Abstract
The food industry faces challenges in maintaining food quality, preservation, and safety. Chemical preservatives like sodium benzoate and potassium sorbate can change the odor, taste, and texture of fruit juices. So, there is consumer demand for safe and eco-friendly food preservatives which are of natural origin. Therefore, in the present study, oregano essential oil–based nanoemulsion has been prepared for use as a natural preservative. Oregano essential oil (OEO)-based nanoemulsion was prepared using of oregano essential oil, Tween80, and water by ultrasonic emulsification. The average droplet diameter of the stable formulated nanoemulsion was 22 nm with polydispersity index (PDI) of 0.107 and spherical morphology. The optimized formulation showed significant antibacterial activity against Escherichia coli, affecting membrane permeability and causing bacterial death and lysis. To test whether the antibacterial activity would translate to food systems, antibacterial activity of selected oregano oil nanoemulsion in fresh fruit juice (mango) was determined using sodium benzoate as positive control. The shelf life of the mango juice was extended up to 120 h by incorporating oregano essential oil nanoemulsion. The nanoemulsion exhibited better antimicrobial activity at 4 °C storage than at 25 °C. This study suggests that oregano oil nanoemulsion could be used as a natural preservative for preserving fruit juice from microbial spoilage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Food industry is growing rapidly and fruit juice is an essential commodity in terms of nutrition to remain healthy. But preservation of a fresh fruit juice is a difficult task due to the activity of food-borne microorganisms leading to its spoilage [1]. A vast population of microorganisms plays a significant role in food spoilage and food-borne illnesses. Examples of these microorganisms include Aeromonas spp, Bacillus cereus, Bacillus circulans, Brochothrix spp, Brucella spp, Campylobacter jejuni, Clostridium spp, Coxiella burnetii, Escherichia coli, lactic acid bacteria (LAB), Leuconostoc spp, Listeria monocytogenes, Mycobacterium bovis, Plesiomonas shigelloides, Salmonella typhi, Staphylococcus aureus, Shigella, Streptococcus spp, Vibrio parahaemolyticus, Weissella spp, Yersinia enterocolitis, as well as various fungi like Alternaria spp, Aspergillus flavus, Aspergillus niger, Bothrytis, Byssochlamys, Candida spp., Dekkera spp., Fusarium spp., Mucor, Penicillium spp., Rhizopus nigricans, Saccharomyces spp., and Zygosaccharomyces spp. [2]. Escherichia coli is one of the most virulent bacteria causing food spoilage due to the shiga-toxin produced by it [3, 4]. It can lead to food-borne illness including fever, stomach pain, bloody diarrhea, and in extreme cases it may lead to life-threatening hemolytic uremic syndrome (HUS) [5].
According to World Health Organization (WHO) estimation, one in 10 people each year falls ill by the consumption of contaminated or spoiled food [6, 7]. There are several methods of preservation of fruit juice such as freezing, thawing, drying, thermal processing, fermentation, etc. Some of the chemicals such as sodium benzoate and potassium sorbate have also been used for fruit juice preservation. But these traditional methods of preservation result in loss of nutrients, odor, texture, and taste. Also, the continuous usage of chemical preservatives over a longer period of time may also lead to drug resistance in bacteria [8].
Plant essential oil (EO) is a rich source of active volatile compounds such as anethole, ascaridole, benzaldehyde, benzoic acid, camphor, carvacrol, carvone, cinnamaldehyde, cinnamic acid, citral, eugenol, geraniol, limonene, linalool, menthol, myrcene, myristic acid, p-cymene, pinene, safrole, terpinene and thymol, etc., which consequences in different application purposes [9, 10]. These essential oils are of hydrophobic nature due to the aromatic volatile constituents that present in different plant parts such as bark, flower, leaf, root, seed, and twig. Burt [11] reported that antimicrobial monoterpenoids interfere in biochemical and physiological processes of microorganisms and result in disruption of growth and development of microorganisms.
Zhang et al. [12] reported the synergistic antifungal effects of cinnamaldehyde and nonanal vapors against Aspergillus flavus for prevention of fungal contamination in agricultural products. Basil and Pimenta dioica essential oils showed antibacterial activity against the food-borne bacteria S. aureus, E. coli, L. monocytogenes, P. aeruginosa, S. Enteritidis, and the food-spoilage mold B. nivea [13]. Oregano essential oil and starch octenylsuccination-based formulation was reported to have antimicrobial and water-resistant property in sweet potato starch films [14]. Siddiqua et al. [15] reported that cinnamaldehyde and clove EO used in watermelon juice showed decrease in microbial count at combination of one-fourth of MIC (at or below 5000 mg/L) of theses oils. Lemon EO applied in apple juice [16] showed positive response but affected the color and taste of the juice which was not accepted by the consumers. But consumers demand for natural, cost effective, and ecofriendly preservative so formulated nanoemulsion using oregano essential oil could be the solution to overcome this problem [17]. Although in the previous research study, essential oils could be directly used as a preservative in food industry, but if applied directly in food, it may incorporate organoleptic properties and also it may alter certain properties such as hydrophobicity, volatility, and reactivity of bioactive molecules [18]. To overcome this problem, nanoemulsion is formulated using essential oil and this formulation is applied in food beverages as a preservative.
