Abstract
Landraces and historical varieties are necessary germplasms for genetic improvement of modern cereals. Allelic variations at the Glu-1 and Glu-3 loci in 300 common wheat landraces and 43 historical varieties from Xinjiang, China, were evaluated by Sodium-dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and allele-specific molecular markers. Among the materials investigated, three, nine, and seven alleles were identified from the Glu-A1, Glu-B1, and Glu-D1 loci, respectively, and a total of 26 high-molecular-weight glutenin subunit (HMW-GS) combinations were found, of which 18 combinations were identified in landraces and historical varieties. Allelic frequency of HMW-GS combinations null, 7 + 8, 2 + 12 was found to be the highest in both the landraces (63.3%) and historical varieties (39.5%). Besides, some distinctive HMW-GS alleles, such as the novel Glu-B1 allele 6.1* + 8.1* and Glu-D1 alleles 2.6 + 12, 2.1 + 10.1, and 5** + 10 were observed in Xinjiang wheat landraces. Among the Glu-A3 and Glu-B3 loci of landraces and historical varieties, a total of eight and nine alleles were found, respectively. At each locus, two novel alleles were identified. A total of 33 low-molecular-weight glutenin subunit (LMW-GS) combinations of Glu-A3 and Glu-B3 were identified, with 31 and 14 combinations occurring in landraces and historical varieties, respectively, but only 10 combinations shared by both of them. As Glu-D1, Glu-A3, and Glu-B3 have highest contribution to the end-use quality and processing properties as compared to Glu-A1, Glu-B1, and Glu-D3 locus, the novel or distinctive HMW-GS and LMW-GS alleles in these loci could potentially be utilized for the improvement in the quality of modern wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The storage proteins of wheat endosperms consist of polymeric glutenins and monomeric gliadins (Shewry et al. 1995). The glutenins are further divided into HMW-GS and LMW-GS, which aggregate together to form large glutenin polymers via intra/inner chain disulfide bonds between and within them (Shewry et al. 2003). The HMW-GS and LMW-GS, comprising 7–15% and 20–35% of the total storage proteins, are major determinants of dough and processing properties of wheat flours (Rasheed et al. 2014).
Hexaploid wheat (Triticum aestivum, 2n = 6x = 42) has three sets of paralogous HMW-GS gene loci, namely, Glu-A1, Glu-B1, and Glu-D1, which are situated on the long arms of group-1 homologous chromosomes. At every locus, the two tightly linked x- and y-type genes encode one x-type and one y-type subunit, with the molecular masses of the former always larger than the latter. Bread wheat cultivars usually express three to five HMW-GS as a consequence of allelic variations and gene silence at a considerable degree among its six genes (Shewry et al. 2003). The quality and quantity of HMW-GS have a profound influence on the bread-making quality and dough properties of wheat flours by forming the backbones of glutenin polymers (Payne 1987).
The gene locus encoding LMW-GS is named complex Glu-3, which is located on the short arms of 1A, 1B, and 1D chromosomes. An extensive survey of LMW-GS compositions in 222 hexaploid wheat cultivars using SDS-PAGE revealed a total of 20 protein alleles, with six (a, b, c, d, e, f), nine (a, b, c, d, e, f, g, h, and i), and five (a, b, c, d, and e) being identified at the Glu-A3, Glu-B3, and Glu-D3 loci, respectively (Jackson et al. 1996). Previous reports have shown that allelic variations of LMW-GS at the Glu-3 locus had a significant influence on dough extensibility of wheat flours, accounting for up to 75% of the total gluten (Gupta et al. 1994; Tsenov et al. 2010; Wang et al. 2016).
Allele discriminations of LMW-GS in hexaplod wheat cultivars using conventional SDS-PAGE are complicated due to the complexity of LMW-GS profiles itself and often overlapped with gliadins by sharing similar molecular weight and electrophoretic mobility (Jackson et al. 1996). Gene-sequence-based functional molecular markers provide a reliable and high-throughput method for screening allelic variations of LMW-GS in a larger number of wheat germplasms. A set of sequence tagged site (STS) markers involving seven Glu-A3 (a, b, c, d, e, f, and g) (Wang et al. 2010) and nine Glu-B3 alleles (a, b, c, d, e, f, g, h, and i) were developed for distinguishing these alleles (Wang et al. 2009). However, no sequence-based functional molecular markers are available for separating six known Glu-D3 protein alleles (a, b, c, d, e, and f) due to limited variation within this locus (Zhao et al. 2006, 2007).
Cereal landraces are necessary germplasms for supporting sustainable agriculture and characterized by heterogeneity and rich genetic diversity. With the development of modern agriculture, the genetic diversity of cultivated wheat has been quickly lost as a consequence of unconstrained use of high inputs and excessive concern of high produce and landraces being replaced by modern cultivars in large scale (Newton et al. 2010).
