Abstract
This study was aimed to synthesize and evaluate the nano starch-based composite films by the addition of nano starch in film formulation at 0.5, 1, 2, 5 and 10% level of total starch. The acid hydrolysis technique was used to reduce the size of starch granules of kidney bean starch. The physicochemical properties of both native and nano starch were determined. Nano starch showed a higher value for swelling power, solubility, water and oil absorption capacity when compared with native starch. The particle size of kidney bean nano starch was 257.7 nm at 100% intensity. The size of starch granule affects various properties of films. The thickness, solubility and burst strength of the composite films were increased significantly (p ≤ 0.05) with an increase in the concentration of nano starch in film formulation. While the moisture content and water vapour transmission rate (WVTR) were decreased significantly (p ≤ 0.05) with an increase in the concentration of nano starch in film formulation. The results suggested that kidney bean starch could be used for the development of packaging films. The utilization of nano starch in film formulations had an additional advantage in improving the film properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Starch is an energy reserve, stored in stems, roots, seeds, and tubers. Commercial starches are abundantly available and isolated from corn, potato, wheat, rice, and tapioca. In addition to that, it is odorless, tasteless and does not require extreme purification (Daudt et al. 2014). Starch granules vary in size, shape and striations or lamellae. The shape of starch granules may be spherical, disk, oval, polygonal or rod-shaped. The granule characteristic varies according to the botanical source and the environment which in turn affects its physicochemical properties. These properties determine the applications and use of starch in a particular industry (Jane 2006).
In the food, textile, pharmaceutical and cosmetic industry, starch is being utilized as a raw material for various formulations. Starch has various applications, like as a binder, thickener, stabilizer, texturizer, foam strengthener, moisture retainer, gel former, film-former and adhesive agent. Starch has been also used for the manufacture of pouches for detergents and insecticides; flushable liners and bags; medical delivery systems and devices (Damodaran and Parkin 2017). The starch percentage varies greatly among different botanical sources i.e. in cereals (60–80%), legumes (25–50%) and a few green immature fruits such as mango and banana (70%) on a dry weight basis (de la Torre-Gutiérrez et al. 2008). Thus, higher demand for starches at the commercial level has created interest in new sources like legumes and fruit kernels. Legumes are consumed all over the world in a variety of forms and processed differently according to the taste and cultural preference of people (Singh et al. 2004). The processing of legumes produces the starch as a major byproduct during the extraction of proteins.
Kidney beans (Phaseolus vulgaris) are an important source of proteins, complex carbohydrates, minerals, vitamins and grown and consumed across various regions of the world (Chung et al. 2008). Beans contain 2–3 times more protein than cereals while carbohydrates content is about 50–60% of dry matter of beans. Significant research has been carried out on the properties of conventional starches such as those from corn (Sandhu et al. 2005), wheat (Nain et al. 2020), and potato (Singh et al. 2006). These are extensively used in the industry. However, in the past, legume starches have not been utilized in the industry due to the lack of intensive research on their properties. Lack of research on legume starches is also due to the scarcity of pulses in many countries. Food scientists have started studying new sources of starch such as legumes to meet a wide range of unique functional properties and consumer demands. Since significant information about the physicochemical properties of kidney beans is still lacking, there is a need to study their structure, composition, and properties to widen their industrial application. Due to the presence of high amylose, legume starches shows high gelation temperature, resistance to shear thinning, fast retrogradation, high resistance starch and high gel elasticity (Ambigaipalan et al. 2011).
The most popular packaging material is made of plastic polymers. The degradation of these plastics is a serious problem because they are derived from petroleum products and cause problems during waste disposal (Avella et al. 2005). More than 8 million tonnes of plastic enters the oceans every year threatening marine life. Plastic waste kills up to 1 million sea birds, 100,000 sea mammals, marine turtles, and countless fish every year. Plastic remains in our ecosystem for years, harming thousands of sea creatures every day. In addition to this, they are derived from fossil fuels which are a non-renewable source of energy and will soon get exhausted in the near future. Biodegradable materials are those materials that are degraded by the enzymatic action of micro-organisms such as bacteria, yeast, fungi and its ultimate degradation into simpler substances that occur under both aerobic and anaerobic conditions (Avella et al. 2005).
