Abstract
The aim of the present work was to study the formulation of edible nanoemulsions containing lemongrass essential oil as an antimicrobial agent, Tween 80 as a non-ionic surfactant and sodium alginate as a stabilizing and texturizing agent, by means of a pseudo-ternary phase experimental design. Polynomial models were satisfactory fitted to experimental data. Nanoemulsions with an average droplet diameter smaller than 100 nm were obtained by mixing oil and Tween 80 at a volume fraction below 0.01 (v/v) and higher than 0.009 (v/v), respectively. However, sodium alginate played a synergistic role regarding the stabilization of oil droplets in the absence of surfactant. In this sense, the higher the sodium alginate concentration, the stronger the negative surface charge of lipid droplets, as well as the higher the viscosity of the mixture. On the other hand, the emulsions’ whiteness decreased after increasing the surfactant and decreasing the oil phase, due to weak light scattering. As expected, the antimicrobial activity of blends was greater at higher amounts of essential oil, reaching a maximum of 7.37 log reduction of Escherichia coli after 30 min of contact time. Nevertheless, solubilizing and stabilizing the oil droplets by adding Tween 80 and sodium alginate might enhance the bactericidal effect of essential oils due to an improved dispersion in the continuous phase. The current work presents relevant information to formulate nanoemulsions incorporating antimicrobial agents for food applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the incorporation of ingredients to food formulations has gone a step forward due to the emergence of new delivery systems that are able to encapsulate, protect and release active compounds in a more efficient way (McClements 2011). Particularly, in the case of lipophilic active ingredients such as certain vitamins, antioxidants or antimicrobials, their incorporation in foods is even more challenging due to their poor water solubility. Nanoemulsions are described as colloidal dispersions of oil droplets (radius between 10 and 100 nm) in suspension within an aqueous phase that can act as carriers of active ingredients entrapped within the lipid core (McClements and Rao 2011). Besides the oil and aqueous phases, the formulation of nanoemulsions requires the use of a stabilizer, which is a surface-active molecule capable of adsorbing to droplet surfaces and protecting droplets against aggregation (McClements 2005; Kralova and Sjöblom 2009). Moreover, the addition of texture modifiers and thickening agents in the aqueous phase, such as biopolymers, is often used to improve emulsion stability, by retarding the droplet movement, as well as to provide specific textural characteristics (McClements and Rao 2011). The characteristics and concentration of all components in the nanoemulsion system will determine its final properties. So far, several advantages have been attributed to nanoemulsions over conventional emulsions. Due to their reduced droplet size, they are considered to be more stable in terms of coalescence, gravitational separation or particle aggregation (Mason et al. 2006). Moreover, they barely scatter the light, appearing as clear or almost transparent systems that might be suitable to incorporate in clear drinks and foods (McClements 2002a). In addition, there are evidence that the reduction of droplet size and the subsequent increase in surface area of nanodroplets might increase the functionality of the bioactive compound delivered. Therefore, the role of intrinsic characteristics of nanoemulsions, such as the droplet size and interfacial composition, is considered to be of crucial importance to design optimized delivery systems of food ingredients with poor water solubility.
Essential oils (EOs) are aromatic oily liquids obtained from natural plant products consisting of concentrated mixtures of terpenoids (Fisher and Phillips 2008). EOs might exert different biological actions and are typically used as flavourings and preservatives in the food industry (Adorjan and Buchbauer 2010). The use of EOs as natural antimicrobials in the food industry is a recent trend that is becoming popular due to the consumer demand of foods free from synthetic additives (Burt 2004). They are able to disrupt and penetrate the lipid structure of the bacterial cell membrane, causing the leakage of cytoplasmatic constituents and therefore the loss of cell viability (Burt 2004; Tajkarimi et al. 2010; Tiwari et al. 2009; Holley and Patel 2005). Lemongrass (Cymbopogon citratus) EO is an aromatic extract rich in terpenoids such as citral and limonene (Friedman et al. 2004) that has been found to be effective against a broad spectrum of microorganisms after in vitro tests (Hammer et al. 1999) and in food products (Rojas-Graü et al. 2007; Raybaudi-Massilia et al. 2008a, 2008b). However, despite the potential benefits related with the use of EOs, their incorporation to foods still presents several drawbacks due to their poor water solubility, intense aroma and potential toxicity at high doses (Sánchez-González et al. 2011). In this sense, nanoemulsions would be a useful tool to design delivery systems of EOs in order to optimize their formulation by achieving an enhanced antimicrobial activity, which in turn would allow minimizing the concentration of EO used.
