Abstract
The objective of this work was to analyze the morphological and physicochemical properties of bean starch and its use in nanoencapsulation by spray drying. Starch purity was 81.21 ± 1.43% db with a resistant starch content higher than a commercial corn starch, but with a high protein content and a low amylose content. Starch granules presented smooth surfaces, polyhedral shape and sizes from ~ 1 to 6.3 µm. Black bean starch exhibited an A-type X-ray diffraction pattern with a crystallinity highest than corn starch. Black bean starch showed higher thermal stability than a commercial corn starch. At 90 °C, solubility was 31.0% and swelling power was 31.2 g g−1. The black bean starch gel showed a high stability under refrigeration and freeze–thaw. Small particles and viscosity profile suggested the potential application of black bean starch as wall material during nano spray drying. Black bean starch tends to form spherical aggregates during nano spray drying due to the protein content. Capsules size was in the range of 1.0–2.5 µm, however were observed agglomerated particles by SEM. The encapsulation efficiency of l-ascorbic acid was 36.88 ± 0.55%. The results indicate that black bean starch possesses properties with potential applications in food industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Starch is a polysaccharide, having amylose, a linear or slightly branched (1 → 4)-α-d-glucan units and amylopectin, a highly branched molecule with (1 → 4)-α-d-glucan short chains which is linked through α (1 → 6) linkages and other minor components such as lipids and proteins that can affect their properties [1,2,3,4]. Starch is used in the food industry as functional ingredient (thickener, stabilizer, emulsifier, and gelifier) in native or modified form [5,6,7,8,9]. Starch also has been considered as an attractive natural polymer for the packaging of materials with varying thickness and flexibility [1, 10, 11] and as wall material for the micro-encapsulation of bioactive compounds and probiotics by spray drying for industrial applications [12,13,14,15]. Encapsulation is a process oriented to protect substances that are susceptible to decomposition or reduction of their functionality due to different degradation reactions (i.e., oxidation, hydrolysis, etc.) [13, 16, 17]. Spray drying is the most common method that is used for encapsulation of food ingredients, because it turns liquid feeds into a powder form, with higher stability, lower storage and transport costs, and easier usage [15, 16, 18]. This consists of the atomization of the active substance, previously dispersed or dissolved in a solution of the wall material, within a dried gas stream, typically air [15, 18]. A much more recent innovation in spray-drying encapsulation has been the design and introduction of the nano-spray dryer, which operates on different principles than conventional spray dryers [17, 19] and is able to generate particles in the size range of 300 nm to 5 µm at high yields (> 90%) [20, 21]. The principles behind this new technology have been described by different authors, briefly, a nano–spray dryer consists of the following elements: (a) an ultrasonic atomizer based on a vibrating mesh technology that can produce very small droplets with a narrow particle-size distribution; (b) a heating gas flow that moves co-currently with atomized droplets into the drying chamber; and (c) a high-voltage electric field at the bottom of the drying chamber that is composed of two electrodes to separate the dried product [17, 19, 21, 22]. In industrial applications, TurboSonic Technologies Inc., and SonoTek Corp., have developed new atomizers that use the ultrasonic coating technology to improve monodispersity and nano- and microcapsule generation [15]. Recently, production of starch-based nano-capsules in the food industry has gained much attention because of their controlled release characteristics, stability, solubility, bioavailability, higher penetration rates through biological barriers and ability to deliver several active ingredients in foods and within the human body [17, 21, 23]. However, the properties of native starches are highly variable depending on the botanical source. Therefore, more studies on physicochemical and morphological characterization of native starches are necessary to identify the potential application in nano-encapsulation by spray drying.
Legumes are the second major food source for human next to cereal and play an important role in the human diet of developing countries. Common beans (Phaseolus vulgaris L.) are the main legumes consumed in the world, which are widely cultivated and have high nutritional quality; they are an excellent source of starch and protein and are fairly good sources of dietary fiber, minerals, vitamins, and polyunsaturated fatty acids [8, 9, 24, 25]. Several studies have reported that starch granules from common beans vary in morphological characteristics, chemical composition, functional properties, physicochemical properties, thermal properties, rheological behavior, freeze–thaw stability and digestibility, depending on the origin of the crops [3, 9, 26, 27], the isolation process [6, 28], the cultivated variety [9, 24, 29,30,31,32], and processing [8, 25]. Thus, the measurement and characterization of the morphologic and physicochemical properties of common bean starch from different varieties and locations, and the effects study of their major chemical constituents on the starch properties, are essential to identify the potential industrial applications of this biopolymer. Moreover, limited information is available on the use of black bean starch as wall material during nano–spray drying. Therefore, the objectives of the present study were to: (1) isolate and characterize the chemical composition, and the morphological and physicochemical properties of starch from black bean Mexican variety (Phaseolus vulgaris L.), to determine potential uses; and (2) evaluate the black bean starch as wall material in the nano-encapsulation by spray drying.
Materials and methods
Materials and chemicals
Black bean (Phaseolus vulgaris L.) seeds were purchased from a local market (Tuxtepec, Oaxaca, México). Both total starch (K-TSTA) and resistant starch (K-RSTAR) assay kits were purchased from Megazyme International (Wicklow, Ireland). Chemicals as corn starch, ascorbic acid, amylose, and amylopectin, were purchased from Sigma Chemical Co. (St. Louis, MO, USA); while all solvents (HPLC and reagent grades) as sulfuric acid, acetone and petroleum ether were acquired from J.T. Baker (CDMX, Mexico). Other reagents were products from Fisher Scientific (Pittsburgh, PA, USA) or Merck Chemical Corporation (Darmstadt, Germany). Both distilled and Milli-Q water (Millipore, Billerica, USA) were used in this work.
Starch isolation
The starch was isolated from black bean seeds using the method reported by Flores-Gorosquera et al. [33], with slight modifications. All impurities and damaged seeds were discarded, the remaining was washed and then suspended in distilled water by 12 h at 25 °C and a 1:6 (w/w) seeds:water ratio. The seed coat from black bean was removed manually. The botanical material was ground with water (1.5 L for each 250 g seeds) into an industrial blender (Waring Commercial, Mod. CB15; Torrington, CT, USA) operated at medium power for 2 min. The paste obtained was sieved (sieve numbers: 40, 100, 200 and 325 US), precipitated (20 °C, 12 h), and washed by resuspension in water and centrifuged (Thermo Fisher Scientific Inc., Heraeus Megafuge 16R; Waltham, MA, USA; 4000×g, 10 min) twice. The fraction resulting from final centrifugation was dried in an air-circulating oven (40 °C, 24 h), grinding and sieved through 100-mesh sieves. The native starch from black bean seeds was stored in hermetically sealed containers until subsequent analyses.
Proximal analysis and total starch content
Proximal chemical composition of black bean starch was determined in triplicate in accordance with AACC International methods for moisture (44-16.01), ash (08-01.01), proteins (46-13.01) and lipids (30-25.01) [34]. Total starch content of native starch was measured enzymatically using a Megazyme assay kit. The analyses were performed according to the instructions supplied with the kits. The analyses also were carried out on a commercial corn starch for reference.