Out of all the biomaterials used for the formation of films, starch is the most widely used as it’s easily available, can be extracted in high yield and is biodegradable and biocompatible. Native starch films generally display poor mechanical and barrier properties. Studies have proven that the use of nano-enforcements improves film properties. The native and nano starch from same botanical sources results in improved mechanical and barrier properties of films due to the identical nature of matrix and filler (Silva et al. 2019a). Starch nanoparticles prepared by acid hydrolysis have relatively high crystallinity and stability and are thus desirable in the food processing industry where temperatures are high (Aldao et al. 2018). The literature on the properties of nano starch obtained from kidney bean starch is limited or not yet studied. The study was carried out to reduce the kidney bean starch granule size by a mild acid hydrolysis method and their utilization in the development of nano starch-based composite films.
Materials and methods
Kidney bean (Phaseolus vulgaris) were procured from the market of Solan, India. The chemicals used in the study were of analytical grades and purchased from Loba Chemical Pvt. Ltd. (Mumbai, India).
Isolation of starch
The steeping method (Sandhu et al. 2005) for starch isolation was used with slight modification. 500 g sample was steeped (20 h) in 1.25 L of distilled water containing 0.1% potassium metabisulphite and ground with distilled water till the development of smooth texture slurry free from any courser particles. The ground slurry was filtered through nylon cloth (100 mesh) and the residue was washed repeatedly with distilled water until it was free of starch. The filtrate was again filtered through a 420 micron-sized screen and the obtained slurry was allowed to stand undisturbed for 4–5 h. The settled starch was separated by removing the supernatant by suction and centrifuged at 3000 rpm for 5 min. After removing the upper non-white layer, starch was obtained, dried in an oven (40 °C for 12 h) and stored in an air-proof container for further study.
Physicochemical properties of starch
The amylose content (Williams et al. 1970); water and oil absorption capacity (Sosulski et al. 1976); the swelling power and solubility (Schoch 1964) of starch were determined by following their respective methods.
Preparation of nano starch
The starch granule size was reduced by acid hydrolysis as per the procedure described by Kim et al. (2012). The starch was kept in the 3.16 M sulphuric acid solution for 7 days at 40 °C and after this the mixture was centrifuged (5000 rpm) for 15 min. The precipitates were washed successively by centrifugation in distilled water until neutralized. Then the neutralized starch was washed in acetone and dried in a hot air oven at 45 °C for 10 h.
Characterization of native and nano starch
Size distribution
The size distribution of starch granules was measured using dynamic light scattering (DLS) at a wavelength of 633 nm. The sample was diluted in de-ionized water (2 mg/10 mL) and then it was subjected for analysis. The nano starch samples were measured at 30 °C without filtering or removing dust (Zhou et al. 2014).
Surface morphology
Field emission-scanning electron microscopy (FE-SEM) system and lithography system (NOVA Nano SEM 450, USA) with an acceleration voltage of 5–30 kV was used to determine the surface morphology of starch granules. The native and nano starch from kidney bean were fixed on the surface of a carbon tape and sputtered with a thin layer of gold for 60 s before analysis. The 650 × magnifications were selected to obtain a representative and clear image.
Preparation of films
For control starch film formulation, 5% (w/w) native starch, 2.5% (w/w) glycerol and 2 g/L soybean refined oil were mixed in distilled water up to a final volume of 100 mL. The composite film formulations were made by using native and nano starch. The nano starch was mixed with native starch at concentrations of 0.5, 1, 2, 5 and 10%. For composite films, a mixture of 5% (w/w) native starch and nano starch, 2.5% (w/w) glycerol and 2 g/L soybean refined oil were mixed in distilled water up to a final volume of 100 mL. The film-forming solution was gelatinized prior to casting in petri dishes. After drying (50 °C for 10 h), films were peeled off (Dularia et al. 2019).