Xinjiang is located in the western border of China, near the wheat origin center in South Asia and Southwest Asia. The ecological condition of Xinjiang is suitable for planting both winter and spring wheat. A larger number of wheat landraces have been preserved under the special ecological condition of Xinjiang by long-term natural and artificial selection. Currently, few reports have targeted genetic diversity analysis of the loci quality of Xinjiang wheat landraces and cultivars (Cong et al. 2005; Wang et al. 2008). The present study focuses on understanding allelic variations at the Glu-1, Glu-A3, and Glu-B3 of Chinese Xinjiang wheat germplasms using SDS-PAGE and allele-specific molecular markers. The results provide basic information for understanding the compositions of glutenin loci in Xinjiang wheat germplasms and also identified some novel glutenin alleles that could be potentially utilized in future breeding programs.
Materials and methods
Plant materials
A total of 300 Xinjiang common wheat (Triticum aestivum L.) landraces and 43 historical varieties belonging to winter or spring wheats were used for characterization of the HMW-GS and LMW-GS (Table S1). Of them, 145 landraces and 22 historical varieties were classified as winter wheat, whereas the remaining 155 landraces and 21 historical varieties were spring wheat. These materials were supplied by the Research Institute of Crop Germplasm Resource, Xinjiang Academy of Agricultural Sciences. All of the landraces were collected from Xinjiang in 1988 and the historical varieties were bred from 1965 to 1999 by major Agricultural institute of Xinjiang-Uygur Autonomous District. These materials were divided into spring or winter wheat according to their heading performance sown in the middle of April in the field after low temperature (0–4 °C) treatment of seeds. Twelve hexaploid wheat cultivars or landraces with known HMW-GS (Table S2), were used as references to estimate the electrophoretic mobility of Xinjiang wheat HMW-GS. The quality scores for Glu-1 were determined as described by Payne et al. (1987).
Electrophoresis of HMW-GS
The HMW-GS was extracted from five individual seeds as described previously (Yan et al. 2007). Briefly, the crushed seed endosperms were extracted with buffer solutions consisting of 0.0625 mM Tris–HCl, pH6.8, 2% (w/v) SDS, 5% (v/v) β-mercaptoethanol, 10% glycerol, and 0.002% (w/v) bromophenol blue at ratios of 25 μl buffer for every microgram of sample. The mixtures were gently shaken under room temperature for about 1 h before denaturing in boiling water for 5 min. The mixtures were then centrifuged at 12,000 rpm for 5 min, and 5 μl of supernatant was loaded on 10% vertical SDS-PAGE gels to separate HMW-GS. The concentrations of separating and stacking gels were 10% (w/v) and 3% (w/v), respectively.
Polymerase chain reaction (PCR) analysis of alleles at the Glu-A3 and Glu-B3 loci
Genomic DNA was extracted from 2 g young seedlings using the 2 × CTAB method (Wang et al. 2010). PCR reaction was run in a Veriti™ 96-well Fast Thermal Cycler (Applied Biosystems, USA) in total volumes of 20 μl consisting of 10 μl 2 × Taq Master Mix (Cat. no. P112, Vazyme Biotech Co, Nanjing, China), 10 pmol each for forward and reverse primer, and 50 ng template DNA. Seven Glu-A3 (a, b, c, d, e, f, and g) and nine Glu-B3 (a, b, c, d, e, f, g, h, and i) allele-specific PCR markers for LMW-GS were based on Wang et al. (2010) and Wang et al. (2009), respectively. These PCR primers were synthesized by Tsingke Biotechnology Co., Ltd (Beijing, China), and their sequences and PCR conditions are listed in Table 1.
Evaluation of genetic diversity at the Glu-1 and Glu-3 loci
Genetic diversity at the Glu-1 and Glu-3 loci was evaluated by allelic richness and genetic dispersion indices. The allelic richness of every locus was indicated by the number of allelic variants, and the total allelic richness of all loci was the summation of three (for Glu-1) or two loci (for Glu-3) at the A, B, and/or D subgenomes (Zhang et al. 2002). The genetic dispersion index (H) was calculated as H = 1–∑P2i (Nei 1973), where Pi represent the allele frequency of a given locus. The mean value of total genetic dispersion indices for all loci indicates the total genetic dispersion index.
