Abstract
Nanoemulsions exhibit a number of advantages to carry and deliver lipophilic compounds such as essential oils (EOs) due to their good stability and high surface area per volume unit. The purpose of this work was to assess the long-term stability of nanoemulsions of clove and lemongrass (LG) EOs and their principal components eugenol and citral (CI), respectively, at 3 different concentrations (2, 5 or 10 times their respective minimum inhibitory concentrations) and at two storage temperatures (1 °C and 21 °C). The initial droplet size of LG and CI-loaded nanoemulsions was below 100 nm and most of them kept droplet sizes in the nano-range until the end of storage at both temperatures. The ζ-potential was lower than − 40 mV, but it increased through storage, indicating a weaker alginate adsorption at the oil surface at both temperatures. The antimicrobial activity increased with the EOs concentration and was negatively affected by the highest storage temperature. Nanoemulsions containing CI and LG were able to significantly decrease Escherichia coli counts during storage, particularly at 1 °C. Nanoemulsions containing 1.0 and 2.0% w/w CI and 2.5% w/w LG were the most efficient in reducing Botrytis cinerea growth through storage, mainly at 1 °C. The nanoemulsions containing 1.0 and 2.0% w/w CI, as well as, 1.25 and 2.5% w/w LG better maintained their stability and antimicrobial effect along 6-months storage mainly when at 1 °C, making those nanoemulsions suitable as edible coatings for food preservation. Future studies should be oriented to evaluate the impact of these nanoemulsions on the organoleptic properties of coated foods and their potential toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fruit and vegetables are important components of the human diet, being their consumption essential for a good nutrition and to prevent a wide number of chronic diseases (Schneeman 2004). However, fruits and vegetables are highly perishable due to physiological processes and microbial spoilage. Losses inflicted throughout the supply chain by pathogen-induced diseases are the major component of food wastage. Among these pathogens, Botrytis cinerea, the cause of grey mold, is considered one of the most important postharvest decays of fresh fruit and vegetables (Wang et al. 2010). The greatest concern with human pathogens on fresh fruits and vegetables are enteric pathogens, as Escherichia coli, that is able to cause disease through contamination of our food supply (Chekabab et al. 2013).
Edible coatings have a high potential to carry active ingredients such as antimicrobial compounds, and can be used to extend fruit and vegetables shelf life (Salvia-Trujillo et al. 2015b). Essential oils (EOs) as Lemongrass (LG) and clove (CL), as well as their main components being citral (CI) and eugenol (EU), respectively, according to Hakkı et al. (2007) and Mohamed Hanaa et al. (2012); have been shown to have inhibitory activities against various bacteria and fungi (Aguilar-González et al. 2015; Guerreiro et al. 2015).
EOs are recognized by the consumers as ‘‘natural’’ components which makes them highly appreciated for application in many commercial food and beverage products since there is an increased demand for natural rather than synthetic additives. However, due to their low water solubility and intense aroma, they present limitations for being incorporated into food products, especially at microbiologically efficient high concentrations (Salvia-Trujillo et al. 2014). Therefore, EOs nanoencapsulation may be an alternative regarding their application in foods (Salvia-Trujillo et al. 2015a). EOs-based nanoemulsions have been applied in food products with the purpose to extend their shelf life keeping or improving the appearance, flavour, aroma as well as nutritional quality (Salvia-Trujillo et al. 2015b). Oil-in-water (O/W) nanoemulsions consist of a lipid phase dispersed in an aqueous continuous phase, being each oil droplet surrounded by a thin interfacial layer of emulsifier molecules, with an average size lower than 200 nm (Tadros et al. 2004). Nanoemulsions exhibit several important advantages over conventional emulsions for certain applications due to their optical clarity, high physical stability, and ability to increase the bioavailability of lipophilic bioactives (Salvia-Trujillo et al. 2015a).
Nevertheless, EOs-based nanoemulsions may present some changes in their dropet size, polydispersity and ζ-potential, mainly if their components show relative high water solubility and mobility (Artiga-Artigas et al. 2018). The main destabilization phenomena suffered by EOs-based nanoemulsions is Ostwald ripening (OR), which is attributed to diffusion of the dispersed phase through the continuous phase and a spontaneous trend toward a minimal interfacial area between the continuous and the dispersed phases (Wooster et al. 2008). Therefore, OR will cause the growth of larger particles at the expense of smaller ones with the dispersed phase being transported through the continuous phase over time. Additionally, nanoemulsions containing EOs are prone to suffer coalescence, phenomenon that occurs when two oil droplets contact due to the weak steric repulsion between them, and they unify in a sole larger droplet (Qian and McClements 2011). These destabilization phenomena may limit the available period for the effective application of nanoemulsions.
EOs-based nanoemulsions require the evaluation of their stability throughout storage (Guerra-Rosas et al. 2016). Therefore, the aim of the present work was to evaluate the stability and antimicrobial activity against E. coli and Botrytis cinerea of nanoemulsions containing LG–EO, CL–EO or their main components CI and eugenol EU, over storage at different temperatures.
Materials and methods
Materials
LG–EO (Cymbopogon citratus), whose major components are CI a and CI b (30–40% w/w and 30–35% w/w, respectively), and CL–EO (Syzygium aromaticum), which contains 80–90% EU, 15% EU acetate and 5–12% beta caryophyllene, were purchased from Oils4life (United Kingdom) (Hakki et al. 2007; Mohamed Hanaa et al. 2012). CI and EU were obtained from Agrós Organics (Portugal) and Fluka (Portugal), respectively. Food-grade sodium alginate (MANUCOL®DH) was supplied by FMC Biopolymer Ltd (Scotland, U.K.). Information provided by the manufacturer indicates that viscosity and pH of a solution 1% is 40–90 mPa and 5.0–7.5, respectively. Tween 80 (Polyoxyethylenesorbitan Monoesterate) (Lab Scharlab, Spain) was used as food-grade nonionic surfactant. Ultrapure water, obtained from Millipore Milli-Q filtration system (0.22 µm) was used for the formulation and analysis of nanoemulsions. All culture mediums used in microbiology assays, Tryptone Soy Broth, McConkey agar and Potato Dextrose Agar were obtained from Bioakar Diagnostics, Beauvais, France.