Characterization of films
The film thickness was measured by a Vernier Calliper (Fan et al. 2016). Moisture Analyser (Mettler Toledo, HE73/01, Switzerland) was used to determine the moisture content of films (Mei et al. 2013). ASTM E96-00 method (ASTM 2000) with slight modification was followed for the estimation of WVTR. A test cell was used having 30 mL distilled water, sealed with films and kept in a plastic cup partially filled with dehydrated calcium chloride. After keeping the test assembly at 25 °C for 24 h, WVTR of the films was calculated according to the formula:
\(\Delta {\text{W}}\) = weight loss of test cell, T = time of storage, A = area of the exposed film.
The water solubility of films was determined by dipping the films in water for 24 h (Gontard et al. 1992). The films were removed from the water and placed in a desiccator to obtain the final dry weight of the film. The loss in weight was expressed as solubility (%).
Bursting strength of films was measured by using Texture Analyser (TA. XT plus, Stable Microsystems, UK). Film pieces (3 × 3 cm2) were placed on film supporting rig and a ball probe (SMS P/0.25S, 1/4th-inch diameter) connected to a load cell was inserted through film sample. The curve of maximum force (g) peak was presented as bursting strength (Roy et al. 2020).
Statistical analysis
Statistical analysis of the data was done by one-way ANOVA using IBM SPSS Statistics Trial Version. Duncan’s multiple range (p ≤ 0.05) was carried out the significant differences in mean.
Results and discussion
Physicochemical properties of native and nano starch from kidney bean
Amylose content of native and nano starch
Genetic factors and growing conditions affect the amylose content in starch. The amylose content of the native kidney bean was 7.18 ± 0.53% (Table 1). Du et al. (2014) reported an amylose content of 32.4% for kidney bean. The difference in amylose content was due to the variation in their climatic and ecological conditions (Bharti et al. 2019). The amylose content of nano starch was not determined. The amylose content becomes undetectable after extensive hydrolysis, indicating that the length of residual linear chains is too short to form a blue iodine complex (Wang and Copeland 2015).
Water and oil absorption capacity of native and nano starch
The water and oil absorption capacity for native and nano starch from kidney bean is shown in Table 1. The water absorption capacity of native kidney bean starch was 2.40 ± 0.34%. Wani et al. (2010) reported the water absorption capacity of kidney bean starch in the range of 1.9–2.1 g/g. The association of amylose and amylopectin molecules affects the water absorption or water-binding capacity of starches. A higher value of water-binding capacity is observed if a loose association is found. Oil absorption capacity is the ability of the dry starch to physically bind fat by capillary attraction and it is of great importance, as fat acts as flavor retainer and also increases the mouthfeel of foods (Singh et al. 2006). The oil absorption capacity of native kidney bean starch was observed to be 15.44 ± 0.40%. The water absorption capacity of kidney bean nano starch was observed to be 54.76 ± 2.14% while the oil absorption capacity was observed to be 38.14 ± 0.38%. The water absorption and oil absorption capacity for nano starch were found to be significantly higher than native starch. This can be associated with the extensive hydrolysis of glycosidic linkages, yielding shorter molecules such as monosaccharides, disaccharides, and small oligosaccharides, which are more hygroscopic than the polysaccharide due to the larger number of free hydroxyl groups (Ali et al. 2016).