Food-grade submicron emulsions have attracted much attention due to its potential applications in food industry [19]. However, food-grade emulsions are limitation in the formulation process for the need of all emulsion components to be generally recognized as safe (GRAS) and also because of the complexity that lies in a typical food system [20]. While considering the surfactants to stabilize food-grade emulsions, there are only a few surfactants available that can be used for food formulations [21]. In this perspective, Tweens/polysorbates (ethoxylated derivatives of sorbitan esters) are an appealing series of surfactants. Owing to its high hydrophilic and lipophilic balance (HLB) value of 15, Tween80 was opted to be used as surfactant. Also, being non-ionic in nature, Tween80 stabilizes nanoemulsion droplets via stearic stabilization. Due to its low molecular weight, Tween80 is proficient in lowering nanoemulsion droplet size efficiently than polymeric surfactants with larger molecular weight [22].
Propolis nanoemulsion is reported for use as natural food preservative by altering its strong and unpleasant flavor that alters the sensory characteristic foods [23]. Reports are available on submicron emulsion formulations, but their application is limited due to the use of the co-surfactants like pentanol, dodecane, etc., which are not recommended in the food and beverage industry [24]. In this study, we formulated co-surfactant (e.g., pentanol, dodecane)-free nanoemulsion from edible oil, i.e., oregano oil with low droplet size. Aim of the present study is to check the antimicrobial activity of selected oregano oil nanoemulsion in vitro and in situ for the food preservation against microbial spoilage.
2 Materials and Methods
2.1 Materials
Oregano essential oil was purchased from local market. Tween80 was purchased from Sigma Aldrich, India. Double distilled water (Cascada Bio Water, Pall Corporation) was used for all the experiments throughout the study. E. coli used in the study was purchased from National Collection of Industrial Microorganisms (NCIM), NCL, Pune, India, with accession no. 5662 [25].
2.2 Nanoemulsion Preparation
Oregano oil nanoemulsion was prepared in two phases. First, organic oil phase was prepared by mixing oregano essential oil (5 %) and Tween80 using a vortex mixer in different ratios of oil and surfactant (1:1, 1:2, 1:3, 1:4, and 1:5) (Table 1) [26]. Water was then added to the organic phase slowly drop wise by keeping the system on a magnetic stirrer (Spinot Model MC O1; Tarsons) at 500 rpm. Coarse emulsion prepared this way was subjected to ultrasonic emulsification for droplet size reduction [27].
2.3 Nanoemulsion Characterization
2.3.1 Droplet Size
Droplet size of oregano oil nanoemulsion was measured by a particle size analyzer (90 plus; Brookhaven Instruments Corporation, USA) [28]. Prior to this experiment, the formulated microemulsion was diluted with deionized double distilled water (Cascada Bio Water, Pall Corporation) to reduce multiple scattering effects and also to get rid of the effect of viscosity caused on account of emulsion ingredients.
2.3.2 Droplet Morphology
TEM was used to visualize the droplet morphology of the formulated oregano oil nanoemulsion. Suspension was prepared by mixing selected formulations in water/ethanol in the ratio of 1:100 and homogenizing using ultrasonicator. One drop of this dispersion was then pipetted out to cast onto carbon-coated grids with 200 meshes. The carbon grid was then air dried and subsequently fixed in a holder-containing specimen. Images were captured by a TEM (Jeol/JEM 2100) operating at 200 kV voltage.