Methods
Determination of minimum inhibitory concentration (MIC)
The antimicrobial activity of CL and LG EOs and their principal components (EU and CI, respectively) against bacteria and fungi was determined by the microdilution method as described previously by Faleiro et al. (2005) (Supplementary material Table 6). The tested concentrations for CL and LG EOs and their major components EU and CI were 0.04, 0.06, 0.08, 0.10, 0.15, 0.20, 0.25, 0.30, 0.35 and 0.40% (w/w) diluted in Tween 20 (2%, w/w). The Escherichia coli S3 and Listeria monocytogenes EGD (Supplementary material Table 6), were maintained at − 80 °C and recovered in TSA plates, which were incubated at 37 °C for 24 h. From each plate a loop was used to inoculate 10 mL of TSB, and the culture was incubated overnight at 37 °C with continuous agitation at 120 rpm. Each well of a flat-bottom microplate (Greiner, abortechnick, Frichenhausen, Germany) was filled with 180 µL of TSB supplemented with CL, LG, EU or CI at the appropriate concentration. Twenty microliters of the bacterial suspension prepared with the culture medium supplemented with the EO at the proper concentration was used to inoculate each well. The growth was followed by spectrophotometry (OD600nm) in a microplate reader (Tecan Infinite M200, Tecan, Austria). Wells containing the culture medium supplemented with Tween 20 or chloramphenicol (30 µg/mL) were included, as control. A set of wells containing the culture medium with no antimicrobial agent represented the negative control. Three biologic and three technical replicates for each strain were used (N = 6). The MIC value was considered the lowest concentration of the essential oil component that caused the inhibition of the bacterial growth (no increase in the OD600nm after 24–48 h) (Faleiro et al. 2005).
The MIC value for fungi was determined as described by Camele et al. (2012). The mould strains were maintained in PDA. The mould culture was prepared by dropping10 µL of single suspensions containing 1 × 104 conidia/mL of each Penicillium digitatum, P. expansum and Botritys cinereal culture into PDA plates supplemented with each compound at appropriate concentration.
PDA plates with 0.2% w/w Tween 20 and with no compound were used as control. Three replicates for each compound dose were performed. The inhibitory effect of the EOs against each tested mould was determined after 3–14 days incubation period at 25 °C, taking into account when control colonies margins reached plate edges.
Primary emulsion formation
Sodium alginate (2% w/w) was dissolved in water at 70 °C, with continuous stirring until it was completely dissolved and the solution was cooled down to 25 °C. A primary emulsion was made by mixing the alginate aqueous solution with the EO and Tween 80 (Supplementary material Table 7) with a laboratory T-25 digital Ultraturrax (IKA, Staufen, Germany) working at 11,000 rpm, for 2 min following the methodology proposed by Artiga-Artigas et al. (2017).
Concentrations of the CL and LG EOs and their main components (EU and CI, respectively) used in the emulsions were based on their minimum inhibitory concentrations (MIC), by using 2, 5 or 10 times higher oil concentrations than their MIC values. Tween 80 concentration was bound an oil/surfactant ratio of 1:3 (Supplementary material Table 7).
Nanoemulsion formation
After primary emulsion preparation, nanoemulsions were obtained by microfluidization (M-110P, Microfluidics, USA) at 150 MPa for 5 cycles following the methodology used by Artiga-Artigas et al. (2017). Nanoemulsions were cooled down at the outlet of the microfluidization unit through an external coil immersed in a water bath with ice, so temperature was kept at 10 °C. For stability studies, aliquots of nanoemulsions were placed in capped plastic test tubes and stored at room temperature (21 °C) or at 1 °C, in the absence of light. Analytical determinations were performed just after preparation and along storage time (2, 4 and 6 months) at both temperatures.
Nanoemulsions characterization
Droplet size, polydispersity and ζ-potential
The average droplet size of the nanoemulsions was determined by dynamic-light-scattering (DLS), using a Zetasizer Nano-ZS laser diffractometer (Malvern Instruments Ltd., Worcestershire, UK), working at 633 nm and equipped with a backscatter detector (173°), which is used to specifically measure submicron particles. Polydispersity index (PdI), which represents the distribution of particle size, was also recorded from the instrument during the DLS measurement. PdI values near 1 indicate a heterogeneous or multimodal distribution of droplet sizes, whereas those near 0 give an idea of monomodal distribution.
The ζ-potential (mV) of the oil droplets in the nanoemulsions was determined by phase-analysis light scattering (PALS) measuring their electrophoretic mobility using an automated capillary electrophoresis device (Zetasizer Nano ZS series, Malvern Instruments Ltd, Worcestershire, UK), working at 633 nm laser at 25 °C. It determines the surface electrical charge of the droplets dispersed in the continuous phase. An aliquot was withdrawn from the storage tube at the mean level of nanoemulsion height and then were diluted 10 times with milli-Q water to avoid multiple scattering effects and stirred to ensure sample homogeneity.
Measurements of both parameters (droplet size and ζ-potential) were performed along storage time (2, 4 and 6 months) at 1 °C and 21 °C.