Morphologic characterization
Transmitted light microscopy and polarized light
The starch was sprayed onto a slide, ensuring an especially thin layer, which was capped with a cover glass, and a drop of distilled water was added to help disperse the sample. The starch granules were observed through a lens with transmitted light and polarized light on a microscope equipped with a color digital camera (Leica Microsystems, DM750; Heerbrugg, Switzerland) [26]. The images were captured with the LAS EZ V4.12 software (Leica Microsystems; Heerbrugg, Switzerland).
Scanning electron microscopy (SEM)
Starch granule morphology was examined by SEM. Granules were sprinkled over a conductive copper tape with double glue, which was covered with a 20 nm thick layer of coal. The layer was deposited in a JEOL evaporator (JEOL, Tokio, Japan) under vacuum conditions. To provide conductivity, the samples were covered with gold. The micrographs were obtained using a scanning electron microscope (Japan Electronic Optical Limited, JSEM 35CX; Tokio, Japan) with an accelerating potential of 20 kV under low vacuum [12]. Magnifications of 4300× were selected for the study.
Particle size distribution
Particle size distribution of native starch was determined on a laser diffraction particle size analyzer (Malvern Instruments Ltd., Mastersizer 3000; Malvern, UK). Powder samples were dispersed using a dip-in wet sample dispersion unit (Malvern Instruments Ltd., Hydro EV; Malvern, UK) at 90% of ultrasound power capacity for agglomerate dispersion. The samples were dispersed in water. The obscuration range was 3–5% [29, 35].
Physicochemical properties
Apparent amylose content
Apparent amylose content was determined using the spectrophotometric method described by Hoover and Ratnayake [3]. The absorbance measurements were carried out at 600 nm at an UV–Vis spectrophotometer (Thermo Scientific, Mod. Genesys 10 S; Waltham, MA, USA). The amylose content was calculated from a standard curve prepared using mixtures of pure potato amylose and amylopectin (over the range 0–100% amylose). Amylopectin content was calculated by difference at 100% of amylose content.
Resistant starch content
A Megazyme kit based on the method approved (32-40.01) by AACCI [34] to determine the resistant starch content in raw black bean starch was used. The absorbance measurements were carried out at 510 nm at an UV–Vis spectrophotometer (Thermo Scientific, Mod. Genesys 10 S; Waltham, MA, USA).
X-ray diffraction (XRD)
XRD patterns of the starch samples were analyzed at room temperature (25 °C) on a diffractometer (Bruker AXS, D8 Advance; Billerica, MA, USA) with copper Kα radiation, operating at 40 kV and 30 mA. The scanning region of the two-theta angle (2θ) was from 5° to 60°, using a scan rate of 1° min−1. The relative crystallinity was quantitatively estimated based on the relationship between the peak area and the total area of diffraction patterns [35, 36]. The Origin software version 7.0 (Microcal Inc., Northampton, MA, USA) was used for integration of the areas.
Pasting properties
Pasting profile of starches was analyzed on a rheometer (TA Instruments, Discovery HR-2 Hybrid; New Castle, USA) equipped with a starch pasting cell (Smart Swap™, SPC 110533; New Castle, USA) according to Ramírez-Hernández et al. [37]. A starch dispersion (10% w/v) was prepared with distilled water. Three cycles of scanning were used (heating-isotherm-cooling). The temperature was conditionate by 60 s at 30 °C and increases at 15 °C min−1 to 90 °C, where it was maintained for 360 s and then allowed to cool to 30 °C at 30 °C min−1. Trios’ software version 4 (TA Instruments; New Castle, USA) to obtain the results was used.
Thermal properties
Thermal properties of the starches were evaluated by differential scanning calorimetry (DSC) according to Paredes-López et al. [38]. The analyses were performed with a DSC250 calorimeter (TA Instruments, Discovery DSC250; New Castle, USA). A sample of approximately 2 mg was inserted into an aluminum pan cell, and then 7 μL of deionized water were added. The pan was hermetically sealed and allowed to equilibrate for 1 h before analysis. The sample was subjected to a heating program from 30 to 140 °C and at a rate of 10 °C min−1. The onset temperature (\({T}_{O}\)), peak temperature (\({T}_{P}\)), conclusion temperature (\({T}_{C}\)) and gelatinization enthalpy (\(\Delta H\)) were determined using the Trios software version 4 (TA Instruments; New Castle, USA). The experiments were performed under nitrogen flow (50 cm3 min−1). An empty pan was used as a reference material.
Water solubility and swelling power
Water solubility and swelling power patterns at 60, 70, 80 and 90 °C were determined according to Pérez-Pacheco et al. [5]. Briefly, weighted starch samples (\({W}_{0}\)) were suspended in deionized water to prepare a 1% starch suspension (w/v). The suspensions were magnetically stirred at a constant temperature (60, 70, 80 or 90 °C) in a water bath for 30 min. The suspensions were then centrifuged at 4000×g for 10 min. The supernatants were decanted carefully, and weight of residues (\({W}_{1}\), gel) were recorded. Subsequently, the supernatants were dried until constant weight (\({W}_{2}\), soluble solids) in an air convection oven (Shel Lab, Mod. 1425; Cornelius, USA) at 120 °C. Water solubility (\(WS\)) and swelling power (\(SP\)) were calculated with Eqs. (1) and (2), respectively.
Refrigeration and freezing stability
The 5% (w/v) starch suspensions were prepared in previously tared centrifuge tubes (\({W}_{ct}\)). The suspensions were magnetically stirred at 85 °C for 30 min. Subsequently, the samples were tempered at room temperature and then stored at 4.4 °C and − 12 °C. After 24 h, the samples were tempered at 25 °C and the weight were recorded (\({W}_{gct}\)). The samples were centrifuged at 8000×g for 10 min. The supernatants were decanted carefully and weighted (\({W}_{sw})\). The water separation from the starch gels at 48, 72, 96 and 120 h also were carried out. The syneresis rate was calculated with Eq. (3) [39].
Preparation of nanoparticles by spray drying
The native starch from black bean was evaluated as wall material for nano-encapsulation of ascorbic acid. An aqueous feed mixture at 0.6% (w/w) of solids content was prepared. 4 mg of starch per 1 mg de ascorbic acid were used (4:1 ratio). The resulting mixture was magnetically stirred at 25 °C for 20 min. The feed mixture (50 g) was magnetically stirred and kept in an ice-bath throughout the spray drying process. Encapsulation was carried out using the Nano Spray Dryer B-90 (Büchi Labortechnik AG, Flawil, Switzerland) which was operated according to recommendations of work previous [19, 21, 22]. Nozzle with 7.0 μm spray-mesh was used. The inlet temperature was set at 80 °C based on preliminary trials and kept constant throughout the process. The air flow rate was 150 L min−1. The relative spray rate was 100% (140 kHz). The feed mixture was fed into the system with a peristaltic pump at level 3. The resulting outlet temperature, under afore mentioned conditions, varied between 38 and 40 °C. The experiment was carried out in triplicate. The powders obtained were kept in Eppendorf tubes (2 mL) that were stored in a desiccator at room temperature (24 °C) in the dark until analysis. Process yield (%) was calculated as the relation between the total recovered product mass and the total solids of the feed mixture [40].