2.4 Antibacterial Activity In Vitro
2.4.1 Inactivation Kinetics
Antibacterial activity of oregano oil nanoemulsion formulation was studied against Escherichia coli (NCIM accession no. 5662) in vitro bacteria growth medium. Inactivation kinetics was performed by the protocol following [29]. Overnight grown culture of E. coli was centrifuged at 5000g for 10 min and the pellet was washed two times in phosphate buffered saline (PBS, pH 7.4). Test bacterial culture was prepared with an inoculum size of 1.5 × 108 CFU/mL by adjusting the absorbance value at 600 nm to 0.132 (McFarland standard no 0.5) [30]. One percent (vol/vol) of this inoculum was then challenged with undiluted and diluted (10-fold, 100-fold, and 1000-fold) oregano oil nanoemulsion formulations for different incubation times (15 min, 30 min, 45 min, and 60 min). Different dilutions (10-fold, 100-fold, and 1000-fold) of oregano oil nanoemulsion in mango juice were prepared by adding 1 mL, 0.1 mL, and 0.01 mL of selected oregano oil nanoemulsion in mango juice for a total 10 mL test volume correspondingly. To estimate the number of live cells at different incubation times, 0.L ml of the interacted sample from each treatment group was spread onto Petri dishes containing nutrient agar medium and incubated in an incubator at 37 °C. Viable E. coli colonies were counted after 24 h.
2.4.2 Effect on the Permeability of Membrane of Bacteria
E. coli culture was inoculated in nutrient broth and incubated overnight on shaker. Next day, centrifugation at 6000 rpm was followed and the pellet was dissolved in PBS (phosphate buffer saline). Pellet was washed twice and then was re-suspended in PBS. Turbidity of culture was adjusted to 108 CFU/mL. This bacterial suspension was then treated with nanoemulsion, sodium benzoate (positive control) in 10 fold, 50 fold, and 100 fold dilutions for 1 h, 2 h, and 4 h, respectively, on shaker at 120 rpm. The bacterial suspension without treatment was used as control. After incubation, the suspension was centrifuged at 6000 rpm for 10 min and supernatant was collected to measure the absorbance at 260 nm by UV-visible spectrophotometer. This experiment was conducted in duplicates to find out the leakage of UV absorbing substances [31].
2.4.3 Evaluation of Surface Modification by Fourier Transform Infrared Spectroscopy (FTIR)
Fourier-transform infrared (FTIR) microscopy is considered one of the sensitive and comprehensive methods for detection of molecular changes in cells of bacteria and fungi. Modification of surface functional groups of E. coli upon treatment with oregano oil nanoemulsion was analyzed by FT-IR spectroscopy. Based on inactivation kinetics study result, 1.5 × 106 CFU/mL of E. coli was treated with 10-fold diluted oregano oil nanoemulsion formulation for 60 min. The bacterial cells were washed two times with phosphate buffered saline (pH 7.4) and cells were lyophilized. Sample for FTIR analysis was prepared by mixing the freeze-dried bacteria cells (control/untreated and microemulsion-treated) with potassium bromide crystals. Spectroscopic analysis was done in the range of 450–4000 cm−1 using Perkin Elmer Spectrum1 FT-IR instrument, which has typical resolution of 1.0 cm−1 to see the modifications in their representative peaks in the spectra [32].
2.4.4 Scanning Electron Microscopy (SEM)
Assessment of morphological alterations of E. coli upon treatment with oregano oil nanoemulsion was done by a scanning electron microscopy (SEM). Overnight grown culture of E. coli in nutrient broth (incubated in a rotary shaker at 37 °C) was harvested by centrifuging at 6000 g for 15 min followed by washing two times with phosphate buffered saline. Bacteria inoculum was prepared by diluting the bacteria pellet in phosphate buffer and 1.5 × 106 CFU/mL was treated with 10-fold diluted oregano oil nanoemulsion for 60 min. Sample for SEM analysis was prepared by mounting the interacted and control/un-interacted bacteria onto aluminum stubs and then the bacteria samples were coated with gold by a sputter coater. Processed samples were then visualized under a scanning electron microscope (high-resolution SEM, FEI Quanta FEG 200).
2.5 Antibacterial Activity In Situ in Mango Juice
Evaluation of in situ antimicrobial activity of oregano oil nanoemulsion formulation was carried out using mango juice as growth medium. Fresh mango juice was prepared aseptically in the laboratory by homogenizing the mangoes and filtering through a sieve. The freshly prepared mango juice was inoculated with 1 × 103 CFU/mL of E. coli and was treated with different dilutions (10-fold, 50-fold, and 100-fold) of oregano oil nanoemulsion. Suitable control was maintained without any treatment (negative control) and positive control was treated with sodium benzoate (equal concentration as nanoemulsion). To study the effect of temperature, the treatment groups were incubated at two different temperatures, i.e., 4 °C and 25 °C. Sample was collected from all treatment groups in regular interval of incubation time (0 h, 6 h, 24 h, 48 h, 72 h, 96 h, and 120 h) and spread onto nutrient agar plates. Viable colonies were counted after 16–24 h of incubation at 37 °C. All the experiments were carried out in duplicate.