Color
The color of nanoemulsions was assessed at room temperature (± 25 °C), using a Minolta Chroma Meter CR-400 colorimeter (Konica Minolta Sensing, Inc., Osaka, Japan) with an illumination D65 and 10° observer angle, to determine the L* value (lightness), a* value (redness) and b* value (yellowness). The device was calibrated with a standard white plate (Y = 94.0; x = 0.3133, and y = 0.3194). The results were expressed as mean values of whiteness index (WI), calculated with Eq. (1) (Salvia-Trujillo et al. 2014):
Antimicrobial activity
The antimicrobial activity of the nanoemulsions was assessed by evaluating the in vitro inactivation of Escherichia coli S3 isolated from a ready-to-eat fruit salad at the Microbiology laboratory of Biomedical Research Center from University of Algarve (Portugal), and Botrytis cinerea DSM 877 (German Collection of Microorganisms and Cell Cultures) provided to the culture collection of the Microbiology laboratory of the University of Algarve (Portugal). The antimicrobial activity determinations were performed just after preparation of the nanoemulsions and along storage time (2, 4 and 6 months) at 1 °C and 21 °C.
The method used to evaluate the inhibition of E. coli growth was adapted from the previously described by Ferreira et al. (2010). Briefly, E. coli was cultured in Tryptone Soy Broth during 18 h at 37 °C with continuous agitation at 120 rpm. The bacterial numbers reached 109–1010 colony-forming units/milliliter (CFU/mL). A 0.5 mL of the bacterial culture was transferred to 0.5 mL of each EO-alginate or essential oils components (EOC)-alginate nanoemulsion (Supplementary material Table 7) and 4.5 mL of sterile Milli-Q water. The bacterial viability was determined by sampling the homogenate after 30 min. Serial dilutions of the bacterial suspension were prepared and inoculated on McConkey Agar. A control was performed with the same method by replacing the nanoemulsion with sterile Milli-Q water.
The inhibition experiments were conducted at room temperature. The inoculated plates were incubated at 37 °C for 24 h. The dilution that showed a minimum of 30 colonies was selected to determine the viable cells.
The CFU number was calculated using the formula: number of colonies × dilution factor/volume unit.
In vitro antifungal activity of the nanoemulsions was determined by measuring the growth inhibition of B. cinerea through a modification of the method described by Abd-Elsalam and Khokhlov (2015). Three wells of 5 mm diameter were punched and aligned in the Petri dishes containing Potato Dextrose Agar (PDA) as shown by (Balouiri et al. 2016) for the agar well diffusion method. A volume of 50 µl of nanoemulsion was dropped in each well. The plates were kept in a refrigerator at 4 °C for 2 h, to allow the diffusion of the nanoemulsion across the PDA medium. The control was performed with the medium without the addition of nanoemulsion. After that, plates were inoculated with Botrytis mycelial disc (4 mm) in the middle well and incubated at 25 ± 1 °C.
Mycelial growth of B. cinerea was expressed as mean values of fungus lag phase (time elapsing until exponential growth starts) and duplication time (td), calculated with Eq. (2) where µ is the specific growth rate (3) (Waites et al. 2001):
where, xo is the initial biomass (or cell density) and x is the biomass (or cell density) at time t.
Statistical analysis
The formation process of each nanoemulsion was repeated 3 times and each replicate was analyzed twice to obtain mean values for the measured parameters. Statistical analysis of experimental data was done by a multifactor analysis of variance using statistical package SPSS version 20 (IBM Corporation, New Orchard Road, Armonk, NY 10504, USA) and was compared using the Tukey test to determine differences among concentrations and storage temperature used for each EO and its main compound, between EOs, between main compounds, among EO and main compounds and along the storage time, at 5% significant level (interval of confidence 95%). All results were expressed as mean ± standard deviation.
Results and discussion
Essential oils and essential oil components antimicrobial activity
The observed MIC values for EOs and EOCs are indicated in the Supplementary material (Table 8). For bacteria tested, E. coli S3 showed a higher MIC value for LG (0.25%) in contrast to and CI that achieved 0.20%. The observed MIC values are higher than those reported for LG (0.12%) and CI (0.15%) by Naik et al. (2010) and Guerreiro et al. (2015). However, Hemaiswarya and Doble (2009) reported a MIC of 20 mM (0.33%) for CI against Gram negative bacteria. This dissimilarity may be associated with several factors, including different strains tested and differences in the methodology used (Faleiro et al. 2005). Both bacteria tested had low MIC values for CL (0.06%) and for EU (0.08%). The same MIC value of EU for Listeria was reported by Apolónio et al. (2014). EOs and EOCs used were active against all the tested moulds. For B. cinerea and P. digitatum, the MIC of LG was slightly higher than that of CI and the MIC of EU was higher than that of the CL for P. digitatum and P. expansum.
According to these results and considering that the nanoemulsions loaded with EOs or EOCs prepared during this work may be applied to foods with long-term storage, where oils volatilization can occur, they were prepared with concentrations of EOs or EOCs higher than the corresponding MIC.
Droplet size
Significant differences were observed in the nanoemulsions droplet size related to the loaded EOs or their main components, (CI or EU) (Table 1). LG and CI-nanoemulsions presented the smallest droplet sizes, followed by CL-nanoemulsions and EU-nanoemulsions, which presented the highest droplets.
For producing emulsions with droplet sizes in the nano-range, it is necessary large amount of surfactant and/or energy (Tadros et al. 2004). For this reason, the nanoemulsions of the present work were obtained from coarse emulsions submitted to microfluidization and in the presence of the surfactant Tween 80. In the present study, CL, LG, CI or EU/Tween 80 ratio of 1/3 was maintained in order to assure the complete coating of lipid droplets surface with adsorbed surfactant molecules, according to Qian and McClements (2011). Moreover, the same processing conditions and sodium alginate concentration was used in the formation of all nanoemulsions, thus the influence of biopolymer on the observed droplet size differences was avoided.