Characterization of nanoparticles
Water activity
Water activity (\({a}_{w}\)) of spray-dried powders was determined using an AquaLab Vapor Sorption Analyzer (Decagon Devices, Inc., AquaLab VSA; Pullman, WA, USA) at 25 °C [41].
Morphological analysis and particle size distribution
Morphology and the particle size distribution of the spray dried particles were analyzed according to Sects. 2.4.2 and 2.4.3, respectively,
Ascorbic acid content and encapsulation efficiency
The ascorbic acid content and the encapsulation efficiency were evaluated according to Leyva-López et al. [41] and Hoyos-Leyva et al. [12], respectively. The dried particles (200 mg) were suspended in distilled water (25 mL) and gently stirred for 15 s to ensure the integrity of the capsules and wash the superficial ascorbic acid (\({AA}_{S}\)). Then, 100 µL of the mixture were transferred to a 10 mL flask, made up with the diluent (4 mL of sulfuric acid, 120 mL of acetone, 876 mL of Milli-Q water), and filtered. For analysis of ascorbic acid, a chromatographic system (Waters, Acquity Arc UHPLC; Massachusetts, USA), equipped with a quaternary pump, a degasser, an auto-sampler, a column oven, and a diode array detector (Waters, Acquity UPLC PDA; Massachusetts, USA), was used. The filtrate was injected into a Luna C18 column (2) 5 µm (4.6 mm internal diameter × 250 mm long), Phenomenex. The samples were conditioned at 28 °C before being injected into the column. The rate flow of the filtrate was 1 mL min−1; it was mixed with sulfuric acid at 0.01% (w/v) as a thinner. The quantification of ascorbic acid was carried out at a wavelength of 245 nm. The calibration curve for ascorbic acid was performed in the range of 10 to 50 ppm. The same procedure was performed to determine the total ascorbic acid (\({AA}_{T}\)); the only difference was that the mixture was brought to sonication (Bandelin, SONOPULS HD 3200; Berlin, Germany) for 10 min at 15 °C (power, 200 W; frequency, 45 kHz) to ensure the total rupture of the capsules. The experiments were performed in triplicates. To determinate the encapsulation efficiency (\(EE\)), the \({AA}_{S}\) and the \({AA}_{T}\) were correlated according to the Eq. (4) [12]. It is expected that \({AA}_{S}\le {AA}_{T}\) since some ascorbic acid could is not captured into the nano-capsules, but only on the surface. In general, ascorbic acid captured into the capsules is protected to degradation by environmental conditions.
Statistical analysis
Statistical analysis was performed by one-way ANOVA to verifying significant differences in the properties between a commercial corn starch and the black bean starch, followed by Tukey’s pairwise test to compare the means. Differences were considered to be statistically significant at a value of probability less than 5% (p < 0.05). The results are given as mean ± standard error. The statistical analysis was carried out with the Minitab 18 statistics package (Minitab Inc., State College, PA, USA).
Results and discussion
Characterization of black bean starch
Starch isolation yield
Extraction yield of black bean starch was 50.94% in dry basis (db), thus 1 kg of dry solids of black bean can produce 509.4 g of starch approximately. This value is lower than extraction yield of starch from rice (75.8%), wheat (71.9%) and corn (79.5%) [42]; but was within the range of starch content (27–60%) reported for common beans flours [31]. The yield value was higher than those obtained by Hoover and Ratnayake [3] (20.1–22.2%) and Ramírez-Jiménez et al. [31] (45.57%) for black bean starch. Roy et al. [1] suggest that the extraction method can affect the extraction yield of starch.
Chemical composition
Table 1 shown the chemical composition of native starches from black bean and corn. The moisture content of black bean starch was accepted range for powdered dry products (< 15%) and is actually lower than the suggested (< 20%) for the other conventional sources [43]. The component principal of black bean starch was the starch content (81.21 ± 1.43 g 100 g−1 db) and the value did not show significant difference with the corn starch. However, the black bean starch had high protein (9.6 ± 0.01 g 100 g−1 db) and ash (1.16 ± 0.03 g 100 g−1 db) content, which values are similar than corn starch. These components in legume starches are present due to the difficulty of separating highly hydratable fine fiber during the wet-isolation process, and the strong adherence of insoluble protein to the starch granules [3, 28]. Neeraj et al. [6] reported that in isolation process of potato starch with cold water retained higher protein content than alkaline steeping method, since NaOH is considered as a good solvent, and it can solubilize the major protein encapsulating the starch. Whereas the fat content (0.46 ± 0.05 g 100 g−1 db) in black bean starch was low. This is consistent with the low-fat content commonly found in the starch granules of tubers and legumes [44]. Similar fat contents were reported for starches from Phaseolus vulgaris (0.20–0.40% db) [3] and Phaseolus lunatus (0.54% db) [28]. Further, the chemical composition of starches could be affected by botanical type of sources, climatic and agronomic conditions, and on the process of harvesting and isolation process [6, 43].
Amylose content has an influence on the functional and physicochemical properties of starch, such as pasting, gelling, retrogradation, and initial gel hardness of cooked starch [1, 9, 30]. The apparent amylose content of black bean starch was lower than of corn starch and in consequence, the amylopectin content was higher in black bean starch (Table 1). The apparent amylose content (7.07 ± 0.91%) was much lower than those of the other legume starches (20.7–30.1%) [36] and common bean starches (27.0–45.4%) [24, 26]. According to Singh et al. [44] the amylose content of black bean studied could be classified as very low range (5–12%), so this starch cannot be used to development a biodegradable packaging film, since an amylose content between ~ 26 and 31% is required to film-forming capacity [11]. The amylose content of starch granules varies depending on the botanical source of starch and is affected by climatic conditions and soil type during growth [30, 43, 44].
Typically, when compared with cereals (corn, wheat, rice), beans show considerable higher amounts of resistant starch (RS), which may be associated with amylose contents, amylopectin branch chain length, degree of crystallinity and size of the starch granules [25]. Table 1 shown that the RS content of raw black bean starch was higher than of raw corn starch. However, the RS content in black bean starch (2.92 ± 0.13%) studied was much lower than in other common bean starches (72.1–78.3%) [24] and cooked legume starches (8.0–10.7%) [30]. Several reports have suggested that the low in vitro digestibility of legume starches and products might be due to the high amylose content, absence of surface pores on the granules, large amounts of B type crystallites, strong interactions between amylose chains, intact cell structures enclosing starch granules and large amounts of soluble dietary fibers [8, 24, 28]. The exact underlying mechanism of RS of starch granules is complicated because those factors are often interconnected [8, 24].