The selection of the appropriate concentration of each individual ingredient in the formulation of nanoemulsions is of crucial importance in order to obtain systems with the desired physicochemical characteristics and functional properties. The construction of conventional pseudo-ternary phase diagrams as a tool to formulate nanoemulsions requires a large amount of mixtures to determine the region where nanoemulsions are formed and often does not provide any explanation of the impact of individual excipients. Response surface mixture experimental design might be an efficient methodology to evaluate the effect of multiple factors, alone or in combination, with a minimum number of experiments (Cornell 2002). The utilization of response surface methodologies may also improve understanding the influence of the emulsion components and their interactions as well as predicting optimized formulations with the desired properties (Li and Chiang 2012; Ren et al. 2013). Thus, the aim of the present work was to explore the effect of the lemongrass essential oil (LEO), surfactant and sodium alginate concentrations on the droplet size, ζ-potential, viscosity, colour and antimicrobial activity of nanoemulsions. For that purpose, a pseudo-ternary phase experimental design based on a response surface methodology was used as a tool to describe their effect on the selected responses.
Material and Methods
Pseudo-Ternary Phase Experimental Design
An experimental mixture design was used to determine the effect of the concentration of sodium alginate, LEO and surfactant on the formation of oil-in-water nanoemulsions. The selected responses were emulsion droplet diameter (z-average), ζ-potential, viscosity, colour and antimicrobial activity. A D-optimal design was used to build up the pseudo-ternary phase diagrams and to define the optimum number of experiments and was set up using the software Design Expert 8.0.4 (Stat Ease Inc., Minneapolis, MN). The concentration of each component was limited according to the following constraints expressed as volume fraction (v/v), except the sodium alginate concentration, which was expressed in weight fraction in the aqueous phase (w/v): 0.001 ≤ LEO ≤ 0.02, 0 ≤ Tween 80 ≤ 0.06, 0 ≤ sodium alginate ≤ 0.02, 0.9 ≤ water ≤ 0.999, and LEO + Tween 80 + sodium alginate + water = 1. The minimum concentration of LEO was set below its minimum inhibitory concentration (Hammer et al. 1999) to see if there is an enhancement of the antimicrobial activity after emulsification, whereas the maximum concentration was set to limit its sensorial impact. Tween 80 concentration was bound to an oil/surfactant ratio of 1:3, assuring a complete coating of lipid droplets surface with adsorbed surfactant molecules (Qian and McClements 2011). Sodium alginate maximum concentration was set according to its viscosity, to avoid the subsequent block of the high-pressure homogenizer microchannels. Oil-in-water nanoemulsions are fully dilutable systems that experience slight changes in their physicochemical characteristics under diluted conditions (McClements and Rao 2011). Therefore, the effect of the water concentration did not have a clear significance in our case of study, so it was not discussed as a mathematical variable. The empirical data of the response surface experimental design is shown in Table 1. All samples were prepared and analysed in random order during the same day without further storage. Two replicate analyses of each sample and parameter were performed, and the mean value was calculated and used for the modelling. Afterwards, experimental data was represented graphically with pseudo-ternary phase diagrams and modelled with a Scheffe polynomial equation (Eq. 1) for the four components to predict their effect in the formulation. In those responses where the differences between experimental values were very high, a Box-Cox transformation was applied to data to normalize the data and stabilize the variance. The statistical significance of the models for each response was considered regarding their adjusted R 2 and p values.
where Y is the response variable, i, j and k are the number of ingredients in the mixture, β i is the first-order coefficient, β ij is the second-order coefficient, β ijk is the third-order coefficient, x 1 is the lemongrass EO, x 2 is the Tween 80, x 3 is the water, and x 4 is the sodium alginate.