2.6 Statistical Analysis
The results of antibacterial activity were analyzed by one-way ANOVA to establish the significant difference between the control and oregano essential nanoemulsion-treated groups using SPSS (IBM SPSS Statistics V23.0) software.
3 Results and Discussion
3.1 Nanoemulsion Droplet Size and Morphology
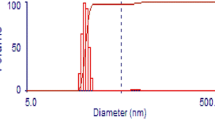
Oregano essential oil nanoemulsion formulation was stabilized by Tween80 (a high HLB surfactant) by reducing oil/water interfacial tension [33]. Droplet size reduced as the surfactant concentration increased in formulations with oil:surfactant ratio 1:1 to 1:2, 1:3, and 1:4 (Table 2). Further increase in surfactant concentration in formulation with 1:5 ratio of oil:surfactant did not result in significant reduction of surfactant concentration. Droplet size of OEO nanoemulsion with 1:4 ratio of oil and surfactant was measured to be 22 nm with a polydispersity index (PDI) value of 0.107 (Fig. 1). The OEO nanoemulsion with 1:1, 1:2, and 1:3 ratios of oil and surfactant was turbid in color, but 1:4 and 1:5 ratios of oil and surfactant were transparent in color. This is due to nanometric size droplet diameter, which leads to weak scattering of light making the emulsion system optically transparent [22, 34, 35].
Mono-modal peak of droplet size distribution and very low PDI of oregano oil nanoemulsion formulation indicates that the droplets in the nanoemulsion formulation are homogenous. The oregano essential oil–based nanoemulsion droplets were spherical in shape (Fig. 2). TEM micrograph also confirmed the data pertaining to droplet size.
3.2 Antibacterial Activity In Vitro
3.2.1 Inactivation Kinetics
Selected oregano oil nanoemulsion with 1:4 ratio of oil and surfactant was tested for its antibacterial efficacy in vitro against food-associated bacteria E. coli. Inactivation kinetics of bacteria population demonstrated dose- and time-dependent E. coli inactivation upon incubation with oregano oil nanoemulsion over a short period of time. All the bacteria cells were killed within 1 min of interaction with undiluted oregano oil nanoemulsion (result not shown). Figure 3 illustrates the reduction of E. coli population upon incubation with diluted oregano oil nanoemulsion.
Approximately 4-log reduction of E. coli cells was observed after 15 min of incubation with 10-fold diluted oregano oil nanoemulsion. Almost 80% E. coli cells were inactivated within 30 min of incubation time and complete loss of E. coli viability occurred in 60 min. Incubation with 100-fold diluted oregano oil nanoemulsion resulted in 2.15, 3.1, 3.9, and 4.7 log reduction of E. coli population in 15 min, 30 min, 45 min, and 60 min, respectively. E. coli incubated with 1000-fold diluted oregano oil nanoemulsion also showed significant bactericidal activity. When E. coli was treated with oregano oil pure form, nearly 25% and 33% bacteria were inactivated after 45 min of treatment, respectively (Table 3). MIC value for antibacterial activity in vitro is 5 μg/mL. The results of a one-way ANOVA analysis of the antibacterial activity of oregano essential oil–based nanoemulsion against E. coli in various treatment groups with differing concentrations of nanoemulsion revealed a statistically significant variance in antibacterial activity compared to the untreated control group. In all instances, the calculated P-values were less than 0.05, confirming that nanoemulsions based on oregano essential oils are indeed efficacious as antibacterial agents. Antibacterial activity of nanoemulsions is due to the active ingredients present in essential oil [22], and also due to the reduced droplet size and increased droplet surface area available to interact with bacteria.
Geraniol nanoemulsion was reported by Feng at al. [36], which demonstrated significant antibacterial activity against food-borne pathogens such as Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, and Listeria monocytogenes (p < 0.05). Ozogul et al. [37] reported antimicrobial effect of laurel essential oil nanoemulsion on food-borne pathogens (Pseudomonas luteola, Staphylococcus aureus, and Enterococcus faecalis) and fish spoilage bacteria. Antibacterial nanoemulsions from cinnamon essential oil co-emulsified with hydroxypropyl-β-cyclodextrin and Tween-80 were also reported [38]. Sage essential oil and its nanoemulsion demonstrated antibacterial activity on food-related pathogens (Enterococcus faecalis ATCC29212, Klebsiella pneumoniae ATCC700603, Salmonella Paratyphi A NCTC13, and Staphylococcus aureus ATCC29213) and spoilage microorganisms (Proteus mirabilis, Photobacterium damselae, Vibrio vulnificus, Enterococcus faecalis, Pseudomonas luteola, and Serratia liquefaciens) [39].