Morphological properties
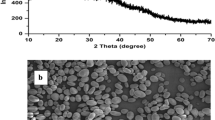
Morphological properties of the starch granules from black bean are shown in Fig. 1. Typically, the bean starches are oval and round, with smooth surface and indentations [24, 26, 29, 32]. However, the starch granules from black bean in the present work presented polyhedral shape and some of them have rounded shape (Fig. 1a–c), which is characteristic of some starches, such as rice, waxy rice, oats, and wrinkled pea [45]. In general, the different morphologies of starches might be attributed to biological origin, amyloplast biochemistry, genotype, and plant physiology [27, 30]. In the cases cited, more than one granule is produced simultaneously in a single amyloplast. The shapes of these starch granules are mostly polyhedral, possibly because of space constraints during the development of starch granules [44, 45]. By SEM (Fig. 1c) starch granules from black bean appeared to be smooth with no evidence of any fissure or rupture. Optical microscopy (bright field and polarized-light) not allowed an observation of granule details including Maltese cross in native starch from black bean (Fig. 1a, b), probably due to granule size and equipment capacity. The particle size distribution (PSD) of starch granules from black bean is presented in Fig. 1d. The black bean starch granules in the present work displayed a bimodal PSD with a main peak at 6.3 µm and a second peak at ~ 1 µm. The highest size obtained stemmed from the presence of agglomerated starch granules, as was observed by optical microscopy (Fig. 1a, b). Maniglia and Tapia-Blácido [46] suggest that starch granules may be trapped within high content of proteins, to form agglomerates with this component. As was showed in Table 1, proteins remained in the isolated starch at 9.6 ± 0.01 g 100 g−1 db. Despite thus, these values were much lower than the average granule diameters of legume starch granules (17.8–27.8 µm) [30, 36] and other bean starches (19.1–33.5 µm) [24, 26, 29]. The diversity in the size of starch granules is a biologically controlled processes, yet not fully understood [29]. It has been suggested that the difference in the structure and quantity of the amylose and amylopectin play a major role in governing the size and shape of starch granule size. Variance in the activity of starch synthase enzymes during grown could be affect the size and shape of the starch granules [30, 43]. It has been reported that small granules tend to have less amylose content [30, 32]. In the present work, the black bean starch showed lower amylose content (Table 1) than other common bean starches as was previously discussed, and so may also support this statement.
X-ray diffraction (XRD) pattern and pasting profile
XRD pattern, crystallinity level and pasting profile of the black bean starch are shown in Fig. 2. Commonly, starch granules with a semi-crystalline structure resultant to diverse polymorphic forms, which are categorized into A, B, and C types, based on their characteristic and distinct XRD patterns [43]. Characteristic peaks of A-type starch were observed in the XRD pattern of black bean starch (2θ around 15°, 17° and 23°), which was same diffraction pattern than of corn starch (Fig. 2a) and is typical of cereal starches [44]. The XRD patterns of starches from beans and legumes have been reported earlier defined to be of the C-type, a mixture of A and B types [3, 29]. Hoover and Ratnayake [3] also reported that black bean starch showed no evidence of a peak at 5.2–5.6, characteristic of B-type starches. However, Zhao et al. [47] reported a diffraction pattern similar to that found in this study (Type A) for mung bean starch. The differences found in the type of crystal of starches is determined by the structural characteristics of amylopectin such as length of the short chains, degree of branching, packing of the double helices [48], that is, the polymorphism (type A, B and C) of starch crystals depend on the organization of amylopectin and amylose within the granule [49] and development conditions and genotype [43]. The relative crystallinity of black bean starch was measured from XRD pattern and was higher (43%) than of corn starch (41%, Fig. 2a) and other starches from legumes (17.0–25.5%) [36] and beans (27.9–35.1%) [29, 43]. It has been specified that the starches with low amylose content exhibit more crystallinity [43], which is consistent with the results obtained in the present work (Table 1). In general, crystallinity of starches is predominantly affected by various factors such as quantity and chain length of amylopectin, moisture content of starches, orientation of the double helices within the crystalline domains and crystallite size [3, 43].
Pasting profile of the black bean starch is shown in Fig. 2b with a peak viscosity (PV) of 1981.2 cP and a final viscosity (FV) of 1753.1 cP. PV is defined as the equilibrium point between swelling and solubility (increases viscosity) and the fracture and placement of polymer chains (reduces viscosity). After reaching the peak viscosity, with increasing temperature and passing the gelatinization temperature, the phenomenon of pasting occurs, which is accompanied by complete destruction of the granular structure and the release of water from the granules to the surrounding environment, resulting in decreased viscosity. During cooling, the starch molecules, especially amylose, are reorganized, and water is trapped inside the starch chains. As a result, viscosity increases due to gel formation, known as the FV [7]. Information about the viscosity during heating contributes to understanding the cooking processes involving starchy products, including soups, sauces, etc., as well as the equipment design and the amount of water required for the process [50]. In the present work the PV value was highest in black bean starch than corn starch (PV, 1750 cP). Moreover, the pasting starts in black bean starch at same time than corn starch (80 °C, 4.5 min), i.e., both starches requires similar cooking time to gelatinize. Starch granules with smaller granule size and lower amylose require less energy to disrupt the polymer interactions within the granules, which facilitate granule swelling and starch pasting [32]. In fact, a small particle size and low amylose content in black bean starch were observed (Fig. 1d and Table 1). Despite thus, the PV temperature (80 °C) indicate that the inclusion of black bean starch studied in products subjected to pasteurization can be feasible, or when gelatinization is not desirable. The pasting profile of black bean starch (Fig. 2b) not showed a typical profile of starches from legumes [3, 30] and other beans [24, 26, 28], which are considered as starches with high amylose content and therefore present more restricted swelling and their granular structure is resistant to mechanical fragmentation. Hence, not shown a clear peak viscosity or breakdowns and gradually increasing viscosities during the holding periods, inclusive sometimes display final viscosity higher than peak viscosity [32]. Amylose content has a significant effect on functional and physicochemical properties, including pasting profile [7, 24]. Typically, the peak viscosity of starch pastes decreases with increasing amylose content along the whole range of amylose content from waxy to high amylose varieties. In contrast, final viscosity increases with increasing amylose content up to a threshold amylose-to-amylopectin ratio, above which final viscosity decreases slightly [7, 51]. Moreover, junction zone formation can be either facilitated or hindered by the presence of other components as lipids, proteins, sugars and acids [2]. Therefore, the high protein content in black bean starch (Table 1) also may influence on the pasting profile (Fig. 2b).
Gelatinization properties
The gelatinization temperatures (\({T}_{O}\), \({T}_{P}\) and \({T}_{C}\)) and gelatinization enthalpy (\(\Delta H\)), for starches from black bean and corn, measured by DSC are presented in Table 2. The significantly highest gelatinization temperatures were observed for black bean starch. As shown in Table 2, the \({T}_{P}\) of corn and black bean starches were found to be 74.62 ± 0.48 °C and 81.24 ± 0.07 °C, respectively. The gelatinization temperature for black bean starch was also higher than those reported for legumes (64.7–76.1 °C) [3, 30] and other bean starches (63.1–76.2 °C) [24, 26, 28, 29, 43]. High gelatinization temperatures indicate that considerable amount of energy is required for starch gelatinization [30]. In general, gelatinization temperatures has been positively correlated with long branch chains of amylopectin, due to higher temperatures being required for dissociation of longer double helices [29]. The gelatinization temperature also can be increased due to the high content of minerals present in the black bean starch (Table 1), since they interact with its structure and make it thermally stable [52, 53]. Some researchers also suggested that the proteins, present in high amounts in black bean starch (Table 1), may increase the gelatinization temperature due to that provide a protective effect and prevent the entrance of water to the starch granules [4].