In all cases, the droplet sizes in our work were higher than those reported by Salvia-Trujillo et al. (2015a). Perhaps, the number of cycles used for obtaining nanoemulsions has contributed to these differences. In the present work, five cycles were used, whereas those authors have used 3 cycles for producing nanoemulsions that incorporate essential oils including those of LG and CL. The droplet size increases with increasing the input energy, either by increasing the microfluidization pressure or number of cycles. In both cases, the probability of collision and coalescence is higher and the timescale of collision is shorter than the timescale of adsorption, consequently, the surfactant loses its capacity for involving the fresh interface of the newly formed droplets (Salvia-Trujillo et al. 2015a). This hypothesis can be supported by the results of Salvia-Trujillo et al. (2015a) for nanoemulsions with CL and LG. Those authors found that the size of CL-nanoemulsions, after microfluidization was similar to those observed in the coarse emulsion. However, it was expected that microfluidization would shorten the size of the droplets. Those results may indicate that for EU or CL–EO, Tween 80 was not the most adequate surfactant as it was for CI or LG nanoemulsions, at least for the concentrations tested. Such different behaviour of CL or EU and LG or CI may be attributed to diverse factors such as those already reported by Salvia-Trujillo et al. (2015a): molecular structure, concentration of volatile compounds in the case of the EOs which are constituted by thousands of compounds, viscosity, interfacial tension, and surfactant affinity. Alginate concentration in our nanoemulsions was twofold higher than that used by Salvia-Trujillo et al. (2015a), and it is suggested that during microfluidization, the excess of alginate molecules that were not adsorbed at oil droplets were repelled, remaining in the emulsion as surfactant micelles or aggregates of the biopolymer molecules that do not contain oil (Artiga-Artigas et al. 2017), with effect on the droplet size measured. Moreover, CI is a mixture of cis- and trans-isomers. The molecular weight of each isomer is 152.12 g/mol and the topological polar surface area (sum of surfaces of polar atoms in the molecule) is 17.07 Å2, which is smaller and less polar than EU (164.08 g/mol and 29.46 Å2, respectively) (Fernandes and Gattass 2009). The higher polarity of EU makes it more soluble in the aqueous phase than CI, which can be other factor that contributed to the larger droplet sizes of CL and EU-nanoemulsions than in the LG and CI-nanoemulsions.
LG-nanoemulsions and CI-nanoemulsions stored at 1 °C showed droplet sizes in the nano-range (< 95 nm) until the end of storage, with the exception of LG 0.5%, which increased significantly after 2 months then decreased, being at the end of the experiment similar to the other concentrations (Table 1). When stored at 21 °C, LG 0.5%, CI 0.4% and CI 2%-nanoemulsions droplet size increased during the first 2 months, and afterwards it decreased (Table 1). This decrease could be explained because sedimentation in the bottom of the bottle was observed in all LG and CI-nanoemulsions, thus indicating nanoemulsion instability.
The higher the temperature of storage, the higher the solubility and diffusivity of the nanoemulsions disperse phases (oils in O/W emulsions), and therefore an increase of the Ostwald ripening rate may occur (Gupta et al. 2016). Thus, at 1 °C, this process was slower and the droplet size was maintained or slightly increased during the 6 months of storage, while at 21 °C it was faster and the larger particles formed by Ostwald ripening were quickly deposited at the bottom of the storage tubes while at the middle height of the tubes, where the aliquots were collected for measurements, the smallest particles remained. Although the density of the dispersed oil phase is lesser than the continuous water, the density of the stabilizing layer around a nanoemulsion droplet is higher than the density of the oil (Rodríguez-Burneo et al. 2017). According to Saberi et al. (2013) the rate of droplet growth due to Ostwald ripening increases with increasing temperature. However, it is usually difficult to establish with accuracy the droplet growth in oil-in-water emulsions because both coalescence and Ostwald ripening led to a net increase in mean droplet size (McClements 2005).
Regarding the stability of EU-nanoemulsions, they showed a large decrease in droplet size during the first 2 months of storage and then stabilization at both temperatures (Table 1). For CL-nanoemulsions stored at 1 °C there was an increased in droplet size, while at 21 °C a pattern cannot be established for the changes of droplet size over time. These results are probably linked to the fact that eugenol and hence the EOs that contain it in their compositions, such as CL, are specifically prone to Ostwald ripening due to its relatively high solubility in water (Wang et al. 2010). In fact, Abd-Elsalam and Khokhlov (2015) obtained EU-nanoemulsions with excellent long-term stability and relatively small particle size, when the surfactant was added together with a ripening inhibitor as sesame and bean oil (long-chain triglycerides). These mixed oil nanoemulsions were stable because the entropy gain associated with the mixing acts as a thermodynamic barrier to Ostwald ripening (Wooster et al. 2008).
Differences in droplet size observed in the present work among EOs or their main compounds-loaded nanoemulsions might be attributed to their molecular structure, concentration of volatile compounds, interfacial tension or surfactant affinity to each type of EO or their main compounds (Salvia-Trujillo et al. 2015a).
Polydispersity
Polydispersity values below 0.2 indicate uniformity among oil droplet sizes or monomodal distributions and therefore better stability, whereas values close to 1 indicate a heterogeneous or multimodal distribution (McClements et al. 2012). In our case, values were closer to 1 indicating heterogeneous distribution.