Swelling power and solubility of native and nano starch
The patterns of progressive swelling and solubility of various starches have been compared over a range of temperatures to provide information about the relative strengths of bonding within the granules. The swelling power and solubility of the native starch and nano starch were observed at four different temperatures i.e. 60, 70, 80 and 90 °C (Fig. 1). The swelling power of native kidney bean starch ranged between 2.26 ± 0.15 and 4.32 ± 0.34 (g/g) while swelling power and solubility of nano starch was ranged between 3.33 ± 0.10 and 8.64 ± 0.85 (g/g). The swelling power was increased with an increase in temperature. When starch granules are heated in excess of water it leads to weakening of the crystalline structure of starch thereby giving a chance to amylose and amylopectin molecules to form a hydrogen bond with water, leading to an increase in the swelling capacity of starch granules (Singh et al. 2003). The solubility of native kidney bean starch at the four temperatures ranged between 4.26 ± 0.15 and 5.8 ± 0.36%. Nano starch showed higher solubility and swelling power than native starch. This might be due to the melting of the crystallites, which may involve a solvation-assisted helix to coil transition of their chains. This, in turn, would result in a gain in enthalpy that would off-set the hydrogen bonding occurring in the crystalline regions leading to increased swelling and solubility (Leach et al. 1959).
Characterisation of native and nano starch
Dynamic light scattering (DLS)
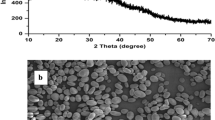
The size distribution of native starch and nano starch was carried out by dynamic light scattering. For native starch, the DLS reading was 433.6 nm at the intensity of 100% while for nano starch it was 257.7 nm at 100% intensity as shown in Fig. 2. Sun (2018) reported that the size of starch nanoparticles (SNPs) ranged between 10 and 300 nm. SNPs with such size ranges are usually lamellar, spherical, rod-like, and irregular in appearance. Aggregation of starch nanocrystals has been attributed to hydrogen bond formation resulting from the interaction of hydroxyl groups on the surface of starch nanocrystals (Mukurumbira et al. 2017).
Field emission: scanning electron microscope (FE-SEM)
FE- SEM was used to study the morphological and surface characteristics of native and nano starch from kidney bean. The native kidney bean starch granules showed round and elliptical shapes and no fissures were observed (Fig. 3). The kidney bean nano starch appeared in agglomerated form due to acid hydrolysis. The surface had cracks and dents and the granules were mostly irregularly shaped except for a few round and elliptical ones. The granules were both small and large in size. With acid hydrolysis, the shape of the SNP represents the original structures present in native starches (Perez Herrera et al. 2016). Variation in the morphology of starch granules is observed owing to the different botanical origins, the biochemistry of the amyloplast, physiology of the plant, plant species and maturity (Jane 2006).
Film preparation
Preparation of native and nano starch-based composite films from kidney bean
The native kidney bean film was transparent with a relatively smooth surface. The nano starch-based composite films (NSCF) were prepared by adding a different concentration of nano starch in native starch i.e. 0.5, 1, 2, 5 and 10%. As the concentration of nano starch was increased in the films, the films became more opaque and thicker in nature. It may be due to the aggregation of nanocrystals during film formation (Silva et al. 2019).
Characterization of films
Thickness of native and nano starch-based composite films
The thickness of the films is shown in Table 2. The thickness of the kidney bean NSCF (0.5%) was lower than the thickness of the native film. The thickness of the kidney bean starch composite films ranged between 0.20 ± 0.005 and 0.34 ± 0.024 mm. The thickness of NSCF (5%) was similar to NSCF (10%). Fan et al. (2016) stated that uniformity in film thickness is important for achieving good repeatability of the results of the mechanical tests because uniform thickness serves as a basis for determining several functional properties of the films.
Moisture content of native and nano starch-based composite films
The moisture content of native and nano starch-based composite films is shown in Table 2. The moisture content of native kidney bean starch film was observed 11.2 ± 1.25%. The moisture content of kidney bean NSCF decreased with an increase in the concentration of nano starch. The most relevant constraint of starch-based materials is their poor mechanical properties, mainly due to the high susceptibility of starch to ambient humidity. Thus, one of the aims is to incorporate fillers to starch matrices to overcome this limitation by reinforcing composites structure (Castillo et al. 2017).