Nanoemulsion Formation
First, the aqueous phase of nanoemulsions was prepared by dissolving the sodium alginate (FMC Biopolymers, UK) in hot water at 70 °C and continuous stirring until it was completely dissolved. Primary emulsions were prepared by mixing the LEO (C. citratus) (Laboratoris Dicana, Spain) and Tween 80 (Scharlau, Spain) as non-ionic surfactant with the aqueous phase. The blend was mixed using a laboratory T25 digital Ultra-Turrax mixer (IKA, Staufen, Germany) working at 3,400 rpm for 2 min. Then, primary emulsions were passed though a microfluidizer (M110P, Microfluidics, Massachusetts, USA), 3 cycles at 150 MPa. The product was refrigerated through a cooling coil immersed in an iced water bath, placed at the outlet of the interaction chamber, so that the temperature of the product was always kept below 20 °C to avoid the loss of volatile compounds in essential oils.
Nanoemulsion Characterization
Particle Size and ζ-Potential
The emulsion droplet size was measured by dynamic light scattering (DLS) with a Zetasizer NanoZS laser diffractometer (Malvern Instruments Ltd., Worcestershire, UK) working at 633 nm at 25 °C and equipped with a backscatter detector (173°) (Brar and Verma 2011). DLS measures the Brownian motion of nanosized droplets and relates this movement to an equivalent hydrodynamic diameter (nm). Average droplet diameter (nm) and size distribution curves in intensity (%) were used to characterize oil droplet dispersion in nanoemulsions. The lemongrass oil absorbance at 633 nm was 0.001 and its refractive index 1.487.
The electrophoretic mobility of oil droplets, also reported as ζ-potential, was measured by phase analysis light scattering (PALS) with a Zetasizer NanoZS laser diffractometer (Malvern Instruments Ltd., Worcestershire, UK). It determines the surface charge at the interface of the droplets dispersed in the aqueous solution.
Viscosity
A SV-10 Vibro-Viscometer (A&D Company, Tokyo, Japan) vibrating at 30 Hz was used to measure the viscosity of 10-mL aliquots of the emulsions. Moreover, the viscosity of sodium alginate solutions of different concentration, according to the experimental design, was also measured. The viscosity of the sodium alginate solutions at 0.01, 0.015 and 0.02 of weight fraction (w/v) was 54.1, 81.3 and 256.0 mPa · s, respectively. The observed values were considered the viscosity of the dispersant phase during the measurement of the particle size by dynamic light scattering.
Whiteness Index
The colour of emulsions and nanoemulsions was measured with a Minolta CR-400 colourimeter (Konica Minolta Sensing, Inc., Osaka, Japan) at room temperature set up for illuminant D65 and 10° observer angle and calibrated with a standard white plate. CIE L*, a* and b* values were determined, and the whiteness index (WI) was calculated with Eq. 2 (Vargas et al. 2008):
Antimicrobial Activity
The antimicrobial activity of nanoemulsions was assessed by evaluating the in vitro inactivation kinetics of Escherichia coli. The method used was a modification of a technique previously described by Ferreira et al. (2010). E. coli 1.107 (Laboratoire de Répression des Fraudes, Montpellier, France) was cultured in tryptone soy broth (Bioakar Diagnostics, Beauvais, France) and incubated at 37 °C with continuous agitation at 120 rpm for 11 h to obtain cells in stationary growth phase. The final concentration reached in the culture was 108–109 colony-forming units/millilitre (CFU/mL). A 0.5-mL aliquot of overnight bacterial culture was mixed with 0.5 mL of the LEO-alginate emulsion or nanoemulsion and 4.5 mL of sterile Milli-Q water. To determine the inactivation kinetics, an aliquot of the homogenate was taken at 5, 15 and 30 min of reaction time to be serially diluted and spread on McConkey agar (Biokar Diagnostics, Beauvais, France) plates. A control was performed with the same method, replacing the emulsion or nanoemulsion by sterile Milli-Q water. The inactivation experiments were conducted at room temperature. Colony count was determined after incubation of agar plates at 37 °C for 24 h.
