance of xanthan gum in enhancing the suspension stability of the cocoa drink, several levels of xanthan gum were then applied and the sedimentation index was periodically observed. The results demonstrate that xanthan gum concentration less than 0.05 (w/v) was not sufficient to stabilize the suspension of the cinnamon-cocoa drink within 24 h of storage, regardless the type of the cocoa powder used in the formulation (Table 3). As shown, the sedimentation index of the sample with 0.025% of xanthan gum was comparable with control indicating that the particles in the sample had fully settled.
Interestingly, after 3 days of storage, the suspension of the cinnamon-cocoa drink formulated natural cocoa powder with 0.05% of xanthan gum was not stable any longer, while the drink prepared with alkalized cocoa powder was still stable with a sedimentation index value of 0. In general, the sedimentation index of the cinnamon-cocoa drink prepared using alkalized cocoa powder was lower than that of the drink with the natural cocoa powder at all xanthan gum concentrations and the day of assessment. It implies that the quality of the raw material has a significant impact on the suspension stability of cocoa drink. The reason behind this occurrence is that the alkalized cocoa powder had a better dispersibility than the natural one. Alkalization is indeed mainly used for improving the dispersibility of the cocoa powder in water (Oracz et al. 2015). The wetting time data obtained in this study (Table 1) which showed the alkalized cocoa powder had a longer wetting time than the natural one confirms this result. As the wettability increases, the particles can be suspended in the drink for a longer period.
The insoluble matter as well as the fat content of cocoa powder may also affect the sedimentation of particles in the cinnamon-cocoa drink. Despite the fact that cocoa fat is less dense than water that might be delaying sedimentation, the suspension of the cinnamon-cocoa drink formulated with natural cocoa powder was less stable than that with alkalized one. The drink formulated with natural cocoa powder which had a higher portion of insoluble matter and a higher fat content than the alkalized cocoa powder was more prone to sedimentation even with the addition of stabilizer. This similar phenomenon was also shown in the study of Dogan et al. (2016). These results emphasize the fact that the stabilization effect of a stabilizer can be significantly affected by the initial conditions of the cocoa powder as the raw material of cocoa drink.
Nevertheless, it was noteworthy that by the addition of xanthan gum at the level of 0.1%, the sedimentation of cocoa particles was fully prevented until the end of the assessment (9 days), regardless the type of cocoa powder as indicated by the Sedimentation Index value of 0 in both cinnamon-cocoa drink formulated with natural and alkalized cocoa powder. According to Yaginuma and Kijima (2006), interaction between the particles and a stabilizing agent induced a stabilization effect. Hydrogen bonding between xanthan gum and other materials has been demonstrated in previous studies (Patel et al. 2013; Outuki et al. 2016).
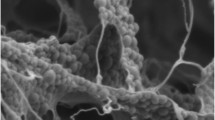
To obtain a better understanding on the ability of xanthan gum to improve the suspension stability of cinnamon-cocoa drink, the microstructural properties of the cinnamon-cocoa drink were then observed. As shown in Fig. 2, the xanthan gum’s coils could arrange a physical network in the system to resist the gravitational force during storage. The network structure originating from xanthan gum did not appear in the sample without the stabilizer. This study agreed the previous research by Katzbauer (1998) that xanthan gum chains forming a three-dimensional network could retard separation of insoluble solid particles in salad dressing matrix.
Xanthan gum networks also caused the improvement of the apparent viscosity of the cinnamon-cocoa drink (Fig. 3). This phenomenon was not only at low shear rate levels, which are typical for gravitational sedimentation, but also at a shear rate level of 50/s, which is often acknowledged as the representation of rheological behaviour in the mouth. For example, at the shear rate of 10/s, the apparent viscosity of the cinnamon-cocoa drinks without xanthan gam was around 2 mPas, while the drinks with xanthan gum 0.1% was higher than 70 mPas. Following the Stokes’ Law, the sedimentation velocity of particles in suspension is slower at a higher viscosity.
By the Pearson product-moment correlation test, strong correlations between xanthan gum concentration and viscosity was found at p < 0.01 at all the tested shear rate levels in both types of the cinnamon-cocoa drink. At the shear rate of 50/s, for instance, the correlation coefficient between xanthan gum concentration and the viscosity of the cinnamon-cocoa drink prepared with natural and alkalized cocoa powder was 0.992 and 0.991, respectively. Thus, it confirms that the viscosity enhancement is a possible mode of action of xanthan gum to stabilize the suspension system of the cinnamon-cocoa drink, in addition to the ability of xanthan gum network to physically entrap particles in the suspension (Muhammad et al. 2019).
Interestingly, Fig. 3 also shows that the addition of xanthan gum resulted in a pseudoplastic behaviour with a shear-thinning model as indicated by the decrease of apparent viscosity by increasing the shear rate level. This behaviour was not detected in the samples without xanthan gum. A possible explanation for this result is that the shearing force disrupts the xanthan gum network resulting in a lower internal friction and a lower viscosity (Dogan et al. 2015).