Results
HMW-GS variations at the Glu-1 locus
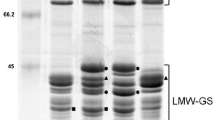
A total of 26 HMW-GS types were identified in 343 Xinjiang wheat landraces and historical varieties (Tables 2, and S1), of which 11, 10, 14, and 7 types occurred in 145 and 155 landraces of spring (Fig. 1a, b) and winter wheat (Fig. 1c. d), respectively, and 22 and 21 historical varieties of spring (Fig. 1e–g) and winter wheat (Fig. 1h), respectively. Null, 7 + 8, 2 + 12 is the predominant type for all four wheat groups, accounting for 81.4% (118 accessions) and 46.5% (72 accessions) of the 145 spring and 155 winter wheat in landraces, respectively, and 22.7% (5 varieties) and 57.1% (12 varieties) of the 22 spring and 21 winter wheat in historical varieties, respectively.
A total of 19 different HMW-GS alleles were found at the Glu-1 locus of Xinjiang wheat landraces and historical varieties, with three (a, b, and c corresponding to subunits 1, 2*, and null), nine (a, b, c, d, e, h, i, aj, and a novel allele encoding subunits 7, 7 + 8, 7 + 9, 6 + 8, 20, 14 + 15, 17 + 18, 8, and 6.1* + 8.1*), and seven (a, c, d, j, v, bq, and a rare allele corresponding to subunits 2 + 12, 4 + 12, 5 + 10, 2 + 12*, 2.1 + 10.1, 2.6 + 12, and 5** + 10) alleles being identified at the Glu-A1, Glu-B1, and Glu-D1 loci, respectively (Table 3). The novel Glu-B1 allele 6.1* + 8.1* was identified from a winter wheat landrace Dm1844 from Miquan (Figs. 1d, and S1). The electrophoretic mobility of Bx 6.1* was lain between subunits 6 and 6** (Yan et al. 2007), and that of By 8.1* was slightly slower than that of By 8. At the Glu-D1 locus, a rare allele was identified in a spring wheat landrace 3929 from Tacheng (Fig. 1b). Further investigation showed that this allele was the same as 5** + 10 in Tibet wheat landrace As1243, and the electrophoretic mobility of the x type subunit (named Dx 5**) in this allele was faster than that of Dx 5 in hexaploid wheat (Fig. S1, Yan et al. 2007).
Allelic frequencies at the Glu-1 are shown in Table 3. Among the 145 spring wheat landraces, three alleles (e.g., a, b, and c) were found at the Glu-A1, of which Glu-A1c (null) was the major allele (95.2%). Six allelic variants, namely, a, b, c, e, i, and aj were identified at the Glu-B1, with Glu-B1b (7 + 8) being the predominant allele (87.6%). Four alleles (a, d, v, and the rare allele 5** + 10) were discovered at the Glu-D1, with Glu-D1a (2 + 12) being the major allele (93.1%). In the 155 winter wheat landraces, three (a, b, and c), four (e.g., b, c, aj, and a novel allele 6.1* + 8.1*), and four (a, c, d, and bq) allelic variants were identified at the Glu-A1, Glu-B1, and Glu-D1 loci, respectively, with Glu-A1c (null, 94.2%), Glu-B1b (7 + 8, 92.3%), Glu-D1a (2 + 12, 49.0%), and bq (2.6 + 12, 45.8%) being predominant at each locus. Among the 22 spring wheat historical varieties, three allelic variants (a, b, and c) were found at the Glu-A1, six (b, c, d, e, i, and h) at the Glu-B1, and four (a, d, j, and v) at the Glu-D1 locus. The Glu-A1c (null, 50.0%), Glu-B1b (7 + 8, 45.5%), and Glu-D1a (2 + 12, 63.6%) alleles had the highest frequency at every Glu-1 locus. Of the 21 winter wheat historical varieties, three allelic variants each were found at the Glu-A1 (a, b, and c), Glu-B1 (b, c, and d), and Glu-D1 (a, d, and bq) loci, with Glu-A1c (null, 71.4%), Glu-B1b (7 + 8, 71.4%), and Glu-D1a (2 + 12, 76.2%) being predominant at each locus.
Glu-1 quality scores
The Glu-1 quality scores of most Xinjiang wheat landraces (73.3%, 220/300) and historical varieties (88.4%, 38/43) ranged from 3 to 10 and were found predominately at 6 (Tables 2, and S1). However, the quality scores of 80 landraces (including 8 spring wheat and 72 winter wheat) and five historical varieties (containing four spring wheat and one winter wheat) that were distributed in 12 HMW-GS combinations (Null, 7 + 8, 2.6 + 12; Null, 7 + 8, 2.1 + 10.1; Null, 8, 2.6 + 12; Null, 20, 2 + 12; 1, 6.1* + 8.1*, 4 + 12; 1, 14 + 15, 5 + 10; 1, 20, 2.1 + 10.1; 1, 7 + 8, 2.1 + 10.1; 1, 7 + 8, 2 + 12*; 2*, 7 + 8, 2.1 + 10.1; 2*, 8, 5** + 10; 2*, 6 + 8, 2.1 + 10.1) were not determined, as some subunits were unique (subunit 2.6 + 12) to Xinjiang wheat landraces or rare (such as 2.1 + 10.1, 2 + 12*, and 5** + 10) in previous reported hexaploid wheat, and the quality contribution of these subunits to bread-making quality has not yet been determined. The frequency of subunits 2.6 + 12 was quite high (46.5%) in Xinjinag winter wheat landraces, and the molecular mass of 2.6 was slower than that of 2.2, one of the largest Dx-type subunits in wheat.