Gelatinization enthalpy (\(\Delta H\)) gives an overall measure of crystallinity (quality and quantity) and is an indicator of the loss of molecular order within the granule which arises in the starches during gelatinization [43, 44]. The \(\Delta H\) in black bean starch (14.24 ± 0.36 J g−1) was the higher than of corn starch (12.19 ± 0.39 J g−1) (Table 2). Du et al. [24] identified the same behavior when they compared the \(\Delta H\) values between common bean starches (13.1–14.9 J g−1) and corn starch (12.8 J g−1). The gelatinization enthalpy change has been reported to be related to characteristics of the starch granule such as degree of crystallinity and particle size [24, 43]. High amylopectin starch is more stable in structure but more difficult to gelatinize due to the requirement of higher gelatinization starting energy [42]. In this study, the black bean starch presented the significant highest amylopectin content, highest relative crystallinity and presented the higher protein content (Fig. 2a and Table 1), which could favorer the presence of agglomerated starch granules (Fig. 1a, b), so could be required more energy to affect the structure of black bean starch granules. In general, the gelatinization properties of starch are related to a variety of factors including the molecular structure of amylopectin (perfection and ordering of amylopectin crystallites, length of the external “A” chains of amylopectin, extent of branching, molecular weight and polydispersity), starch composition (amylose/amylopectin ratio, lipid complexed amylose chains) and granule architecture (crystalline to amorphous ratio) [3].
Water solubility and swelling power
The water solubility and swelling power can be used to measure the interaction between starch chains, within the amorphous and crystalline regions of the starch granule [43]. The water solubility and swelling power of black bean and corn starches are presented in Fig. 3. Based on the behavior of starch swelling, it can be classified in to three types (1) rapid swelling (e.g. waxy maize, potato starch) (2) slow swelling that can be converted to rapid swelling by extracting surface protein and lipid fraction (3) limited swelling (it will not affect lipid/protein fractions) [27]. Black bean starch showed a slow swelling behavior according to it high protein content. For both starches evaluated, the granules did not swell and soluble significantly at temperatures between 60 and 70 °C. However, at temperatures above 70 °C, they swelled and solubilized rapidly, due to the breaking of intermolecular hydrogen bonds in amorphous areas, thus permitting irreversible and progressive water absorption, as was reported by Betancur-Ancona et al. [28] for Phaseolus lunatus, Pelissari et al. [4] for ‘‘Terra’’ banana starch and Reddy et al. [43] for Vigna angularis L. and Pueraria thomsonii Benth. This behavior could be due to both high protein content and gelatinization temperature (Tables 1, 2); therefore, these results are correlated with the pasting profile and the thermal properties above described. From the results (Fig. 3) can be observed that the swelling power and water solubility were higher in black bean starch as compared to corn starch. It could be possible due to low amylose content and subsequently higher amylopectin in the black bean starch (Table 1) which is predominantly responsible for swelling [27, 43]. The greater the amylose content, the more compact the starch granules, making it more difficult for amylose to escape from the granules which favors lower solubility and swelling power values [4, 51]. In this study, the corn starch had an amylose content higher than of black bean starch (Table 1), in agreement with the results of solubility and swelling power (Fig. 3).
Refrigeration and freeze–thaw stability
The stability under refrigeration and freeze–thaw of black bean starch gel, measured as percentage of syneresis (water exuded), was determined after the 1st–5th cycles, and shown in Fig. 4. The results showed high syneresis in the 1st cycle under freeze–thaw; however, above 2th cycle starch did not present greater syneresis. The syneresis of black bean starch gel was not changed significantly during the refrigeration cycles. This information is important to maintain the sensory quality of refrigerated and frozen products [3, 50, 54]. These results are different from those reported by Hoover and Ratnayake [3] and Pérez-Pacheco et al. [5]. Hoover and Ratnayake [3] reported an increase in the syneresis values (66–72%) of black bean starch gel for five freeze–thaw cycles. Pérez-Pacheco et al. [5] also observed an increase of the syneresis for corn and Brosimum alicastrum seeds starches under refrigeration (56.00–73.67%; 76.33–80.00%) and freeze–thaw (70.33–78.00%; 72.33–76.33%), where Brosimum alicastrum seeds starch showed the highest syneresis values under refrigeration. The results of the present study suggest that the black bean starch gel is more stable than the samples cited under refrigeration and freeze–thaw (Fig. 4). Srichuwong et al. [54] evaluated native starches from twenty-six botanical sources and they reported that the syneresis was not observed for starch gels of cassava, normal and waxy japonica rice up to the 1st, 3rd and 5th cycle, respectively. Srichuwong et al. [54] suggest that the gels prepared from starches with relatively high distribution of amylopectin branch chains with degree of polymerization 6–12 (APC ratio: 0.432–0.457) and small apparent amylose content (AAC: 0–13%) are more resistant to syneresis than the others. In the present study the black bean starch showed a low apparent amylose content (Table 1), which support the behavior observed under refrigeration and freeze–thaw stability (Fig. 4).
Evaluation of black bean starch as wall material
One of the most recent trends in encapsulation of bioactive food ingredients is nanoencapsulation [17]. Nanoencapsulation techniques could be effective candidates to enhance bioactivity and bioaccessibility of bioactive compounds as well as to increase their storage stability against harsh treatments due to nano size of particles. Also, this approach could provide proper absorption and bioavailability of nanoencapsulated ingredients [18, 23]. Thus, researchers need special spray-drying technologies and wall materials to produce powder nanoparticles from the feed. The Büchi Company in Switzerland introduced the first nano-spray dryer in 2009 to extend spray drying to the submicron scale. Its technological novelty lies in the gentle flow of laminar drying gas, the vibrating mesh spray technology to form fine droplets, and the highly efficient electrostatic precipitator to collect nanoparticles. However, the equipment requires feed mixtures at low solid concentration, with maximum viscosity of 10 cP and maximum particle size of 7 µm [21]. From morphological characterization (Fig. 1d) and pasting profile analysis (Fig. 2b), it was concluded that the present black bean starch studied showed these properties. For these reasons in the present study was decided to evaluate the potential application of black bean starch as wall material during nano spray drying. Ascorbic acid was used as bioactive compound (core material) because is a thermolabile compound and is used commonly as a “marker” to evaluate the effect on the chemical compounds from food under high-temperature treatments [55].