3.2.2 Effect on the Permeability of Membrane of Bacteria
To the study the mechanism of antibacterial action of oregano essential oil nanoemulsion, the effect of oregano essential oil nanoemulsion on the permeability of membrane of bacteria was investigated. Bacterial membrane gets ruptured by treating with OEO nanoemulsion, which results in release of UV absorbing substances such as DNA and the quantification can be done by measuring the absorbance at 260 nm.
A rapid increase in the leakage of 260 nm absorbing substances was observed in E. coli when treated with 10-fold dilution of OEO nanoemulsion. By treating with 100-fold dilution of OEO nanoemulsion, 50% release was observed after 30 min and 100% release was observed after 60 min (Fig. 4). After treating with 1000-fold dilution of OEO nanoemulsion, approximately 60% release was obtained after 60 min. The result proves that nanoemulsion affects the membrane integrity of bacteria and results in lysis and death of pathogen.
3.2.3 Fourier Transform Infrared Spectroscopy (FTIR)
FT-IR is one of the imperative methods to study the modification of bacterial surface functional groups. Spectral changes in the region of 400–4000 cm−1 were revealed in the E. coli cells treated with oregano oil nanoemulsion (Fig. 5). Bands were assigned according to previous reports. In bacteria control cells, the spectral region 2800–3000 cm−1 corresponds to C–H lipid region. The dominant bands at 1641 cm–1 and 1535 cm–1 can be attributed to protein amide I and II bands. Bands at 1238 cm–1 and 1078 cm–1 can be attributed to asymmetric and symmetric stretching vibrations of PO–2 and phospholipids.
Alteration in spectral features was observed in the E. coli cells treated with oregano oil nanoemulsion. In treated cells, bands at 2926 cm–1, 1535 cm–1, 1238 cm–1, 966 cm–1, and 862 cm–1 shifted to 2924 cm–1, 1543 cm–1, 1247 cm–1, 948 cm–1, and 860 cm–1, respectively. Change FTIR spectra can be attributed to O–H, N–H, C–H, C–O, C–CI, and C–S stretching vibrations of various functional groups of lipid, protein, carbohydrate, and nucleic acid. The FTIR results propose the modification of functional groups present on bacterial surface upon incubation with oregano oil nanoemulsion [40,41,42].
3.2.4 Scanning Electron Microscopy (SEM)
Microscopy is a vital tool to assess the morphological changes of bacteria. SEM images visualized cell membrane distortion in oregano oil nanoemulsion-interacted E. coli cells. The untreated/control cells of E. coli (Fig. 6a) exhibited intact cell structure. However, significant morphological changes were observed in bacteria cells exposed to oregano oil nanoemulsion. Intact control (untreated) E. coli cells became distorted after treatment with 10-fold diluted oregano oil nanoemulsion for 60 min. Cell membrane was remarkably distorted leading to leakage of intracellular contents and lysis of bacteria. As a result, some of the cells appeared approximately oval in shape (Fig. 6b), when compared to characteristic rod shape of E. coli.
The above result corroborates with the work carried out by Ghosh et al. [43], where interaction with nano-dispersions of cinnamon essential oil resulted in significant disintegration of bacterial (Staphylococcus aureus) membrane. Hwang. et al [44] also utilized scanning electron microscopy to investigate the effect of nanoemulsion on morphology of A. baumannii ATCC BAA-1605 (antibiotic-resistant strain) biofilms resulting in disruption and dispersion of the bacterial biofilms, with the reduction in overall bacteria present.
3.3 In situ Antibacterial Activity in Fresh Mango Juice
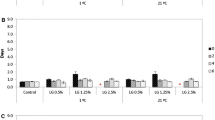
Oregano oil nanoemulsion added fresh mango juice showed a significant reduction in the bacterial population up to a time period of 6 h, followed by a gradual increase in the bacteria population (Table 4).
Approximately, 50% bacteria cells were killed within 6 h of interaction with 10-fold diluted oregano oil nanoemulsion. However, interaction with 50-fold diluted oregano oil nanoemulsion resulted in 50% reduction in bacterial population after 24 h. One log reduction in bacterial population was observed after 48 h of interaction with oregano oil nanoemulsion and a gradual increase in bacteria population was observed thereafter (Fig. 7).
Control group without treatment demonstrated increase in bacteria population with increase in incubation time. Positive control (i.e., treatment with sodium benzoate) exhibited significant reduction in bacterial population with respect to time and concentration. However, the bactericidal effect of oregano oil nanoemulsion was better than sodium benzoate (Table 2).