LMW-GS alleles at the Glu-A3 and Glu-B3 loci
The composition and frequency of LMW-GS at the Glu-A3 and Glu-B3 loci are shown in Table 2. A total of 36 combinations were found, of which 25 and 16, and 9 and 11 combinations were identified from the landraces and historical varieties in spring and winter wheat, respectively. Only three combinations, namely, b/new 3 (Glu-A3/Glu-B3), c/i, and c/new3 were common to landraces and historical varieties. Three major LMW-GS types that account for 42.1% (61/145) and 64.5% (100/155) of the total spring and winter wheat landraces were b/g (32 accessions), c/i(18), and c/g (11), and c/i (44), b/i (35), and c/new3 (21), whereas the predominant three or two types that contribute to totals of 68.2% (15/22) and 47.6% (10/21) in the spring and winter wheat historical varieties were c/new 3 (8 accessions), c/new 4 (4), and d/new3 (3), and b/a (5) and c/i (5), respectively.
A total of eight Glu-A3, namely, a, b, c, d, e, f, new 1, and new 2, and nine Glu-B3 alleles, viz., a, b, c, d, g, h, i, new 3, and new 4 (Table 4), were identified from landraces (Fig. 2a, b, e, and f) and historical varieties (Fig. 2c, d, g, and h) in spring (Fig. 2a, c, e, and g) and winter wheat (Fig. 2b, d, f, and h), of which four alleles (new 1, new 2, new 3, and new 4) were not reported previously (Table 4). The two novel Glu-A3 alleles, new 1 and new 2, were unique to spring or winter wheat landraces, whereas the two novel Glu-B3 alleles (new 3 and new 4) were shared by the landraces and historical varieties in spring and winter wheat. The novel allele new 1 was negative to seven known Glu-A3 PCR markers (from a to g), but new 2 gave a larger PCR fragment (about 1100 bp) than the expected 967-bp size for Glu-A3d (Fig. 2b). The new 3 given a positive amplification for marker Glu-B3bef but negative for any of the Glu-B3b, gf, and e (Fig. 2e, h) or only with a larger faint band (about 900 bp) for Glu-B3e (expected size 158 bp) (Fig. 2f, g). The new 4 was negative to nine known Glu-B3 markers from a to i.
Major alleles at Glu-A3 and Glu-B3 loci of landraces and historical varieties are depicted in Table 4. It was shown that the three major Glu-A3 alleles contributing to 92.4% and 95.5% of the landraces in spring and winter wheat, respectively, were b (63 accessions), c (50), and a (21), and c (82), b (43), and new2 (23). At the Glu-B3 locus, alleles g (49), i (38), and d (21), and i (86), a (30), and new 3 (27) were the predominant three alleles that account for 74.5% and 92.3% of landraces in spring and winter wheat, respectively. For 22 spring wheat historical varieties, alleles c (16 entries) at Glu-A3 and new 3 (12 entries) at Glu-B3 were the highest alleles of each locus. Of the 21 winter wheat historical varieties, alleles b (8 entries), and a (7) and i (7) were the predominant types for Glu-A3, and Glu-B3, respectively.
Genetic diversity at the Glu-1 and Glu-3 loci
Allelic richness and genetic dispersion indices (H) at the Glu-1 and Glu-3 loci of landraces and historical varieties in spring and winter wheat are shown in Table S3.
For total allelic richness at the Glu-1 locus, the spring wheat was higher than the winter wheat for both the landraces (13 vs. 11) and historical varieties (13 vs. 9) due to more allelic variations occurring at the Glu-B1 (for landraces and historical varieties) and Glu-D1 loci (for historical varieties). Analysis of the total allelic richness at the Glu-3 locus (Glu-D3 not included) has shown that landraces exhibited richer allelic profile compared to historical varieties for both the spring and winter wheat, as more allelic variations were found at the Glu-A3 (for both the spring and winter wheat landraces) or Glu-B3 loci (for spring wheat landraces).
For total genetic dispersion indices at the Glu-1 locus, the landraces were lower than historical varieties for both the spring (0.1506 vs. 0.6260) and winter wheat (0.2679 vs. 0.4187). It was shown that the total genetic dispersion indices at the Glu-3 locus of landraces were slightly larger than those of the historical varieties (0.7235 vs. 0.5543) for spring wheat, whereas those of the landraces were smaller than historical varieties for winter wheat (0.6215 vs. 0.6984).