Water activity and morphological properties
Water activity (\({a}_{w}\)) has long been considered as an important index for spray dried powders due to its effects on the microbiological safety and physicochemical stability [56, 57]. It can be observed in Table 3 that spray dried particles with black bean starch presented a \({a}_{w}\) above 0.6, which is higher than the safety limit of 0.5, this maximum value is desired to avoid microbial growth and to assure the physicochemical stability of the capsules [56]. Increasing \({a}_{w}\) indicates increasing amount of free water available for deteriorative reactions, thus shortens the shelf-life and increases the possibility of a capsules collapse during storage [56, 57]. This result suggests that the spray drying conditions proposed were not feasible to obtain particles sufficiently dried. During nano spray drying the \({a}_{w}\) of the particles could be controlled principally by the drying gas flow rate, drying gas humidity and the type of solvent used in the feed mixture, but also may be considered the inlet temperature, spray rate intensity and the solids concentration [22]. Furthermore, has been reported that the moisture content in biofilms based in starch depend on the botanical source and the interaction with the bioactive compounds. The addition of the bioactive compounds may decrease the intra- and intermolecular interactions between starch macromolecules facilitating water adsorption from surrounding [10, 11].
SEM image that details the particle shape and surface aspect of spray dried particles with black bean starch is showed in Fig. 5a. In general, the external surfaces of the particles have continuous walls with no fissures, cracks, or interruptions, which suggest that starch granules were not damage during the spray drying process. In general, has been reported that with conventional spray drying, the granular structure of native starches remained almost unchanged [13]. The result obtained in the present work demonstrates the capacity of the nano spray drying process to preserve the integrity of native starch. Both irregular and spherical aggregates particles were observed in the SEM image (Fig. 5a). The results of the present study showed that black bean starch has small granules and high protein content (Fig. 1d and Table 1). These properties in starches have been reported as useful for encapsulation purposes by conventional spray drying of bioactive compounds [12] and probiotics [14] due to that favor the formation of spherical aggregates [13]. However, a largest number of agglomerated particles in form irregular were also observed (Fig. 5a). This suggests high cohesion between particles, which would be favored by small particles and in consequence by higher contact area. The high \({a}_{w}\) value obtained also would increase the formation of agglomerated and collapsed particles. Figure 5b shown the PSD of spray dried particles with black bean starch and is correlated with the observed by SEM (Fig. 5a). A higher volume of particles at ~ 2.5 µm was observed in spray dried particles than of black bean native starch (Fig. 1d). This could indicate the redistribution of the agglomerated native starch granules of 6.3 µm to spherical aggregates of ~ 2.5 µm during nano spray drying. However, this small size in spray dried particles with the high \({a}_{w}\) value allow the formation of agglomerated irregular with a size of ~ 80 µm (Fig. 5b) as was also observed by SEM (Fig. 5a). Without the presence of agglomerated irregular, the PSD obtained in the present work (1.0–2.5 µm) is in the range of particles obtained by nano spray drying (0.2 to 16 µm) previously reported [20, 22]. In general, during nano spray drying the particle size could be controlled principally by the spray mesh size and solid concentration (viscosity), and in less proportion by the inlet temperature, spray rate intensity, circulation pump rate, surfactant/stabilizer in feed and type of solvent [22].
Process yield and encapsulation efficiency
Process yield is an important indicator for the industry since higher yield means more benefit [57]. The process yield obtained is shown in Table 3 and was not feasible, because a successful spray drying process must have yield higher than 50% [40, 57]. The presence of an important protein fraction (~ 5–7% relative to starch) into starch-based feed mixtures can led to flocculation, which hampers the operation of the nozzle and the structure of spherical aggregates. Indeed, positively charged proteins would form complex coacervates with negatively charged starch chains, which are unstable under aqueous conditions and precipitate under aggregation effects [12]. These factors could affect the process yield, as the result obtained with black bean starch suggest has a high protein content (Table 1), so a fouling on spray-mesh can be carried out during nano spray drying and could avoid the continuous atomization and then affect the process yield. During nano spray drying, variations in the yield may occur due to particle depositions around the spray cap and the chamber walls, nozzle blockage, or due to losses during the manual collection of the powder with a rubber spatula [22]. In microencapsulation by conventional spray drying the proteins are efficient to increase product yield in low concentration (< 5% w/w) into the fed solids [40, 57, 58]. Therefore, a reduction in the protein content of black bean starch could be interesting to evaluate the effect on the process yield in nano spray drying. In general, the process yields in conventional spray drying depends on the operating conditions such as inlet and outlet air temperatures, feed flow rate, atomization speed or pressure, feed concentration, carrier types, and feed to carrier ratio [40, 58]. While that in nano spray drying, also the surfactant/stabilizer in feed and solvent instead of water into fed mixture [21, 22] could be studied.
The encapsulation efficiency of ascorbic acid with black bean starch was above 30% (Table 3), this value was higher than reported by Hoyos-Leyva et al. [12] for ascorbic acid microcapsules obtained with taro starch (20.9 ± 0.30%) by conventional spray drying (Tin, 145 °C; Tout, 80 °C; starch:compound ratio, 10:1), despite that in the present work the starch:compound ratio was lower (4:1). When the coating material concentration is increase in the feed mixture, more material is available for the encapsulation, and hence higher encapsulation percentage could be obtained [16]. However the relatively low value of encapsulation efficiency obtained by Hoyos-Leyva et al. [12] was attributed to the porous structure of taro starch spherical aggregates. Pores with relative high size permit the easy solvent flow across microcapsules, resulting in the rapid extraction of ascorbic acid retained into spherical aggregates. Pores in the particles obtained in the present work were not observed by SEM. The uniform spheres are desirable for the stability of the encapsulated compounds and for their controlled release; the more intact and regular the microsphere wall, better has been the encapsulation process [59]. Spherical aggregates more sealed with black bean starch than taro starch may be obtained, which avoid the thermal degradation of ascorbic acid during spray drying and allow a less release in water dispersion. The high protein content showed an effect on the process yield as was discussed above, the fouling on the spray mesh also could affect the droplet generation and in consequence the nanocapsules formation. The nano spray drying conditions evaluated may also affect the encapsulation efficiency, therefore a design of experiments should be carried out to determine the working conditions that allow both maximum encapsulation efficiency and maximum process yield using black bean starch as wall material during the nano spray drying.
Conclusions
The results suggest that the properties of starch from black bean were affected principally by the amylose content and the high protein content, which were not into the range of other legume starches previously studied. The present black bean starch may have numerous applications as an ingredient in food systems and other industrial applications, e.g., the native starch could be used in food systems requiring high processing temperatures, and the starch gel could be used under refrigeration and freezing. The characteristics of the black bean starch, principally the viscosity profile and the presence of protein and small particles, suggests on their application as wall material in nano-encapsulation purposes and therefore was assessed in this study. The nano spray drying conditions used produced spherical aggregates of black bean starch, evidenced by SEM, and allowed encapsulate a thermolabile compound (l-ascorbic acid), which is used as marker during the evaluation of high-temperature processes. Spherical aggregates of black bean starch can be an alternative for micro or nano-encapsulation of substances used in the food and pharmaceutical industries. More studies are necessary to determine the conditions of nano spray drying that allow both maximum yield and the maximum encapsulation efficiency. Further investigations also could be carried out to evaluate the physicochemical stability of the dried particles and the controlled and gradual release of bioactive compounds from nano-capsules obtained with this black bean starch by spray drying. Overall, the micro or nanoencapsulation of bioactive compounds with the black bean starch evaluated is an alternative for the future formulation of functional foods.