Effect of temperature on fruit juice preservation against microbial spoilage was studied by incubating all the treatment groups at two different temperatures, i.e., 4 °C and 25 °C. Antibacterial activities of both oregano oil nanoemulsion and sodium benzoate-treated groups were better at 4 °C than that at 25 °C (Fig. 7). MIC value for antibacterial activity in situ in mango juice is 10 μg/mL. The antibacterial activity was due to the bioactive compounds present in the essential oil. Oregano essential oil contained carvacrol (73%) as the key bioactive compound and other bioactive compounds were thymol (4.3%) and p-cymene (7.1%) as analyzed by GC-MS chromatography. Also, size reduction in the nanoemulsion enhanced the antibacterial activity. Also, the inclusion of mango juice did not have any significant effect on the sensory profile of mango juice.
4 Conclusions
Oregano oil nanoemulsion demonstrated significant antibacterial activity in vitro and in situ in mango juice against E. coli. Oregano oil nanoemulsion exhibited time and concentrated killing of bacteria. Considerable bactericidal activity was observed even at higher dilution (i.e., 1000-fold diluted) of oregano oil nanoemulsion. FTIR studies illustrate alteration in surface functional groups of bacteria upon treatment with oregano oil nanoemulsion, which would have resulted in membrane damage and consequent leakage of intracellular constituents. SEM images revealed the morphological changes in E. coli upon treatment with oregano oil nanoemulsion. It also visualized distortion of characteristic rod shape of E. coli to oval. In situ antibacterial evaluation in mango juice demonstrated time-, concentration-, and temperature-dependent bactericidal activity of oregano oil nanoemulsion. Antibacterial activities of both oregano oil nanoemulsion and sodium benzoate–treated mango juice were better at 4 °C than that at 25 °C.
Data Availability
Not applicable.
References
Aneja, K. R., Dhiman, R., Aggarwal, N. K., & Aneja, A. (2014). Emerging preservation techniques for controlling spoilage and pathogenic microorganisms in fruit juices. International Journal of Microbiology, 2014, 758942.
Joe, M. M., Bradeeba, K., Parthasarathi, R., Sivakumaar, P. K., Chauhan, P. S., Tipayno, S., Benson, A., & Sa, T. (2012). Development of surfactin based nanoemulsion formulation from selected cooking oils: Evaluation for antimicrobial activity against selected food associated microorganisms. Journal of the Taiwan Institute of Chemical Engineers, 43, 172–180.
Chen, H., Li, Y. K., Zhang, T. T., Bi, Y., Shu, M., Zhong, C., Tang, K. J., & Wu, G. P. (2021). A novel real-time loop-mediated isothermal amplification combined with immunomagnetic beads separation and ethidium bromide monoazide treatment for rapid and ultrasensitive detection of viable Escherichia coli O157: H7 in milk. Food Analytical Methods, 14(5), 944–956.
Evran, S., Tayyarcan, E. K., Acar-Soykut, E., Guven, B., Durakli-Velioglu, S., & Boyaci, I. H. (2022). Investigation of phage and molasses interactions for the biocontrol of E. coli O157: H7. Canadian Journal of Microbiology, 68(1), 55–65.
Stratakos, A. C., & Grant, I. R. (2018). Evaluation of the efficacy of multiple physical, biological and natural antimicrobial interventions for control of pathogenic Escherichia coli on beef. Food Microbiology, 76, 209–218.
Falleh, H., Jemaa, M. B., Saada, M., & Ksouri, R. (2020). Essential oils: A promising eco-friendly food preservative. Food Chemistry, 330, 127268.
Fung, F., Wang, H.-S., & Menon, S. (2018). Food safety in the 21st century. Biomedical Journal, 41(2), 88–95.
Yang, J., Goksen, G., & Zhang, W. (2023). Rosemary essential oil: Chemical and biological properties, with emphasis on its delivery systems for food preservation. Food Control, 154, 110003.
Koul, O., Walia, S., & Dhaliwal, G. S. (2008). Essential oils as green pesticides: Potential and constraints. Biopestic International, 4, 63–84.
Mohapatra, P., Singh, P., Singh, D., Sahoo, S., & Sahoo, S. K. (2022). Phytochemical based nanomedicine: A panacea for cancer treatment, present status and future prospective. OpenNano, 7, 100055.
Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in food – A review. International Journal of Food Microbiology, 94, 223–253.
Zhang, W., Li, B., Lv, Y., Wei, S., Zhang, S., & Hu, Y. (2023). Synergistic effects of combined cinnamaldehyde and nonanal vapors against Aspergillus flavus. International Journal Food Microbiology, 402, 110277.