Water vapor transmission rate of native and nano starch-based composite films
The WVTR of the kidney bean native and composite films is presented in Table 2. The WVTR of the native starch film was significantly higher than the composite film containing nano starch. The WVTR for kidney bean NSCF decreased significantly from 6.9 × 10−3±2 to 1.4 × 10−3±0.05 g/m2/s with an increase in the concentration of nano starch. WVTR of kidney bean films decreases with an increase in concertation of nano starch. This decrease in WVTR values in the composite films with nano starch can be due to nanometric sizes, which increase the surface-volume ratio and interfere with the mobility of polymer chains (Fan et al. 2016).
Solubility of native and nano starch-based composite films
The solubility of starch films is shown in Table 2. The solubility of kidney bean starch films increased with an increase in the concentration of nano starch from 0.5 to 10%. No significant difference was observed in the solubility of NSCF (5%) and NSCF (10%). Native kidney bean film showed the lowest solubility of 10.56 ± 2.71%. Higher concentrations of amylopectin, decreased film solubility in water, leading to aggregation of starch micro-granules (Thakur et al. 2019).
Burst strength of native and nano starch-based composite films
Burst strength measures the amount of force required to puncture the films and is a measure of mechanical strength. Native starch films showed the lowest burst strength. The burst strength of kidney bean NSCF ranged between 1255.7 ± 2.08 and 2777.7 ± 4.50 g (Table 2). The burst strength of native kidney bean starch film and NSCF (0.5%) was similar while with increase in the concentration of nano starch it was observed that the burst strength was also increased. The results indicate that the incorporation of nano starch improved the burst strength of the films. The nanoparticle presents a high specific surface area which results in better interfacial interaction between filler and polymeric matrix, which results in an increase of the nanocomposite strength.
Conclusion
The synthesized nano starch showed higher water solubility, swelling power, water and oil absorption capacity than native starch. The addition of nano starch in film formulation resulted in improved film properties of composite films. The nano starch in film formulation reduced the WVTR and increased the burst strength of composite films. Thus, the addition of nano starch has proved an advantage in improving composite film properties. In relation to nano starch and packaging films, the scientific information available on the kidney bean starch is limited. Therefore, this needs to be explored more as a potential source of starch besides proteins and other nutrients. Further, utilization of kidney bean starch as a raw material for the development of food packaging films require more emphasis in future research.
References
Aldao DC, Šárka E, Ulbrich P, Menšíková E (2018) Starch nanoparticles–two ways of their preparation. Czech J Food Sci 36(2):133–138. https://doi.org/10.17221/371/2017-CJFS
Ali A, Wani TA, Wani IA, Masoodi FA (2016) Comparative study of the physico-chemical properties of rice and corn starches grown in Indian temperate climate. J Saudi Soc Agric Sci 15(1):75–82. https://doi.org/10.1016/j.jssas.2014.04.002
Ambigaipalan P, Hoover R, Donner E, Liu Q, Jaiswal S, Chibbar R, Nantanga KK, Seetharaman K (2011) Structure of faba bean, black bean and pinto bean starches at different levels of granule organization and their physicochemical properties. Food Res Int 44(9):2962–2974