The different volatile compounds (CI and EU) or volatile oils (LG and CL) tested and their concentrations had different effects on polydispersity of their nanoemulsions (Table 2). LG and CI-nanoemulsions had the lowest polydispersity at the highest EO concentrations (Table 2). For CL- and EU-nanoemulsions, the decrease of polydispersity index with the increase of EO concentration did not happen. For example, the highest concentration of EU (0.8%) produced the EU-nanoemulsion with the highest polydispersity index, whereas for CL-nanoemulsion, the concentration of CL–EO was 0.3% (Table 2). When the concentration of EU or CL increases (the ratio EO/surfactant is always 1/3), a higher number of droplets will be formed, including those with large size due to the reasons aforementioned. The DLS technique for measuring particle size analyses the fluctuations in scattered light intensity. Large particles scatter light more strongly than small particles (Salvia-Trujillo et al. 2015a). The formation of high amounts of large droplets, affect the homogeneity of droplet size distribution, which results on increased polydispersity index. In the case of LG or CI-emulsions, with the increase of the EO concentration and that of surfactant able to adsorb them, more and more small droplets were formed. The concentrations of Tween 80 used in the present work to keep the ratio EO/surfactant 1/3 are above its critical micellar concentration (0.012 mM, that is, ≈ 15 mg/L of this surfactant) (Qian and McClements 2011), therefore, micellar phases are formed and when larger concentrations of surfactant are used, the micellar phases lead to an increase of the effective viscosity of the continuous phase and, according to the ratio ηd/ηc (dispersed to continuous phases), smaller droplets are originated (Table 1), beyond the reduction of interfacial tension (McClements 2005). As small droplets scatter light less strongly than large particles the polydispersity index decreased for CI and LG-nanoemulsions (Table 2).
During storage, LG nanoemulsions showed a decrease in polydispersity at both temperatures, but starting later when storage took place at 1 °C. CI-nanoemulsions maintained the polydispersity values at 1 °C, while at 21 °C, it decreased (Table 2). CL-nanoemulsions polydispersity remained almost constant at both temperatures. At 21 °C, EU-nanoemulsions polydispersity had a tendency to decrease with time. Although it is not possible to establish a pattern for polydispersity at 1 °C, values at the end of the experiment were 3 times higher than at 21 °C.
The polydispersity change throughout storage seems to be linked to the maintenance or increase of particle size when stored at 1 °C and the decrease through storage at 21 °C. The deposition of the larger particles generated by coalescence and/or Ostwald ripening would have allowed that particles with the closest sizes have remained in the middle zone of storage tubes where the aliquots for analysis were collected, thus explaining the higher reduction of polydispersity for nanoemulsions maintained at 21 °C. Also, Saberi et al. (2013) reported a polydispersity decrease in nanoemulsions composed by 10% oil phase (100% vitamin E acetate) + 10% surfactant phase (Tween 80) + 80% aqueous phase (40% glycerol in buffer), with increased storage temperature (5, 20 and 37 °C). The origin of this effect can be related to progressive dehydration of the polar head group of the non-ionic surfactant molecules (Tween 80) as the temperature raised. This process reduces the hydration repulsion between the droplets thereby allowing them to get closer. In addition, head group dehydration means that the optimum curvature of the surfactant monolayer moves closer to unity, which favors an ultra-low interfacial tension and promotes droplet coalescence and droplet growth due to Oswald ripening (McClements 2005; Saberi et al. 2013).
ζ-potential
Particles with ζ-potential more positive than + 30 mV or more negative than − 30 mV are usually considered to be stable, since electrical charge of droplets is strong enough to assume that repulsive forces between droplets are predominant in the nanoemulsion (Salvia-Trujillo et al. 2015a). In the beginning of the experiment, almost all nanoemulsions of the present study fulfill this requirement (Table 3). All nanoemulsions tested presented negative ζ-potential values, despite the fact that a neutral or slightly negative electrical charge was expected at the oil–water interface, according to the non-ionic low-mass nature of Tween 80 (Artiga-Artigas et al. 2017). It has been reported that a nonionic emulsifier/surfactant can give a negative charge to oil droplets, due to preferential adsorption of hydroxyl ions from the aqueous phase or to the presence of anionic impurities such as free fatty acids in the surfactant or oil phases (McClements, 2005). Moreover, the anionic groups of sodium alginate molecules dispersed in the aqueous phase have a strong influence in the ζ-potential values. Mechanical treatment of emulsions such as microfluidization, it might cause the opening of the biopolymer chain by mechanical shear, releasing free hydroxyl and carboxyl groups from their molecular structures available to bind with water (Artiga-Artigas et al. 2017). These deprotonated alcohols or carboxylic acids (R–O− or R′CO2−, respectively) contributed to increase the negative charge in the interface of the droplets (Artiga-Artigas et al. 2017).
Generally, LG-nanoemulsions exhibited the less negative and CL-nanoemulsions the more negative ζ-potential values.
According to Guerra-Rosas et al. (2016) differences observed in the ζ-potential of emulsions and nanoemulsions formulated with different essential oils might be attributed to differences in the dissociation degree and number of ionizable compounds of oils. Therefore, the differences in the ζ-potential observed among essential oils might be due to differences between the adsorption of the surface-active compounds in the oil–water interface (Salvia-Trujillo et al. 2015a). Moreover, ζ-potential varied significantly depending on the concentration of the different components of the emulsions and the type of oil used (Artiga-Artigas et al. 2018). Just made LG and CI-nanoemulsions showed the more negative ζ-potential values at the lowest concentration tested. The ζ-potential values reported by Salvia-Trujillo et al. (2015b) for LG-nanoemulsions stabilized with the same nonionic surfactant had more negative ζ-potential than the obtained in this work and did not presented differences among the EO concentration incorporated. However, our ζ-potential values for LG and CL-nanoemulsions were similar to results obtained by Salvia-Trujillo et al. (2015a) for nanoemulsions containing 1% (v/v) lemongrass or 1% (v/v) clove + 1% (w/v) of sodium alginate + 1% (v/v) of Tween 80. CL-loaded nanomeulsions did not show significant differences in ζ-potential values regardless the tested concentrations.