Results and Discussion
Particle Size
The experimental results of droplet diameter of oil-in-water emulsions are shown in Table 1. The average droplet size of emulsions ranged between 2 and 1,300 nm depending on the concentration of LEO, Tween 80 and sodium alginate. Contour plots and 3D surface response graphs throughout the manuscript are represented setting the concentration of water constant at 0.98 to facilitate the representation of the data in a two-dimensional plane. It was observed that the droplet size of the emulsions was smaller with decreasing concentrations of oil and increasing amounts of Tween 80 (Fig. 1). In this sense, mixtures with concentrations of oil and Tween 80 lower than 0.01 and higher than 0.009 (v/v), respectively, led to nanoemulsions with particle sizes smaller than 100 nm. However, in the region of the mixture design representing high concentrations of LEO (≥0.01 v/v) and low concentrations of surfactant (≤0.005 v/v), the droplet size of emulsions was above 500 nm. Low-mass surfactants, such as Tween 80, which has a molar mass of 1,310 g/mol, are known to rapidly coat the surface of the created oil-water interface during emulsification (Kralova and Sjöblom 2009). Therefore, there must be a sufficient amount of surface-active molecules to cover the surface area of oil droplets generated during the formation of nanoemulsions. Moreover, the concentration of sodium alginate had a less pronounced influence on the droplet size of the obtained nanoemulsions. However, mixtures with absence of a surfactant (Tween 80) and a concentration of sodium alginate dissolved in the aqueous phase higher than 0.015 in weight fraction (w/v) exhibited droplet sizes between 200 and 300 nm, thus confirming the role of sodium alginate as an emulsifier in the formation of nanoemulsions. This effect might be due to the increase of the continuous phase viscosity produced by the addition of sodium alginate. This effect has also been observed by other authors (Qian and McClements 2011) who reported a reduction on the nanoemulsion droplet size as the viscosity of the continuous phase increased. When nanoemulsions are produced by microfluidization, the viscosity of the continuous phase may affect the droplet size throughout an increased droplet fragmentation due to an increased disruptive shear stress, or a decreased droplet re-coalescence due to a decreased droplet collision frequency.
The coefficients of the polynomial equation to predict the droplet size and the statistic significance of the model are shown in Table 2. A quadratic model was successfully fitted to experimental data, which was previously transformed using a logarithmic power law as recommended by the Box-Cox method, being significant with a p value of 0.0004 and a R 2 of 0.91 (Table 2). It is considered that the higher the magnitude of the coefficient parameter of the linear components in the fitted equation, the higher the response (Cornell 2002). The emulsion droplet size is determined by the individual components and the interaction between them, given by the estimate coefficients of the linear or quadratic terms. Positive coefficients of the linear terms indicate that the higher the concentration of the component, the higher the emulsion droplet size and vice versa. On the other hand, positive (>0) coefficients of the quadratic terms indicate a synergistic effect, whereas negative coefficients indicate an antagonistic effect (Cornell 2002). The largest coefficient was seen in the interaction between the lemongrass essential oil (component A) and the Tween 80 (component B) concentrations, with an AB coefficient of −53.74. Thus, the large negative coefficient for the AB term (Table 2) shows the effect of the concentration of LEO and Tween 80 on the droplet size of nanoemulsions, meaning that when increasing the concentration of both ingredients, the droplet size is reduced. Recently, two other research works by Hessein and co-workers (2011) and Maher and co-workers (2011) have used ternary phase diagrams as a tool to formulate nanoemulsions.