Discussion
To reveal allelic variations at the Glu-1 and Glu-3 loci of 343 accessions of Xinjiang wheat landraces and historical varieties, we used SDS-PAGE and allele-specific STS molecular markers to characterize the HMW-GS and LMW-GS, respectively. It was shown that null, 7 + 8, 2 + 12 was the predominant HMW-GS pattern for wheat landraces (63.3%, 190 out of 300 accessions) from Xinjiang (Table 2), Sichuan (97.8%, 87/89), Tibet (76.4%, 175/229), Yangtze-River regions of China (32.2%, 156/485) (Wei et al. 2000; Yan et al. 2007; Zheng et al. 2011), Japan (57.5%, 100/174) (Nakamura 2000), and also for historical varieties (39.5%, 17/43) from Xinjiang, China, but not for wheat landraces from Pakistan and India (Niwa et al. 2008; Goel et al. 2018), which were predominant for 2*, 17 + 18, 2 + 12 (41.8%, 71/170), and null, 17 + 18, 2 + 12 (24.75%, 128/517), respectively. Glu-A1c (null) was the most frequently occurring Glu-A1 alleles in Xinjiang wheat landraces (94.7%, 284/300) and historical varieties (62.8%, 27/43). Previous reports showed that null was the major Glu-A1 allele for other Chinese wheat landraces, and also for Japanese and Indian wheat landraces (Nakamura 2000; Wei et al. 2000; Yan et al. 2007; Zheng et al. 2011; Goel et al. 2018). However, among the 170 Pakistan wheat landraces, the subunit 2* contributed to the major Glu-A1 allele with a frequency of 53.5% (Niwa et al. 2008).The frequently occurring Glu-B1 allele for wheat landraces from China and Japan, and improved wheat varieties from China was 7 + 8, whereas it was 17 + 18 for wheat landrace from India and Pakistan (Niwa et al. 2008; Goel et al. 2018). For wheat landraces and improved varieties from different sources, the most frequently occurring Glu-D1 allele was 2 + 12 (Nakamura 2000;Yan et al. 2007; Zheng et al. 2011; Goel et al. 2018). Present analyses of Xinjiang wheat landraces and historical varieties are consistent with these previous reports, suggesting that 2 + 12 was distributed extensively among different wheat materials.
The composition of HMW-GS and LMW-GS play an important role in determining the dough strength and the processing properties of wheat flours (Ram 2003; Kaur et al. 2013; Katyal et al. 2016, 2017, 2018). For example, the HMW-GS combinations 20 and 2 + 12 showed very weak dough stability and 17 + 18 with 2 + 12 or 5 + 10 and 7 + 8 with 5 + 10, and 2*, 17 + 18 and 5 + 10 were very strong strength, whereas 2 + 12 and 7 + 9 as well as 5 + 10 with 7 or 7 + 9 were intermediated between them (Ram 2003; Kaur et al. 2013). In durum wheat, the HMW-GS 13 + 16 and 6 + 8 showed stronger dough strength than 20 (Ram 2003). Meanwhile, the dough strength of wheat flours correlated with more quality associated parameters such as sedimentation value, gluten content as well as grain hardness and particle size distribution and finally resulted in difference in elastic and viscous properties for making variable food products (Katyal et al. 2016, 2017, 2018).
Average Glu-1 quality score of Xinjiang wheat landraces and historical varieties (about 6) were similar to that of the wheat landraces from Tibet and cultivars from India and Japan but lower than that of the varieties from Russia, Canada, and Serbia (Yan et al. 2007; Nakamura 2000; Novoselskaya-Dragovich et al. 2011; Goel et al. 2018). Asia is a major noodle-consuming area, and only a small amount of wheat is used for making bread. In contrast, wheat in Europe is mainly used for making bread. Japanese wheat landrace and commercial wheat are characterized by a very high frequency of HMW-GS Glu-D1f (145kD +12), which is one of the ideal subunits for making Japanese Udon noodles (Nakamura 2000). As deduced from the HMW-GS compositions, only two landraces (3986 and 3998A) and four historical varieties (Yinong 3, Xinchun 7, Xinchun 23, and Baidong 1) of Xinjiang wheat are expected to have very good bread-making quality for possessing subunits 1, 7 + 8, 5 + 10; 2*, 7 + 8, 5 + 10; and 2*, 17 + 18, 5 + 10, with high-quality scores up to 10 (Payne et al. 1987). With the exception of making bread, Xinjiang wheat has largely been used to make some distinctive food products such as Nan bread and Xinjiang stretched noodles. Previous reports regarding Chinese Lanzhou alkaline stretched noodles (LASN) have shown that many noodle quality parameters were significantly influenced by Glu-1 alleles (Meng and Cai 2008). It should be noted that Xinjiang wheat possesses some distinctive subunits (such as 2.1 + 10.1, 2.6 + 12) that were rare or absent in wheat from other sources. We also identified a novel Glu-B1 (6.1* + 8.1*) and two rare Glu-D1 alleles (2 + 12* and 5** + 10) that were not described in Xinjiang wheat landraces (Cong et al. 2005, 2007). As mentioned in a previous report, some of these subunits or subunit combinations might have been favorable for making special Xinjiang foods such as Nan bread and noodles (Cong et al. 2007). Currently, the quality association between the HMW-GS compositions and the special Xinjiang wheat food products such as noodles and Nan bread has not been well established.