In all nanoemulsions tested, less negative ζ-potential values were observed throughout storage at both temperatures (Table 3). This may be due to a reduction of adsorption of alginate molecules at the droplets interface (Guerra-Rosas et al. 2016). So, the initial, more negative ζ-potential values for all EOs nanoemulsions were not sufficient to achieve electrostatic stabilization and therefore, being highly unstable, prone to particle aggregation and sedimentation.
Whiteness index
All fresh nanoemulsions showed a decrease in WI values with increased oil concentration, except LG-nanoemulsions in which the decrease was not significant (Table 4). It is known that the emulsion color depends mainly of light scattered, the refractive index of continuous and dispersed phase, oil concentration, droplet concentration and size (McClements 2002). Large particles scatter the light more intensely than smaller ones, which cause an increase in the lightness, opacity and WI of emulsions (Qian and McClements 2011). In this regard, transparency of nanoemulsions is highly desirable. Salvia-Trujillo et al. (2015a, b) reported lower WI values for nanemulsions containing clove (1%) + alginate 2% and lemongrass EO (0.1, 0.5 and 1%) + alginate 2% than the values found in the present study. The higher size of droplets obtained in this study for lemongrass and clove-nanoemulsions may explain these differences.
According to Salvia-Trujillo et al. (2014) the color of the emulsions was basically determined by the oil and Tween 80 concentrations, namely, the lower the lemongrass essential oil and the higher the Tween 80 concentrations, the lower the WI. However, in this study, although the proportionality of essential oil to Tween 80 was the same, the WI decreased as the EO concentration increased. This can be explained by reduction of droplet size with increasing oil concentration (Table 1), since small particles scatter light less strongly.
During storage at both temperatures, the WI value of the nanoemulsions containing LG and CI remained practically constant. CL and EU-nanoemulsions maintained WI values at both temperatures, except EU at the higher concentration, which increased in the first 2 months. There was no significant difference between the two temperatures for all EO (Table 4). These results are not explained only by the reduction of average droplet size that occurred through storage especially at the highest temperature. Probably, others droplet characteristics, as droplet concentration and droplet aggregation, that influence the optical properties of oil-in-water emulsions, have intervened (Qian and McClements 2011).
Antimicrobial activity
EOs antimicrobial effectiveness depends, not only, on their active compounds but also on their concentration (Guerra-Rosas et al. 2016). In addition, the storage temperature may interfere with the antimicrobial properties of the EOs (Salvia-Trujillo et al. 2014). Therefore, the antibacterial action against E. coli of all nanoemulsions was evaluated immediately after being prepared and along storage period.
LG and CI-nanoemulsions were effective in reducing E. coli growth at 5X and 10X the MIC, in a similar way for both storage temperatures. Nevertheless, LG at 2X MIC (0.5%) was effective in reducing E. coli but only in fresh made nanoemulsions.
All CL- and EU-nanoemulsions showed little or no antimicrobial activity from the beginning and throughout storage at both temperatures (Table 5). This means that CL and EU suffered a significant loss in their antimicrobial efficacy when incorporated into nanoemulsions. It was found that the MIC of CL and EU against E. coli S3 was 0.06% and 0.08%, (w/w), respectively. However, just prepared nanoemulsions containing CL 0.6% and EU 0.8% (10X the MIC) reduced the bacterial viability only by 0.16 and 0.2 log CFU/ml, respectively. This was unexpected since antimicrobial efficacy of clove and eugenol based O/W emulsion has been confirmed previously by various studies (Abd-Elsalam and Khokhlov 2015; Salvia-Trujillo et al. 2015a). Nevertheless, Terjung et al. (2012) reporting EU-nanoemulsions with Tween 80 surfactant, found that small droplets (200 nm) were less effective than larger droplets (3000 nm). They suggest that sequestration of phytophenols at the interface resulted in less availability in the aqueous phase and ultimately limited their efficacy as antimicrobials. On the other hand, Ma et al. (2016) reported a negative effect of Tween 80 on the antimicrobial activity of EU which was attributed to a strong hydrophobic binding between Tween 80 and EU reducing the amount of EO available to interact with bacteria. Moreover, Majeed et al. (2016) referred that MIC values obtained against GNB (E. coli) and GPB strains (Listeria monocytogenes and Staphylococcus aureus) were less for CL-nanoemulsion in comparison with CL + Tween80-nanoemulsion, and also time kill dynamic experiment results showed that CL-nanoemulsion was more effective at inhibiting the growth of GPB and GNB strains in comparison with CL + Tween80-nanoemulsion. In fact, it has been observed that antimicrobial activity of EU was affected by the emulsifier. EU-nanoemulsions containing Tween80 (Terjung et al. 2012) or whey protein-maltodextrin conjugates (Shah et al. 2013) exhibited a higher MIC or MBC than free EU. The emulsions prepared with gum arabic could lower the MIC and MBC or not adversely affect the antimicrobial activity (Hu et al. 2016).
For most of nanoemulsions, the results showed that antimicrobial effectiveness of EO-nanoemulsions against E. coli increased as their EO-concentration increased and this effect was higher at 1 °C than at 21 °C (Table 5). It can be observed that nanoemulsions decreased their antimicrobial capacity through storage time. It is known that the overall antibacterial effect of EO largely depends on their volatile composition, which may be an explanation for the loss of EO-nanoemulsion antimicrobial capacity over the storage period. Recently, Guerra-Rosas et al. (2016) confirmed that the loss of EO-nanoemulsions antimicrobial activity was related to the loss of their volatile compounds during long term storage. Generally, antimicrobial capacity of EO-nanoemulsions against E. coli presented the following order: LG > CI > EU = CL-nanoemulsions (Table 5).