ASTM (2000) Standard test method for water vapour transmission of materials. E 96-00. In: Annual book of ASTM standards. ASTM International, Philadelphia, Pa, pp 907–914
Avella M, De Vlieger JJ, Errico ME, Fischer S, Vacca P, Volpe MG (2005) Biodegradable starch/clay nanocomposite films for food packaging applications. Food Chem 93(3):467–474. https://doi.org/10.1016/j.foodchem.2004.10.024
Bharti I, Singh S, Saxena DC (2019) Influence of alkali treatment on physicochemical, pasting, morphological and structural properties of mango kernel starches derived from Indian cultivars. Int J Biol Macromol 125:203–212. https://doi.org/10.1016/j.ijbiomac.2018.12.034
Castillo LA, López OV, Ninago MD, Versino F, Barbosa SE, García MA, Villar MA (2017) Composites and nanocomposites based on starches. Effect of mineral and organic fillers on processing, structure, and final properties of starch. In: Starch-based materials in food packaging. Academic Press, pp 125–151
Chung HJ, Liu Q, Pauls KP, Fan MZ, Yada R (2008) In vitro starch digestibility, expected glycemic index and some physicochemical properties of starch and flour from common bean (Phaseolus vulgaris L.) varieties grown in Canada. Food Res Int 41(9):869–875. https://doi.org/10.1016/j.foodres.2008.03.013
Damodaran S, Parkin KL (2017) Fennema’s food chem. CRC Press, Boca Raton
Daudt RM, Külkamp-Guerreiro IC, Cladera-Olivera F, Thys RCS, Marczak LDF (2014) Determination of properties of pinhão starch: analysis of its applicability as pharmaceutical excipient. Ind Crops Prod 52:420–429. https://doi.org/10.1016/j.indcrop.2013.10.052
de la Torre-Gutiérrez L, Chel-Guerrero LA, Betancur-Ancona D (2008) Functional properties of square banana (Musa balbisiana) starch. Food Chem 106(3):1138–1144. https://doi.org/10.1016/j.foodchem.2007.07.044
Du SK, Jiang H, Ai Y, Jane JL (2014) Physicochemical properties and digestibility of common bean (Phaseolus vulgaris L.) starches. Carbohyd Polym 108:200–205. https://doi.org/10.1016/j.carbpol.2014.03.004
Dularia C, Sinhmar A, Thory R, Pathera AK, Nain V (2019) Development of starch nanoparticles based composite films from non-conventional source-Water chestnut (Trapa bispinosa). Int J Biol Macromol 136:1161–1168. https://doi.org/10.1016/j.ijbiomac.2019.06.169
Fan H, Ji N, Zhao M, Xiong L, Sun Q (2016) Characterization of starch films impregnated with starch nanoparticles prepared by 2, 2, 6, 6-tetramethylpiperidine-1-oxyl (TEMPO)-mediated oxidation. Food Chem 192:865–872. https://doi.org/10.1016/j.foodchem.2015.07.093
Gontard N, Guilbert S, Cuq JL (1992) Edible wheat gluten films: influence of the main process variables on film properties using response surface methodology. J Food Sci 57(1):190–195. https://doi.org/10.1111/j.1365-2621.1992.tb05453.x
Jane JL (2006) Current understanding on starch granule structures. J Appl Glycosci 53(3):205–213
Kim HY, Lee JH, Kim JY, Lim WJ, Lim ST (2012) Characterization of nanoparticles prepared by acid hydrolysis of various starches. Starch-Stärke 64(5):367–373. https://doi.org/10.1002/star.201100105
Leach HW, McCowen LD, Schoch TJI (1959) Structure of the starch granule. Swelling and solubility patterns of various starches. Cereal Chem 36:534–544
Mei J, Yuan Y, Guo Q, Wu Y, Li Y, Yu H (2013) Characterization and antimicrobial properties of water chestnut starch-chitosan edible films. Int J Biol Macromol 61:169–174. https://doi.org/10.1016/j.ijbiomac.2013.06.026
Mukurumbira A, Mariano M, Dufresne A, Mellem JJ, Amonsou EO (2017) Microstructure, thermal properties and crystallinity of amadumbe starch nanocrystals. Int J Biol Macromol 102:241–247. https://doi.org/10.1016/j.ijbiomac.2017.04.030