The LMW-GS compositions at the Glu-A3 and Glu-B3 loci play major roles in determining the quality of wheat flours (Zhang et al. 2012). It was shown that alleles Glu-A3d and Glu-B3d had slightly better dry white Chinese noodle quality compared to those of the others alleles (He et al. 2005). The Glu-B3h contributed to superior dough strength and bread-making quality (Wang et al. 2016), and the Glu-A3b, Glu-A3d, Glu-B3g, and Glu-B3f alleles had a significant impact on the mixograph property of wheat flours (Jin et al. 2013). At the Glu-A3 locus, Xinjiang wheat landraces and historical cultivars had a considerably high frequency of alleles b (35.3%, 106/300), and b (20.9%, 9/43) and d (25.6%, 11/43), although the most frequently occurring alleles at this locus for both were c, with a frequency of 44.0% (132/300) and 51.2% (22/43). At the Glu-B3 locus, Xinjiang wheat landraces had a relatively high frequency of g (19%, 57/300) but absence of f and h, whereas historical cultivars lacked all the three known Glu-B3 alleles (g, f, and h) that contribute to superior dough strength, mixograph properties, and bread-making quality of wheat flours. The predominant Glu-B3 allele for landraces and historical cultivars was i and new3, with a frequency of 41.3% (124/300) and 37.2% (16/43), respectively. As shown in Chinese LASN, Glu-A3d and Glu-B3g were highly related to high protein content, high volume of SDS-sediment, and super dough strength, all of which prove beneficial to the quality of LASN (Meng et al. 2007). We could expect that the second frequently occurring Glu-B3g allele in Xinjiang wheat landraces had an important role in determining the quality of Xinjiang wheat food products such as noodles. It should be noted that Xinjiang wheat landraces (12.7%, 38/300) and historical varieties (37.2%, 16/43) had a relatively high frequency of Glu-B3 new 3, although its quality contribution to wheat has not been determined. Favorability of this allele regarding Xinjiang wheat quality needs further investigation. Due to limited variations within the genes among Glu-D3 alleles, we were not able to investigate allelic variations at Glu-D1 locus for the absence of allele-specific PCR markers (Zhao et al. 2006, 2007). Previous reports also showed that allelic variations at Glu-D3 exhibited a minor impact on the quality of wheat flours in comparison with those of the Glu-A3 and Glu-B3 loci (Zhang et al. 2012).
Conclusion
Xinjiang wheat landraces and historical varieties have rich diversity at the Glu-1, Glu-A3, and Glu-B3 loci. A novel Glu-B1 allele, 6.1* + 8.1*, two Glu-D1 rare alleles, 2.1 + 10.1 and 5** + 10, and one distinctive allele, 2.6 + 12, were observed in Xinjiang wheat landraces in addition to the HMW-GS previously reported. The results also showed that the LMW-GS compositions at the Glu-A3 and Glu-B3 locus of Xinjiang wheat landraces and historical varieties between spring and winter wheat were different from each other. Interestingly, two novel Glu-A3 (new1 mad new 2) and two novel Glu-B3 alleles (new 3 and new 4) were identified from landraces and historical varieties at a considerable frequency using known allele-specific PCR markers. It could be worthwhile to understand the quality contribution of these distinctive HMW-GS and LMW-GS to specific Xinjiang wheat food products. Our further works will focus on elucidation the quality contribution of these novel or distinctive HMW-GS and LMW-GS alleles identified from Xinjiang landraces on the quality parameters and the end-use quality of food products.