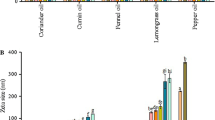
Clove and LG–EO and their principal components EU and CI were active against B. cinerea DSM 877. These EOs showed MIC values of 0.06% and 0.25% (w/w), respectively (unpublished data). In the current work, B. cinerea mycelial growth was initially completely inhibited by nanoemulsions containing LG 2.5% (Fig. 1a, b) or CI at 1 and 2% (Fig. 1c, d). Nevertheless, after 2 months of storage, these nanoemulsions did not inhibit the fungal growth, but the fungus showed longer lag phases (Fig. 1a, c) when exposed to nanoemulsions stored at 1 °C, although similar duplication times at both storage temperatures was achieved (Fig. 1b, d).
Growth of Botritys cinerea in the presence of nanoemulsions with different concentrations of: lemongrass EO, lag phase (a) and duplication time (b); citral, lag phase (c) and duplication time (d); clove EO, lag phase (e) and duplication time (f) and eugenol, lag phase (g) and duplication time (h). The effect of nanoemulsions in Botrytis growth, were tested when they were produced (day 0) and after 2, 4 and 6 months of storage at 1 °C and at 21 °C. * Without mycelial growth. Data shown represent the mean ± standard deviation
It has been shown that nanoemulsions loading LG–EO clearly improved the essential oil antimicrobial activity towards nine bacteria and ten fungal strains, including B. cinerea (Bonferoni et al. 2017). In that case, Minimum Fungicidal Concentration (MFC) value for nanoemulsion was 0.5% (of solution with 1.5 mg lemongrass/mL).
In this study, nanoemulsions containing CL and EU were not so effective as LG and CI in reducing the fungus growth (Fig. 1e–h). During storage, only the nanoemulsions containing the highest concentrations of those EOs caused increased lag phases up to 4 months storage (Fig. 1e, g), and similar fungus doubling time up to 6 months for both storage temperatures (Fig. 1f, h). This slightly inhibition in fungus growth can be attributed to the negative effect of Tween 80 on the antimicrobial activity of EU as reported by Ma et al. (2016). Effectively, the mycelial growth of B. cinerea was very susceptible to free EU with EC50 values of 38.6 µg/mL (Wang et al. 2010). And also, the CL–EO vapors effectiveness against B. cinerea was demonstrated by in vitro and in vivo assays (Aguilar-González et al. 2015).
Conclusion
This study demonstrates that the oil phase composition (oil type and concentration), as well as, storage temperature had a significant influence on physical stability and antimicrobial activity of nanoemulsions.
Throughout storage, LG 1.25% and 2.5%-nanoemulsions and CI 1.0% and 2%-nanoemulsions maintained the values of droplet size, polydispersity, ζ-potential and WI close to their initial values and prolonged their inhibitory effect on bacterial and fungi growth, making them suitable for commercial purposes.
CL and EU-nanoemulsions had droplet size higher than 100 nm and with low antimicrobial effect even when they were fresh due to a strong hydrophobic binding between Tween 80 and these EOs.
All tested EO-nanoemulsions become less stable and lost antimicrobial activity faster when stored at high temperature (21 °C), thus storage at low temperature is preferable.
Based on the analyzed parameters (droplet size, polydispersity, ζ-potential, whiteness índex, E. coli viability, induction of B. cinerea lag phase and duplication times) the LG and CI nanoemulsions at the 2 higher concentrations and stored at 1 °C, maintained better for long term storage (6 months) their properties. Therefore, further studies need to be done to confirm their effectiveness on the preservation of food products, maintaining their organoleptic properties and safety.
References
Abd-Elsalam KA, Khokhlov AR (2015) Eugenol oil nanoemulsion: antifungal activity against Fusarium oxysporum f. sp. vasinfectum and phytotoxicity on cottonseeds. Appl Nanosci 5:255–265
Aguilar-González AE, Palou E, López-Malo A (2015) Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innov Food Sci Emerg Technol 32:181–185
Apolónio J, Faleiro ML, Miguel MG, Neto L (2014) No induction of antimicrobial resistance in Staphylococcus aureus and Listeria monocytogenes during continuous exposure to eugenol and citral. FEMS Microbiol Lett 354:92–101
Artiga-Artigas M, Acevedo-Fani A, Martín-Belloso O (2017) Effect of sodium alginate incorporation procedure on the physicochemical properties of nanoemulsions. Food Hydrocoll 70:191–200
Artiga-Artigas M, Guerra-Rosas MI, Morales-Castro J et al (2018) Influence of essential oils and pectin on nanoemulsion formulation: a ternary phase experimental approach. Food Hydrocoll 81:209–219
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71–79
Bonferoni MC, Sandri G, Rossi S et al (2017) A novel ionic amphiphilic chitosan derivative as a stabilizer of nanoemulsions: improvement of antimicrobial activity of Cymbopogon citratus essential oil. Colloids Surf B Biointerfaces 152:385–392
Camele L, Altieri L, DeMartino L et al (2012) In vitro control of post harvest fruit rot fungi by some plant essential oil components. J Mol Sci 13:2290–2300
Chekabab SM, Paquin-Veillette J, Dozois CM, Harel J (2013) The ecological habitat and transmission of Escherichia coli O157:H7. FEMS Microbiol Lett 341:1–12