At the end of this study, the physical and chemical properties of cinnamon-cocoa drink containing xanthan gum as stabilizer were investigated. The sample with xanthan gum at a concentration of 0.1% (w/v) was selected for further investigation. This selection was based on the supposition that a lower concentration of xanthan gum has less effect on the physical and chemical properties of cinnamon-cocoa drink. As shown in Table 2, it can be concluded that the addition of xanthan gum (0.1% (w/v)) had no effect on the pH, total phenolic content and antioxidant activity. However, a slight increase, with significant difference (p < 0.05), in the lightness of the cinnamon-cocoa drink formulated with alkalized cocoa powder was observed.
Several studies show that xanthan can have effect on the phenolic compound and antioxidant activity of a specific matrix (Hu et al. 2019; Widelska et al. 2019). Therefore, in this study we considered to analyse the total phenolic content and the antioxidant activity of the cinnamon-cocoa drink both with and without xanthan gum. However, we found in this study that xanthan gam at a concentration of 0.1% (w/v) had no significant effect on the total phenolic content and the antioxidant activity of the cinnamon-cocoa drink. A minor effect on the characteristic of cinnamon-cocoa drink is rational since there was only a small amount of xanthan gum added to the formulation.
Conclusion
This study shows that a stabilized cinnamon-cocoa drink was obtained by the addition of xanthan gum as it can effectively create a network preventing sedimentation of cinnamon and cocoa particles. Nevertheless, the suspension stability of cinnamon-cocoa drink was also affected by the initial fquality of the cocoa powder. In the production of cocoa drink, the quality of raw material was directly proportional to the quality of the end-product. Findings in this study are useful for the food industry to define a strategy to produce “ready-to-drink” cocoa beverage with prolonged suspension stability.
References
Amer A, Schaumann GE, Diehl D (2017) The effect of pH modification on wetting kinetics of a naturally water-repellent coniferous forest soil. Eur J Soil Sci 68:317–326
Benković M, Belščak-Cvitanović A, Komes D, Bauman I (2013) Physical properties of non-agglomerated cocoa drink powder mixtures containing various types of sugar and sweetener. Food Bioprocess Technol 6:1044–1058
Casas JA, Santos VE, Garcıa-Ochoa F (2000) Xanthan gum production under several operational conditions: molecular structure and rheological properties. Enzyme MicrobTechnol 26:282–291
Ching SH, Bansal N, Bhandari B (2017) Alginate gel particles: a review of production techniques and physical properties. Crit Rev Food SciNutr 57:1133–1152
Dogan M, Aktar T, Toker OS, Tatlisu NB (2015) Combination of the simple additive (saw) approach and mixture design to determine optimum cocoa combination of the hot chocolate beverage. Int J Food Prop 18:1677–1692
Dogan M, Aslan D, Aktar T, Sarac MG (2016) A methodology to evaluate the sensory properties of instant hot chocolate beverage with different fat contents: multi-criteria decision-making techniques approach. Eur Food Res Technol 242:953–966
Dogan M, Toker OS, Aktar T, Goksel M (2013) Optimization of gum combination in prebiotic instant hot chocolate beverage model system in terms of rheological aspect: mixture design approach. Food Bioprocess Technol 6:783–794
Fäldt P, Bergenståhl B (1996) Spray-dried whey protein/lactose/soybean oil emulsions. 2. Redispersability, wettability and particle structure. Food Hydrocoll 10:431–439
Food and agriculture organisation (2020) FAOSTAT. https://www.fao.org/faostat/en/#data/QC Accessed 8 May 2020
Hardjohutomo H (1967) Mustika Rasa. DepartemenPertanian, Jakarta
Holkar CR, Jadhav AJ, Pinjari DV (2019) A critical review on the possible remediation of sediment in cocoa/coffee flavored milk. Trends Food SciTechnol 86:199–208
Hu X, Wang K, Yu M, He P, Qiao H, Zhang H, Wang Z (2019) Characterization and antioxidant activity of a low-molecular-weight xanthan gum. Biomolecules 9(11):730
Ilmi A, Praseptiangga D, Muhammad DRA (2017) Sensory attributes and preliminary characterization of milk chocolate bar enriched with cinnamon essential oil. In: IOP Conference Series—materials science and engineering 193: 012031. IOP Publishing
Jaeger SR, Axten LG, Wohlers MW, Sun-Waterhouse D (2009) Polyphenol-rich beverages: insights from sensory and consumer science. J Sci Food Agric 89:2356–2363
Katzbauer B (1998) Properties and applications of xanthan gum. PolymDegrad Stab 59:81–84
Liu J, Zhan X, Wan J, Wang Y, Wang C (2015) Review for carrageenan-based pharmaceutical biomaterials: favourable physical features versus adverse biological effects. CarbohydrPolym 121:27–36