Data availability
Not applicable.
Code availability
Not applicable.
References
K. Roy, R. Thory, A. Sinhmar, A.K. Pathera, V. Nain, Int. J. Biol. Macromol. (2020). https://doi.org/10.1016/j.ijbiomac.2019.12.113
J.N. BeMiller. in: Carbohydrate Chemistry for Food Scientists, ed. by J.N. BeMiller (AACC International Press, 2019) pp. 159–189 https://doi.org/10.1016/B978-0-12-812069-9.00006-6
R. Hoover, W.S. Ratnayake, Food Chem. 78, 4 (2002). https://doi.org/10.1016/S0308-8146(02)00163-2
F.M. Pelissari, M.M. Andrade-Mahecha, P.J.d.A. Sobral, F.C. Menegalli, Starch - Stärke 64, 5 (2012) https://doi.org/10.1002/star.201100133
E. Pérez-Pacheco, V.M. Moo-Huchin, R.J. Estrada-León, A. Ortiz-Fernández, L.H. May-Hernández, C.R. Ríos-Soberanis, D. Betancur-Ancona, Carbohydr. Polym. (2014). https://doi.org/10.1016/j.carbpol.2013.10.012
Neeraj, S. Siddiqui, N. Dalal, A. Srivastva, A.K. Pathera, J. Food Meas. Charact. 15, 3 (2021). https://doi.org/10.1007/s11694-021-00862-5
N. Javadian, A. Mohammadi Nafchi, M. Bolandi, Food Sci. Nutr. (2021). https://doi.org/10.1002/fsn3.2506
D. Jeong, J.-A. Han, Q. Liu, H.-J. Chung, Food Hydrocoll. (2019). https://doi.org/10.1016/j.foodhyd.2018.12.039
R.L. Zapata-Luna, T. Ayora-Talavera, N. Pacheco, E. García-Márquez, H. Espinosa-Andrews, Á. Ku-González, J. Ruiz-Ruiz, J.C. Cuevas-Bernardino, J. Food Meas. Charact. 15, 2 (2021). https://doi.org/10.1007/s11694-020-00739-z
S. Ekramian, H. Abbaspour, B. Roudi, L. Amjad, A. Mohammadi Nafchi, J. Food Meas. Charact. 15, 4 (2021). https://doi.org/10.1007/s11694-021-00895-w
L. Xue Mei, A. Mohammadi Nafchi, F. Ghasemipour, A. Mat Easa, S. Jafarzadeh, A.A. Al-Hassan, Int. J. Biol. Macromol. (2020). https://doi.org/10.1016/j.ijbiomac.2020.09.082
J.D. Hoyos-Leyva, A. Chavez-Salazar, F. Castellanos-Galeano, L.A. Bello-Pérez, J. Álvarez-Ramírez, Food Hydrocoll. (2018). https://doi.org/10.1016/j.foodhyd.2018.05.002
J.D. Hoyos-Leyva, L.A. Bello-Pérez, J. Álvarez-Ramírez, H.S. García, Food Rev. Int. 34, 2 (2018). https://doi.org/10.1080/87559129.2016.1261298
O. Alfaro-Galarza, E.O. López-Villegas, N. Rivero-Perez, D. Tapia-Maruri, A.R. Jiménez-Aparicio, H.M. Palma-Rodríguez, A. Vargas-Torres, LWT-Food Sci. Technol. (2020). https://doi.org/10.1016/j.lwt.2019.108686
T.J. Gutiérrez. in: Handbook of Nanomaterials for Industrial Applications, ed. by C. Mustansar Hussain (Elsevier, 2018) pp. 989–1011 https://doi.org/10.1016/B978-0-12-813351-4.00057-2
S. Goyal, S.K. Sonawane, N. Nachal, S.S. Arya, J. Food Meas. Charact. 14, 6 (2020). https://doi.org/10.1007/s11694-020-00599-7
E. Assadpour, S.M. Jafari, Annu. Rev. Food Sci. 10, 1 (2019). https://doi.org/10.1146/annurev-food-032818-121641
F. Garavand, S. Rahaee, N. Vahedikia, S.M. Jafari, Trends Food Sci. Tech. (2019). https://doi.org/10.1016/j.tifs.2019.05.005
X. Li, N. Anton, C. Arpagaus, F. Belleteix, T.F. Vandamme, J. Control. Release 147, 2 (2010). https://doi.org/10.1016/j.jconrel.2010.07.113
S.H. Lee, D. Heng, W.K. Ng, H.-K. Chan, R.B.H. Tan, Int. J. Pharm. 403, 1 (2011). https://doi.org/10.1016/j.ijpharm.2010.10.012
C. Arpagaus, P. John, A. Collenberg, D. Rütti. in: Nanoencapsulation Technologies for the Food and Nutraceutical Industries, ed. by S.M. Jafari (Academic Press 2017) pp. 346–401. https://doi.org/10.1016/B978-0-12-809436-5.00010-0
C. Arpagaus, A. Collenberg, D. Rütti, E. Assadpour, S.M. Jafari, Int. J. Pharm. 546, 1 (2018). https://doi.org/10.1016/j.ijpharm.2018.05.037
Q. Sun. in: Starch in Food, ed. by M. Sjöö, L. Nilsson (Woodhead Publishing, Sawston, Cambridge, 2018) pp. 691–745. https://doi.org/10.1016/B978-0-08-100868-3.00018-4
S.-K. Du, H. Jiang, Y. Ai, J.-L. Jane, Carbohydr. Polym. (2014). https://doi.org/10.1016/j.carbpol.2014.03.004
F.G.B. Los, A.A.F. Zielinski, J.P. Wojeicchowski, A. Nogueira, I.M. Demiate, Curr. Opin. Food Sci. (2018). https://doi.org/10.1016/j.cofs.2018.01.010
I.M. Demiate, A.M. Figueroa, M.E.B. Zortéa Guidolin, T.P. Rodrigues dos Santos, H. Yangcheng, F. Chang, J.-l. Jane, Food Hydrocoll. (2016) https://doi.org/10.1016/j.foodhyd.2016.07.014
C. Sudheesh, K.V. Sunooj, J. George, S. Kumar, V.A. Sajeevkumar, . Food Meas. Charact. 13, 2 (2019). https://doi.org/10.1007/s11694-018-0016-x
D. Betancur-Ancona, J. López-Luna, L. Chel-Guerrero, Food Chem. 82, 2 (2003). https://doi.org/10.1016/S0308-8146(02)00515-0
P. Li, S. Dhital, B. Zhang, X. He, X. Fu, Q. Huang, Food Hydrocoll. (2018). https://doi.org/10.1016/j.foodhyd.2018.07.007
M. Ma, Y. Wang, M. Wang, J.-L. Jane, S.-K. Du, Food Hydrocoll. (2017). https://doi.org/10.1016/j.foodhyd.2016.09.004
A.K. Ramírez-Jiménez, R. Reynoso-Camacho, S. Mendoza-Díaz, G. Loarca-Piña, Food Chem. (2014). https://doi.org/10.1016/j.foodchem.2014.04.008
H.M. Romero, Y. Zhang, J. Agric. Food Res. (2019). https://doi.org/10.1016/j.jafr.2019.100001
E. Flores-Gorosquera, F.J. García-Suárez, E. Flores-Huicochea, M.C. Núñez-Santiago, R.A. González-Soto, L.A. Bello-Pérez, Acta Cient. Venez. 55, 1 (2004)