Faleiro L, Miguel G, Gomes S et al (2005) Antibacterial and antioxidant activities of essential oils isolated from Thymbra capitata L. (Cav.) and Origanum vulgare L. J Agric Food Chem 53:8162–8168
Fernandes J, Gattass CR (2009) Topological polar surface area defines substrate transport by multidrug resistance associated protein 1 (MRP1/ABCC1). J Med Chem 1:1–5
Ferreira JP, Alves D, Neves O et al (2010) Effects of the components of two antimicrobial emulsions on food-borne pathogens. Food Control 21:227–230
Guerra-Rosas MI, Morales-Castro J, Ochoa-Martínez LA et al (2016) Long-term stability of food-grade nanoemulsions from high methoxyl pectin containing essential oils. Food Hydrocoll 52:438–446
Guerreiro AC, Gago CML, Faleiro ML et al (2015) The effect of alginate-based edible coatings enriched with essential oils constituents on Arbutus unedo L. fresh fruit storage. Postharvest Biol Technol 100:226–233. https://doi.org/10.1016/j.postharvbio.2014.09.002
Gupta A, Eral HB, Hatton TA, Doyle PS (2016) Nanoemulsions: formation, properties and applications. Soft Matter 12:2826–2841. https://doi.org/10.1039/c5sm02958a
Hakkı Alma M, Murat Ertaş SN, Kollmannsbergerb H (2007) Chemical composition and content of essential oil from the bud of cultivated Turkish clove. BioResources 2:265–269
Hemaiswarya S, Doble M (2009) Synergistic interaction of eugenol with antibiotics against gram negative bacteria. Phytomedicine 16:997–1005
Hu Q, Gerhard H, Upadhyaya I et al (2016) Antimicrobial eugenol nanoemulsion prepared by gum arabic and lecithin and evaluation of drying technologies. Int J Biol Macromol 87:130–140
Ma Q, Davidson PM, Zhong Q (2016) Antimicrobial properties of microemulsions formulated with essential oils, soybean oil, and Tween 80. Int J Food Microbiol 226:20–25
Majeed H, Liu F, Hategekimana J et al (2016) Bactericidal action mechanism of negatively charged food grade clove oil nanoemulsions. Food Chem 197:75–83
McClements DJ (2002) Colloidal basis of emulsion color. Curr Opin Colloid Interface Sci 7:451–455
McClements DJ (2005) Food emulsions: principles, practices, and thecniques, vol 2. CRC Press, Boca Raton
McClements DJ, Henson L, Popplewell LM et al (2012) Inhibition of Ostwald ripening in model beverage emulsions by addition of poorly water soluble triglyceride oils. J Food Sci 77:33–38
Mohamed Hanaa AR, Sallam YI, El-Leithy AS, Aly SE (2012) Lemongrass (Cymbopogon citratus) essential oil as affected by drying methods. Ann Agric Sci 57:113–116
Naik MI, Fomda BA, Jaykumar E, Bhat JA (2010) Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacterias. Asian Pac J Trop Med 3:535–538
Qian C, McClements DJ (2011) Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocoll 25:1000–1008
Rodríguez-Burneo N, Busquets M, Estelrich J (2017) Magnetic nanoemulsions: comparison between nanoemulsions formed by ultrasonication and by spontaneous emulsification. Nanomaterials 7:190
Saberi AH, Fang Y, McClements DJ (2013) Effect of glycerol on formation, stability, and properties of vitamin-E enriched nanoemulsions produced using spontaneous emulsification. J Colloid Interface Sci 411:105–113
Salvia-Trujillo L, Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O (2014) Formulation of antimicrobial edible nanoemulsions with pseudo-ternary phase experimental design. Food Bioprocess Technol 7:3022–3032
Salvia-Trujillo L, Rojas-Graü A, Soliva-Fortuny R, Martín-Belloso O (2015a) Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll 43:547–556
Salvia-Trujillo L, Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O (2015b) Use of antimicrobial nanoemulsions as edible coatings: impact on safety and quality attributes of fresh-cut fuji apples. Postharvest Biol Technol 105:8–16
Schneeman BO (2004) Plenary lecture 6. J Food Sci 69:123–126
Shah B, Davidson PM, Zhong Q (2013) Nanodispersed eugenol has improved antimicrobial activity against Escherichia coli O157:H7 and Listeria monocytogenes in bovine milk. Int J Food Microbiol 161:53–59
Tadros T, Izquierdo P, Esquena J, Solans C (2004) Formation and stability of nano-emulsions. Adv Colloid Interface Sci 108–109:303–318
Terjung N, Löffler M, Gibis M et al (2012) Influence of droplet size on the efficacy of oil-in-water emulsions loaded with phenolic antimicrobials. Food Funct 3:290–301
Waites MJ, Morgan NL, Rockey JS, Higton G (2001) Industrial microbiology: an introduction. Blackwell, London
Wang C, Zhang J, Chen H et al (2010) Antifungal activity of eugenol against Botrytis cinerea. Trop Plant Pathol 35:137–143
Wooster TJ, Golding M, Sanguansri P (2008) Impact of oil type on nanoemulsion formation and ostwald ripening stability. Langmuir 24:12758–12765
Acknowledgements
Author Custódia Maria Luís Gago thanks Fundação para a Ciência e a Tecnologia (Portugal) for their postdoctoral fellowship SFRH/BPD/108831/2015. Author María Artiga-Artigas thanks the University of Lleida for her predoctoral fellowship. This study was funded by the Ministry of Economy, Industry and Competitiveness (MINECO/FEDER, UE) throughout Project AGL2015-65975-R.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gago, C.M.L., Artiga-Artigas, M., Antunes, M.D.C. et al. Effectiveness of nanoemulsions of clove and lemongrass essential oils and their major components against Escherichia coli and Botrytis cinerea. J Food Sci Technol 56, 2721–2736 (2019). https://doi.org/10.1007/s13197-019-03762-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03762-1