Manuhara GJ, Praseptiangga D, Muhammad DRA, Maimuni BH (2016). Preparation and characterization of semi-refined kappa carrageenan-based edible film for nano coating application on minimally processed food. In: AIP conference proceedings 1710: 030043. AIP Publishing
Menggala SR, Vanhove W, Muhammad DRA, Hendri J, Speelman S, Van Damme P (2019) Sustainable harvesting of Cinnamomum burmannii (Nees and T. Nees) blume in Kerinci Regency, Indonesia. Sustainability 11(23):6709
Muhammad DRA, Dewettinck K (2017) Cinnamon and its derivatives as potential ingredients in functional foods: a review. Int J Food Prop 20:2237–2263
Muhammad DRA, Doost AS, Gupta V, Bin-Sintang MD, Van de Walle D, Van der Meeren P, Dewettinck K (2020) Stability and functionality of xanthan gum–shellac nanoparticles for the encapsulation of cinnamon bark extract. Food Hydrocoll 100:105377
Muhammad DRA, Gonzalez CG, Doost AS, Van de Walle D, Van der Meeren P, Dewettinck K (2019) Improvement of antioxidant activity and physical stability of chocolate beverage using colloidal cinnamon nanoparticles. Food Bioprocess Technol 12:976–989
Muhammad DRA, Lemarcq V, Alderweireldt E, Vanoverberghe P, Praseptiangga D, Juvinal JG, Dewettinck K (2020) Antioxidant activity and quality attributes of white chocolate incorporated with Cinnamomum burmanniiblume essential oil. J Food SciTechnol 57:1731–1739
Muhammad DRA, Praseptiangga D, Van de Walle D, Dewettinck K (2017) Interaction between natural antioxidants derived from cinnamon and cocoa in binary and complex mixtures. Food Chem 231:356–364
Muhammad DRA, Tuenter E, Patria GD, Foubert K, Pieters L, Dewettinck K (2021) Phytochemical composition and antioxidant activity of cinnamomumburmanniiblume extracts and their potential application in white chocolate. Food Chem. https://doi.org/10.1016/j.foodchem.2020.127983
Oracz J, Zyzelewicz D, Nebesny E (2015) The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region, and processing operations: a review. Crit Rev Food SciNutr 55:1176–1192
Outuki PM, de Francisco LMB, Hoscheid J, Bonifácio KL, Barbosa DS, Cardoso MLC (2016) Development of arabic and xanthan gum microparticles loaded with an extract of Eschweilera nana Miers leaves with antioxidant capacity. Colloids Surf A PhysicochemEng Asp 499:103–112
Patel AR, Remijn C, Cabero AIM, Heussen P, ten-Hoorn JWM, Velikov KP (2013) Novel all-natural microcapsules from gelatin and shellac for biorelated applications. AdvFunct Mater 23:4710–4718
Ribeiro-Santos R, Andrade M, Madella D, Martinazzo AP, Moura LdAG, de Melo NR, Sanches-Silva A (2017) Revisiting an ancient spice with medicinal purposes: cinnamon. Trends Food SciTechnol 62:154–169
Rivas JCM, Dietze M, Zahn S, Schneider Y, Rohm H (2018) Diversity of sensory profiles and physicochemical characteristics of commercial hot chocolate drinks from cocoa powders and block chocolates. Eur Food Res Technol 244:1407–1414
Selamat J, Hussin N, Zain AM, Man YBC (1998) Effects of alkalized cocoa powder and soy lecithin on physical characteristics of chocolate beverage powders. J Food Processing Preserv 22:241–254
Toker Ö, Dogan M, Göksel M (2012) Prediction of rheological parameters of model instant hot chocolate beverage by adaptive neuro fuzzy inference system. Milchwissenschaft 67:22–25
Toker OS, Dogan M, Canıyılmaz E, Ersöz NB, Kaya Y (2013) The effects of different gums and their interactions on the rheological properties of a dairy dessert: a mixture design approach. Food Bioprocess Technol 6:896–908
Widelska G, Wójtowicz A, Kasprzak K, Dib A, Oniszczuk T, Olech M, Oniszczuk A (2019) Impact of xanthan gum addition on phenolic acids composition and selected properties of new gluten-free maize-field bean pasta. Open Chem 17(1):587–598
Yaginuma Y, Kijima T (2006) Effects of microcrystalline cellulose on suspension stability of cocoa beverage. J DispersSciTechnol 27:941–948
Żyżelewicz D, Krysiak W, Nebesny E, Budryn G (2014) Application of various methods for determination of the color of cocoa beans roasted under variable process parameters. Eur Food Res Technol 238:549–563
Acknowledgements
Hercules Foundation is acknowledged for its financial support in the acquisition of the Scanning Electron Microscope JEOL JSM-7100F equipped with cryo-transfer system Quorum PP3010T (Grant Number AUGE-09-029). We would like to thank Universitas Sebelas Maret for supporting this research through international joint research and publication grant (No. 452/UN27.21/PN/2020). The authors also thank Jan Cromphout, Alina Rupprecht, Konstantinos Kios, Lore Lybeert and Stijn Vanderroost for assisting to collect some data in this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muhammad, D.R.A., Kongor, J.E. & Dewettinck, K. Investigating the effect of different types of cocoa powder and stabilizers on suspension stability of cinnamon-cocoa drink. J Food Sci Technol 58, 3933–3941 (2021). https://doi.org/10.1007/s13197-020-04855-y
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04855-y