Marques, C. S., Carvalho, S. G., Bertoli, L. D., Villanova, J. C. O., Pinheiro, P. F., Dos Santos, D. C. M., Yoshida, M. I., de Freitas, J. C. C., Cipriano, D. F., & Bernardes, P. C. (2019). β-Cyclodextrin inclusion complexes with essential oils: Obtention, characterization, antimicrobial activity and potential application for food preservative sachets. Food Research International, 119, 499–509.
Li, J., Ye, F., Lei, L., & Zhao, G. (2018). Combined effects of octenylsuccination and oregano essential oil on sweet potato starch films with an emphasis on water resistance. International Journal of Biological Macromolecules, 115, 547–553.
Siddiqua, S., Anusha, B. A., Ashwini, L. S., & Negi, P. S. (2015). Antibacterial activity of cinnamaldehyde and clove oil: effect on selected foodborne pathogens in model food systems and watermelon juice. Journal of Food Science and Technology, 52(9), 5834–5841.
Tserennadmid, R., Takó, M., Galgóczy, L., Papp, T., Pesti, M., Vágvölgyi, C., & Krisch, J. (2011). Anti-yeast activities of some essential oils in growth medium, fruit juices and milk. International Journal of Food Microbiology, 144(3), 480–486.
Barrett, D. M., & Lloyd, B. (2012). Advanced preservation methods and nutrient retention in fruits and vegetables. Journal of the Science of Food and Agriculture, 92(1), 7–22.
Sugumar, S., Singh, S., Mukherjee, A., & Chandrasekaran, N. (2016). Nanoemulsion of orange oil with non-ionic surfactant produced emulsion using ultrasonication technique: evaluating against food spoilage yeast. Applied Nanoscience, 6(1), 113–120.
Flanagan, J., & Singh, H. (2006). Conjugation of sodium caseinate and gum Arabic catalyzed by transglutaminase. Journal of Agricultural and Food Chemistry, 54, 7305–7310.
Garti, N., Yaghmur, A., Leser, M. E., Clement, V., & Watzke, H. J. (2001). Improved oil solubilization in oil/water food grade microemulsions in the presence of polyols and ethanol. Journal of Agricultural and Food Chemistry, 49, 2552–2562.
Dungan, S. R. (1997). In C. Solans & H. Kunieda (Eds.), Industrial applications of microemulsions (pp. 148–170). Dekker.
Ghosh, V., Mukherjee, A., & Chandrasekaran, N. (2013). Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrasonics Sonochemistry, 20, 338–344.
Seibert, J. B., Bautista-Silva, J. P., Amparo, T. R., Petit, A., Pervier, P., Dos Santos Almeida, J. C., Azevedo, M. C., Silveira, B. M., Brandão, G. C., de Souza, G. H. B., de Medeiros Teixeira, L. F., & Dos Santos, O. D. H. (2019). Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chemistry, 287, 61–67.
Fu, X., Feng, F., & Huang, B. (2006). Physicochemical characterization and evaluation of a microemulsion system for antimicrobial activity of glycerol monolaurate. International Journal of Pharmaceutics, 321, 171–175.
https://www.ncl-india.org/files/ncim/CatalogueDetails.aspx?NCIMNo=5662. Accessed 16 Jan 2017.
Bouchemal, K., Briançon, S., Perrier, E., & Fessi, H. (2004). Nano-emulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimisation. International Journal Pharmacology, 280(1–2), 241–51.
Setya, S., Talegaonkar, S., & Razdan, B. K. (2014). Nanoemulsions: Formulation methods and stability aspects. World Journal Pharmaceutica Pharmaceutica Science, 3(2), 2214–2228.
Groves, M. J., & Freshwater, D. C. (1968). Particle-size analysis of emulsion systems. Journal of Pharmaceutical Sciences, 57(8), 1273–1291.
Al-Adham, I. S. I., Khalil, E., Al-Hmoud, N. D., Kierans, M., & Collier, P. J. (2000). Microemulsions are membrane-active, antimicrobial, self-preserving systems. Journal of Applied Microbiology, 89, 32–39.
McFarland, J. (1907). Nephelometer: An instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. Journal of the American Medical Association, 14, 1176–1178.
Ghosh, V., Saranya, S., Mukherjee, A., & Chandrasekaran, N. (2013). Cinnamon oil nanoemulsion formulation by ultrasonic emulsification: Investigation of its bactericidal activity. Journal of Nanoscience and Nanotechnology, 13(1), 114–122.
Erukhimovitch, V., Pavlov, V., Talyshinsky, M., Souprun, Y., & Huleihel, M. (2005). FTIR microscopy as a method for identification of bacterial and fungal infections. Journal of Pharmaceutical and Biomedical Analysis, 37(5), 1105–8.