Abbreviations
- HMW-GS:
-
High-molecular-weight glutenin subunits
- LMW-GS:
-
Low-molecular-weight glutenin subunits
- SDS-PAGE:
-
Sodium-dodecyl-sulfate polyacrylamide gel electrophoresis
- STS:
-
Sequence tagged site
- PCR:
-
Polymerase chain reaction
- H:
-
The genetic dispersion index
- LASN:
-
Lanzhou alkaline stretched noodles
References
Cong H, Takata K, Zong YF, Ikeda TM, Yanaka M, Nagamine T, Hiroshi F (2005) Novel high molecular weight glutenin subunits at the Glu-D1 locus in wheat landraces from the Xinjiang district of china and relationship with winter habit. Breeding Sci 55:459–463
Cong H, Takata K, Ikeda TM, Yanaka M, Fujimaki H, Nagamine T (2007) Characterization of a novel high molecular weight glutenin subunit pair 2.6 + 12 in common wheat landraces in the Xinjiang uygur autonomous district of China. Breed Sci 57:253–255
Goel S, Yadav M, Singh K, Jaat RS, Singh NK (2018) Exploring diverse wheat germplasm for novel alleles in HMW-GS for bread quality improvement. J Food Sci Technol 55:3257–3262
Gupta RB, Paul JG, Cornish GB, Palmer GA, Békés F, Rathjen AJ (1994) Allelic variation at glutenin subunit and gliadin loci, Glu-1, Glu-3 and Gli-1 of common wheats. 1. Its additive and interaction effects on dough properties. J Cereal Sci 19:9–17
He ZH, Liu L, Xia XC, Liu JJ, Peña RJ (2005) Composition of HMW and LMW glutenin subunits and their effects on dough properties, pan bread, and noodle quality of Chinese bread wheats. Cereal Chem 82:345–350
Jackson EA, Morel MH, Sontag-Strohm T, Branlard G, Metakovsky EV, Redaelli R (1996) Proposal for combining the classification systems of alleles of Gli-1 and Glu-3 loci in bread wheat (Triticum aestivum L.). J Genet Breed 50:321–336
Jin H, Zhang Y, Li GY, Mu PY, Fan ZR, Xia XC, He ZH (2013) Effects of allelic variation of HMW-GS and LMW-GS on mixograph properties and Chinese noodle and steamed bread qualities in a set of Aroona near-isogenic wheat lines. J Cereal Sci 57:146–152
Katyal M, Virdi AS, Kaur A, Singh N, Kaur S, Ahlawat AK, Singh AM (2016) Diversity in quality traits amongst Indian wheat varieties I: flour and protein characteristics. Food Chem 194:337–344
Katyal M, Virdi AS, Kaur A, Singh N, Kaur S, Ahlawat AK, Singh AM (2017) Extraordinarily soft, medium-hard and hard Indian wheat varieties: composition, protein profile, dough and baking properties. Food Res Int 100:306–317
Katyal M, Virdi AS, Singh N, Kaur A, Rana JC, Jyoti K (2018) Diversity in protein profiling, pasting, empirical and dynamic dough rheological properties of meal from different durum wheat accessions. J Food Sci Technol 55:1256–1269
Kaur A, Singh N, Ahlawat AK, Kaur S, Singh AM, Chauhan H, Singh GP (2013) Diversity in grain, flour, dough and gluten properties amongst Indian wheat cultivars varying in high molecular weight subunits (HMW-GS). Food Res Int 53:63–72
Meng X, Cai S (2008) Association between glutenin alleles and Lanzhou alkaline stretched noodle quality of northwest China spring wheats. II. Relationship with the variations at the Glu-1 loci. Cereal Res Commun 36:107–115
Meng X, Xie F, Shang X, An L (2007) Association between allelic variations at the Glu-3 loci and wheat quality traits with Lanzhou alkaline stretched noodles quality in northwest China spring wheats. Cereal Res Commun 35:109–118
Nakamura H (2000) The high-molecular-weight glutenin subunit composition of Japanese hexaploid wheat landraces. Aust J Agric Res 51:673–677
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 70:3321–3323
Newton AC, Akar T, Baresel JP, Bebeli PJ, Bettencourt E, Bladenopoulos KV, Czembor JH, Fasoula DA, Katsiotis A, Koutis K, Koutsika-Sotiriou M, Kovacs G, Larsson H, Pinheiro de Carvalho MAA, Rubiales D, Russell J, Dos Santos TMM, Vaz Patto MC (2010) Cereal landraces for sustainable agriculture, a review. Agron Sustain Dev 30:237–269
Niwa K, Suzuki H, Tominaga T, Nasim S, Anwar R, Ogawa M, Furuta Y (2008) Evaluation of genetic variation in high molecular weight glutenin subunits of seed storage protein using landraces of common wheat from Pakistan. Cereal Res Commun 36:327–332