AACCI, Approved Methods of Analysis, 10th edn. (American Association of Cereal Chemists International, St. Paul, MN, USA, 2000)
J.D. Hoyos-Leyva, L.A. Bello-Pérez, H. Yee-Madeira, M.E. Rodriguez-Garcia, A. Aguirre-Cruz, Starch - Stärke 69, 1600370 (2017). https://doi.org/10.1002/star.201600370
M.E.B. Zortéa-Guidolin, I.M. Demiate, R.C.B.d. Godoy, A.d.P. Scheer, D. Grewell, J.l. Jane, Food Hydrocoll. (2017). https://doi.org/10.1016/j.foodhyd.2016.08.022
A. Ramírez-Hernández, C.E. Hernández-Mota, D.E. Páramo-Calderón, G. González-García, E. Báez-García, G. Rangel-Porras, A. Vargas-Torres, A. Aparicio-Saguilán, Carbohydr. Res. (2020). https://doi.org/10.1016/j.carres.2020.107907
O. Paredes-López, L.A. Bello-Pérez, M.G. López, Food Chem. 50, 4 (1994). https://doi.org/10.1016/0308-8146(94)90215-1
A.-C. Eliasson, H.R. Kim, J. Texture Stud. 23, 3 (1992). https://doi.org/10.1111/j.1745-4603.1992.tb00526.x
G.Ç. Koç, S.N. Dirim, J. Food Meas. Charact. 12, 3 (2018). https://doi.org/10.1007/s11694-018-9781-9
R. Leyva-López, H.M. Palma-Rodríguez, A. López-Torres, J. Capataz-Tafur, L.A. Bello-Pérez, A. Vargas-Torres, Food Hydrocoll. (2019). https://doi.org/10.1016/j.foodhyd.2019.04.056
C. Wijaya, Q.D. Do, Y.H. Ju, S.P. Santoso, J.N. Putro, L. Laysandra, F.E. Soetaredjo, S. Ismadji, Heliyon 5, 5 (2019). https://doi.org/10.1016/j.heliyon.2019.e01622
C.K. Reddy, F. Luan, B. Xu, Int. J. Biol. Macromol. (2017). https://doi.org/10.1016/j.ijbiomac.2017.07.052
N. Singh, J. Singh, L. Kaur, N. Singh Sodhi, B. Singh Gill, Food Chem. 81, 2 (2003). https://doi.org/10.1016/S0308-8146(02)00416-8
J.-l. Jane. in: Starch, ed. by J. BeMiller, R. Whistler (Academic Press, San Diego, 2009) pp. 193–236. https://doi.org/10.1016/B978-0-12-746275-2.00006-9
B.C. Maniglia, D.R. Tapia-Blácido, Food Hydrocoll. (2016). https://doi.org/10.1016/j.foodhyd.2015.11.001
K. Zhao, B. Zhang, C. Su, B. Gong, J. Zheng, H. Jiang, G. Zhang, W. Li, Food Bioprocess Technol. 13, 3 (2020). https://doi.org/10.1007/s11947-020-02405-0
M.G. Sajilata, R.S. Singhal, P.R. Kulkarni, Compr. Rev. Food Sci. Food Saf. 5, 1 (2006). https://doi.org/10.1111/j.1541-4337.2006.tb00076.x
E. Besbes, A. Le Bail, K. Seetharaman, Food Bioprocess Technol. 7, 12 (2014). https://doi.org/10.1007/s11947-014-1347-1
M.T. Pedrosa Silva Clerici, U.M. Sampaio, M. Schmiele. in: Starches for Food Application, ed. by M.T.P. Silva Clerici, M. Schmiele (Academic Press, 2019) pp. 23–69. https://doi.org/10.1016/B978-0-12-809440-2.00002-2
J. Blazek, L. Copeland, Carbohydr. Polym. 71, 3 (2008). https://doi.org/10.1016/j.carbpol.2007.06.010
M. Mondragón, L.A. Bello-Pérez, E. Agama-Acevedo, D. Betancur-Ancona, J.-L. Peña, Starch - Stärke 56, 6 (2004). https://doi.org/10.1002/star.200200190
R.R. Robles, E.D. Murray, O. Paredes-López, Int. J. Food Sci. Tech. 23, 1 (1988). https://doi.org/10.1111/j.1365-2621.1988.tb00554.x
S. Srichuwong, N. Isono, H. Jiang, T. Mishima, M. Hisamatsu, Carbohydr. Polym. 87, 2 (2012). https://doi.org/10.1016/j.carbpol.2011.09.004
M.W. Davey, M.V. Montagu, D. Inzé, M. Sanmartin, A. Kanellis, N. Smirnoff, I.J.J. Benzie, J.J. Strain, D. Favell, J. Fletcher, J. Sci. Food Agric. 80, 7 (2000). https://doi.org/10.1002/(sici)1097-0010(20000515)80:7%3c825::Aid-jsfa598%3e3.0.Co;2-6
A. Soottitantawat, H. Yoshii, T. Furuta, M. Ohgawara, P. Forssell, R. Partanen, K. Poutanen, P. Linko, J. Agric. Food Chem. 52, 5 (2004). https://doi.org/10.1021/jf035226a
I. Tontul, A. Topuz, Trends Food Sci. Tech. (2017). https://doi.org/10.1016/j.tifs.2017.03.009
R. Wang, Y. Zhao, L. Zhu, Z. Fang, Q. Shi, J. Food Meas. Charact. 14, 3 (2020). https://doi.org/10.1007/s11694-019-00369-0
T. Kinalski, C.P.Z. Noreña, J. Food Meas. Charact. 13, 3 (2019). https://doi.org/10.1007/s11694-019-00164-x
Acknowledgements
The authors express their gratitude to Adolfo López-Torres for the technical support provided during the UHPLC analysis. Author Lucio Abel Vázquez-León thanks to National Council for Science and Technology (CONACyT-Mexico) for the Cátedras-CONACYT project No. 768.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by RMM-M, LAV-L, VC-T, and AA-S. The first draft of the manuscript was written by DEP-C and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors of the article entitled "Physicochemical and morphological characterization of black bean (Phaseolus vulgaris L.) starch and potential application in nano-encapsulation by spray drying", declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vázquez-León, L.A., Aparicio-Saguilán, A., Martínez-Medinilla, R.M. et al. Physicochemical and morphological characterization of black bean (Phaseolus vulgaris L.) starch and potential application in nano-encapsulation by spray drying. Food Measure 16, 547–560 (2022). https://doi.org/10.1007/s11694-021-01181-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-01181-5