Tadros, T., Izquierdo, P., Esquena, J., & Solans, C. (2004). Formation and stability of nano-emulsions. Advances in Colloid and Interface Science., 108–109, 303–318.
McClements, D. J. (2002). Colloidal basis of emulsion color. Current Opinion in Colloid & Interface Science, 7, 451–455.
McClements, D. J. (2002). Theoretical prediction of emulsion color. dvances in Colloid and Interface Science, 97, 63–89.
Feng, X., Feng, K., Zheng, Q., Tan, W., Zhong, W., Liao, C., Liu, Y., Li, S., & Hu, W. (2022). Preparation and characterization of geraniol nanoemulsions and its antibacterial activity. Frontiers in Microbiology, 13, 1080300.
Özogul, Y., El Abed, N., & Özogul, F. (2022). Antimicrobial effect of laurel essential oil nanoemulsion on food-borne pathogens and fish spoilage bacteria. Food Chemical, 368, 130831.
Hou, K., Xu, Y., Cen, K., Gao, C., Feng, X., & Tang, X. (2021). Nanoemulsion of cinnamon essential oil Co-emulsified with hydroxypropyl-β-cyclodextrin and Tween-80: Antibacterial activity, stability and slow release performance. Food Bioscience, 43, 101232.
Yazgan, H. (2020). Investigation of antimicrobial properties of sage essential oil and its nanoemulsion as antimicrobial agent. LWT, 130, 109669.
Dukor, R. R., Liebman, M. N., & Johnson, B. L. (1998). A new, non-destructive method for analysis of clinical samples with FT-IR microspectroscopy, breast cancer tissue as an example, Cell Mol. Biology, 44, 211–217.
Herrero, A. M., Carmona, P., Pintado, T., Jiménez-Colmenero, F., & Ruíz-Capillas, C. (2011). Olive oil-in-water emulsions stabilized with caseinate: elucidation of protein-lipid interactions by infrared spectroscopy. Food Hydrocolloids, 25, 12–18.
Huleihel, M., Pavlov, V., & Erukhimovitch, V. (2009). The use of FTIR microscopy for the evaluation of anti-bacterial agents activity. J. Photochem. Photobiol. B, 96, 17–23.
Ghosh, V., Saranya, S., Mukherjee, A., & Chandrasekaran, N. (2013). Antibacterial microemulsion prevents sepsis and triggers healing of wound in wistar rats. Colloids and Surfaces B: Biointerfaces, 105, 152–157.
Hwang, Y. Y., Ramalingam, K., Bienek, D. R., Lee, V., You, T., & Alvarez, R. (2013). Antimicrobial activity of nanoemulsion in combination with cetylpyridinium chloride in multidrug-resistant Acinetobacter baumannii. Antimicrobial agents and chemotherapy, 57(8), 3568–3575.
Acknowledgements
Authors acknowledge Sophisticated Analytical Instrumentation Facility (SAIF), Department of Science and Technology (DST), Govt. of India, at Indian Institute of Technology (IIT), Madras, for Fourier transform infrared spectroscopy (FT-IR) and high-resolution scanning electron microscopy (HRSEM) analysis facility used for this work.
Funding
This work was supported by Uka Tarsadia University, Bardoli, Gujarat (Research Promotion Scheme Grant Number: UTU/RPS/2703/2017).
Author information
Authors and Affiliations
Contributions
VG conceptualized the idea, supervised, analyzed, and interpreted data apart from acquisition of the funding. SC carried out the experiments and wrote the original draft. AL revised and edited the manuscript. NSB revised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Oregano essential oil (OEO) nanoemulsion was formulated with droplet diameter of 22 nm with polydispersity index (PDI) of 0.107 and spherical morphology.
• The optimized formulation exhibited effective antibacterial activity against E. coli.
• Nanoemulsion altered E. coli membrane permeability, confirmed by quantifying the leakage of 260 nm absorbing materials.
• FTIR spectra also confirmed alteration in functional groups on nanoemulsion treated bacteria surface.
• SEM micrographs demonstrated distortion of membrane in nanoemulsion treated bacteria.
• OEO nanoemulsion also exhibited significant antibacterial activity in situ in fresh mango juice.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghosh, V., Chandwani, S.K., Lonhare, A. et al. Antibacterial Nanoemulsion of Oregano Oil for Food Preservation: In Vitro and In Situ Evaluation Against Escherichia coli. BioNanoSci. 14, 1340–1350 (2024). https://doi.org/10.1007/s12668-024-01300-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-024-01300-8