Novoselskaya-Dragovich AY, Fisenko AV, Yankovsky NK, Kudryavtsev AM, Yang QJ, Lu ZH, Wang DW (2011) Genetic diversity of storage protein genes in common wheat (Triticum aestivum L.) cultivars from China and its comparison with genetic diversity of cultivars from other countries. Genet Resour Crop Evol 58:433–543
Payne PI (1987) Genetics of wheat storage proteins and the effect of allelic variation on breadmaking quality. Annu Rev Plant Physiol 38:141–153
Payne PI, Nightingale MA, Krattiger AF, Holt LM (1987) The relationship between HMW glutenin subunit composition and the breadmaking quality of British-grown wheat varieties. J Sci Food Agric 40:51–65
Ram S (2003) High molecular weight glutenin subunit composition of Indian wheats and their relationships with dough strength. J Plant Biochem Biot 12:151–155
Rasheed A, Xia XC, Yan YM, Appels R, Mahmood T, He ZH (2014) Wheat seed storage proteins: advances in molecular genetics, diversity and breeding applications. J Cereal Sci 60:11–24
Shewry PR, Tatham AS, Barro F, Barcelo P, Lazzeri P (1995) Biotechnology of breadmaking: unraveling and manipulating the multi-protein gluten complex. Biol Tech 13:1185–1190
Shewry PR, Halford NG, Tatham AS, Popineau Y, Lafiandra D, Belton PS (2003) The high molecular weight subunits of wheat glutenin and their role in determining wheat processing properties. Adv Food Nutr Res 45:219–302
Tsenov N, Atanasova D, Todorov I, Ivanova I, Stoeva I (2010) Quality of winter common wheat advanced lines depending on allelic variation of Glu-A3. Cereal Res Commun 38:250–258
Wang L, Li GY, Xia XC, He ZH, Mu PY (2008) Molecular characterization of Pina and Pinb allelic variations in Xinjiang landraces and commercial wheat cultivars. Euphytica 164:745–752
Wang LH, Zhao XL, He ZH, Ma W, Appels R, Peña RJ, Xia XC (2009) Characterization of low-molecular-weight glutenin subunit Glu-B3 genes and development of STS markers in common wheat (Triticum aestivum L.). Theor Appl Genet 118:525–539
Wang LH, Li GY, Peña RJ, Xia XC, He ZH (2010) Development of STS markers and establishment of multiplex PCR for Glu-A3 alleles in common wheat (Triticum aestivum L.). J Cereal Sci 51:305–312
Wang YP, Zhen SM, Luo NN, Ha CX, Lu XB, Li XH, Xia XC, He ZH, Yan YM (2016) Low molecular weight glutenin subunit gene Glu-B3h confers superior dough strength and breadmaking quality in wheat (Triticum aestivum L.). Sci Rep 6:27182
Wei YM, Zheng YL, Liu DC, Zhou YH, Lan XJ (2000) Genetic diversity of Gli-1, Gli-2 and Glu-1 alleles in Sichuan wheat landraces. Acta Bota Sin 42:496–501
Yan ZH, Dai SF, Liu DC, Wei YM, Zheng YL (2007) Allelic variation of high molecular weight glutenin subunits in the hexaploid wheat landraces of Tibet, China. Int J Agric Res 2:838–843
Zhang X, Pang B, You G, Wang L, Jia J, Dong Y (2002) Allelic variation and genetic diversity at Glu-1 loci in Chinese wheat (Triticum aestivum L.) gernplasm. Sci Agric Sin 35:1302–1310
Zhang XF, Jin H, Zhang Y, Liu DC, Li GY, Xia XC, He ZH, Zhang AM (2012) Composition and functional analysis of low-molecular-weight glutenin alleles with Aroona near isogenic lines of bread wheat. BMC Plant Biol 12:243
Zhao XL, Xia XC, He ZH, Gale KR, Lei ZS, Appels R, Ma W (2006) Characterization of three low-molecular-weight Glu-D3 subunit genes in common wheat. Theor Appl Genet 113:1247–1259
Zhao XL, Xia XC, He ZH, Lei ZS, Appels R, Yang Y, Sun QX, Ma W (2007) Novel DNA variations to characterize low molecular weight glutenin Glu-D3 genes and develop STS markers in common wheat. Theor Appl Genet 114:451–460
Zheng W, Peng YC, Ma JH, Rudi A, Sun DF, Ma WJ (2011) High frequency of abnormal high molecular weight glutenin alleles in Chinese wheat landraces of the Yangtze-River region. J Cereal Sci 54:401–408
Acknowledgements
This study was supported by the National Natural Science Foundation of China (U1403185, 31771783), and Sichuan Science and Technology Program (No. 2018HH0113 and 2018HH0130), and Xinjiang Urumqi Science and Technology Bureau program (No. Z16120002).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dai, S., Xu, D., Yan, Y. et al. Characterization of high- and low-molecular-weight glutenin subunits from Chinese Xinjiang wheat landraces and historical varieties. J Food Sci Technol 57, 3823–3835 (2020). https://doi.org/10.1007/s13197-020-04414-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04414-5