Nain V, Kaur M, Sandhu KS, Thory R, Sinhmar A (2020) Development, characterization, and biocompatibility of zinc oxide coupled starch nanocomposites from different botanical sources. Int J Biol Macromol 162:24–30. https://doi.org/10.1016/j.ijbiomac.2020.06.125
Perez Herrera M, Vasanthan T, Hoover R (2016) Characterization of maize starch nanoparticles prepared by acid hydrolysis. Cereal Chem 93(3):323–330. https://doi.org/10.1094/CCHEM-08-15-0175-R
Roy K, Thory R, Sinhmar A, Pathera AK, Nain V (2020) Development and characterization of nano starch-based composite films from mung bean (Vigna radiata). Int J Biol Macromol 144:242–251. https://doi.org/10.1016/j.ijbiomac.2019.12.113
Sandhu KS, Singh N, Malhi NS (2005) Physicochemical and thermal properties of starches separated from corn produced from crosses of two germ pools. Food Chem 89(4):541–548. https://doi.org/10.1016/j.foodchem.2004.03.007
Schoch TJ (1964) Swelling power and solubility of granular starches. M Carbohydr Chem 4:106–108
Silva APM, Oliveira AV, Pontes SM, Pereira AL, Rosa MF, Azeredo HM (2019) Mango kernel starch films as affected by starch nanocrystals and cellulose nanocrystals. Carbohydr Polym 211:209–216. https://doi.org/10.1016/j.carbpol.2019.02.013
Singh N, Singh J, Kaur L, Sodhi NS, Gill BS (2003) Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem 81(2):219–231. https://doi.org/10.1016/S0308-8146(02)00416-8
Singh N, Kaur M, Sandhu KS, Guraya HS (2004) Physicochemical, thermal, morphological and pasting properties of starches from some Indian black gram (Phaseolus mungo L.) cultivars. Starch-Stärke 56(11):535–544. https://doi.org/10.1002/star.200400290
Singh J, McCarthy OJ, Singh H (2006) Physico-chemical and morphological characteristics of New Zealand Taewa (Maori potato) starches. Carbohydr Polym 64(4):569–581. https://doi.org/10.1016/j.carbpol.2005.11.013
Sosulski F, Humbert ES, Bui K, Jones JD (1976) Functional Propreties of Rapeseed Flours, Concentrates and Isolate. J Food Sci 41(6):1349–1352. https://doi.org/10.1111/j.1365-2621.1976.tb01168.x
Sun (2018) Starch in food (second edition), structure, function and applications. In: Woodhead Publishing Series in Food Science, Technology and Nutrition, pp 691–745
Thakur R, Pristijono P, Scarlett CJ, Bowyer M, Singh SP, Vuong QV (2019) Starch-based films: major factors affecting their properties. Int J Biol Macromol 132:1079–1089. https://doi.org/10.1016/j.ijbiomac.2019.03.190
Wang S, Copeland L (2015) Effect of acid hydrolysis on starch structure and functionality: a review. Crit Rev Food Sci 55(8):1081–1097. https://doi.org/10.1080/10408398.2012.684551
Wani IA, Sogi DS, Wani AA, Gill BS, Shivhare US (2010) Physico-chemical properties of starches from Indian kidney bean (Phaseolus vulgaris) cultivars. Int J Food Sci 45(10):2176–2185. https://doi.org/10.1111/j.1365-2621.2010.02379.x
Williams PC, Kuzina FD, Hlynka I (1970) Rapid colorimetric procedure for estimating the amylose content of starches and flours. Cereal Chem 1:413–420
Zhou G, Luo Z, Fu X (2014) Preparation of starch nanoparticles in a water-in-ionic liquid microemulsion system and their drug loading and releasing properties. J Agric Food Chem 62(32):8214–8220. https://doi.org/10.1021/jf5018725
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, I., Sinhmar, A., Thory, R. et al. Synthesis and characterization of nano starch-based composite films from kidney bean (Phaseolus vulgaris). J Food Sci Technol 58, 2178–2185 (2021). https://doi.org/10.1007/s13197-020-04728-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04728-4