ζ-Potential
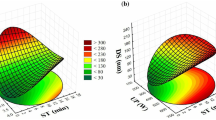
The electrical charge at oil-water interface of lipid droplets of the different formulations is shown in Table 1. The ζ-potential of oil droplets with different formulations varied significantly according to their composition, ranging between 0 and −98.65 mV. The surface electrical charge of lipid droplets was clearly influenced by the addition of sodium alginate in the mixture. In the contour plot and 3D surface response graph (Fig. 2), it can be observed that blends with a low concentration of sodium alginate and high concentration of oil phase resulted in ζ-potential close to 0. However, slightly increasing the concentration of sodium alginate beyond 0.001 of weight fraction (w/v) led to oil droplets with ζ-potential around −30 mV. In general, particles with ζ-potentials more positive than +30 mV or more negative than −30 mV are considered to be stable, since electrical charge of droplets is strong enough to assume that repulsive forces between droplets are predominant in the system (Heurtault et al. 2003). Increasing the weight fraction of sodium alginate in the aqueous phase up to 0.02 (w/v) decreased the surface electrical charge of lipid particles up to −90 mV. Alginates are linear polymers of mannuronic acid and guluronic acids, which are monomers with a low-dissociation constant (Tønnesen and Karlsen 2002). Therefore, alginic acid and its salts tend to be negatively charged in a wide range of pH. However, oil droplets exhibited negative charges even in the absence of sodium alginate (axis of component D, Fig. 2). This fact could be due to the fact that despite being non-ionic surfactants, polysorbate molecules (Tween 80) adsorbed to droplet surface might lead to negative electrical charges when pH of the emulsions are higher than 4 (Hsu and Nacu 2003). A cubic model was fitted to experimental data with a p value of <0.0001 and a R 2 of 0.9982 (Table 2). Consistently, the negative coefficients for Tween 80 (−74.76) and sodium alginate (−928.00) indicate their contribution to lower the ζ-potential of the blend, the latter being the one exerting the larger influence. However, the interaction of Tween 80 and sodium alginate seems to be synergistic in terms of the ζ-potential, as shown by the positive coefficient BD (2420.57). As can be observed by the curvature of the contour lines (Fig. 2), at constant concentration of alginate, the addition of a moderate amount of Tween 80 increases the ζ-potential of the mixture. This fact suggests competitive adsorption of polymer and surfactant molecules on the oil/water interface, which in turn might be concentration-dependent. Low-surfactant concentrations might not be enough to cover the oil/water interface. This could lead to the adsorption of polymer molecules, thus conferring a more negative ζ-potential (Goddard 2002). The behaviour observed at higher-surfactant concentrations might be explained by two reasons: (i) the surfactant molecules adsorbed at the oil/water interface might repel polymer molecules (De Gennes 1990), and (ii) the hydrophilic sites of polymer molecules might bind surfactant molecules, thus lowering the adsorption of sodium alginate to the oil surface (Neumann et al. 2003). Nevertheless, the large negative coefficient ABD (−10704.75) shows that mixing lemongrass essential oil, Tween 80 and sodium alginate causes a decrease in the ζ-potential of emulsions, thus conferring a higher stability to the oil-in-water system (Table 2).
Viscosity
The viscosity values of the mixtures ranged between 0.97 and 135.00 mPa · s depending on the formulation (Table 1). As expected, the viscosity of blends was mainly governed by the concentration of sodium alginate dissolved in the aqueous phase. A quadratic model was fitted to experimental data, which was transformed using a log10 power law, recommended by the Box-Cox method. The fitting of the quadratic model was statistically significant with a R 2 of 0.999 and p value of <0.0001 (Table 2). As observed in the contour plot and 3D surface graph (Fig. 3), the higher the concentration of sodium alginate, the higher the viscosity. The horizontal trend of the contour lines regarding the alginate axis indicates a weak influence of other compounds on the emulsion viscosity. The positive coefficients of the quadratic terms of alginate with oil, Tween 80 or water (AD, BD, CD) indicate the increase of viscosity after adding alginate to the mixtures. It can be observed (Fig. 3) that concentrations of sodium alginate lower than 0.01 in weight fraction (w/v) led to emulsions with viscosities lower than 15 mPa · s. However, increasing the weight fraction of sodium alginate in the aqueous phase from 0.01 (w/v) to 0.02 (w/v) caused a remarkable increment of the viscosity. The thickening properties of sodium alginate have been previously described (Tønnesen and Karlsen 2002; Rinaudo 2008) and, more recently, its use as a stabilizer and texturizer in the formulation of nanoemulsions has been pointed out (McClements and Rao 2011). Previously reported data concluded that alginates present non-Newtonian behaviour in most cases and exhibited typical pseudo-plastic behaviour (King 1994; Mancini et al. 1996). Also, the viscosity of sodium alginate depends on the molecular weight and increases with increasing the concentration (Mancini et al. 1996; Gómez-Díaz and Navaza 2003). The vibro-viscometer used to determine the viscosity of emulsions in the present study does not allow varying the shear rate of the determination. Therefore, due to the non-Newtonian behaviour of sodium alginate, the viscosity increase is more pronounced at higher concentrations.
Whiteness Index
The whiteness index of oil-in-water emulsions was determined as an indicator of their milky appearance. Therefore, the lower the whiteness index, the more transparent the emulsions. The whiteness index of blends ranged between 25.48 and 72.20 (Table 1) depending on the formulation. A quadratic model was fitted to experimental results, which was statistically significant with a R 2 and p value of 0.9246 and 0.0002, respectively (Table 2). In this sense, in the contour plot and 3D surface graph (Fig. 4), it can be observed that the concentration of Tween 80 and LEO significantly affected the colour of the blends. In this regard, the higher the concentration of LEO, the higher the whiteness index. This was confirmed by the positive coefficients of the quadratic terms in the polynomial equation that includes the LEO component (AC, AD) (Table 2). Moreover, in general, at increasing concentration of surfactant, the whiteness index of the mixture is decreased. This fact was reflected by the negative coefficients of the quadratic terms in the polynomial equation (AB, BC) (Table 2). Emulsion colour is given as a consequence of the scattering of light colliding with droplets. It is described that the light scattering of oil droplets depends on the refractive index of continuous and dispersed phase, oil concentration and droplet size (McClements 2002a, 2002b). Large particles scatter the light more intensely than smaller ones, which causes an increase in the lightness, opacity and whiteness index of emulsions (McClements 2011). In this sense, oil and surfactant concentrations might affect emulsion whiteness index due to their influence on droplet size. Similarly to our results, Rao and McClements (2012) also reported an increase on the turbidity of lemon oil nanoemulsion when increasing the oil content or decreasing the Tween 80 concentration.
Antimicrobial Activity
The inactivation of E. coli population after 5, 15 or 30 min of contact with the mixtures containing LEO is shown in Table 1. The reduction of log-units strongly depended on the formulation of emulsions and nanoemulsions, being the concentration of oil the ingredient with the greatest effect on the antimicrobial activity. In this sense, blends with a volume fraction of LEO lower than 0.006 (v/v) did not reduce the microorganism after 30 min of contact time. On the contrary, a maximum reduction of 7.37 log-units was observed after 30 min of contact with formulations containing a volume fraction of LEO higher than 0.01 (v/v), up to 0.02 (v/v). Quadratic models were fitted to the inactivation experimental results. In all cases, the fitting of the model was statistically significant with R 2 values higher than 0.78 (Table 2). In the contour plots and 3D surface response graphs (Fig. 5), it can be observed that, as expected, the higher the concentration of LEO, the higher the antimicrobial activity, and this behaviour was more pronounced after 15 or 30 min of contact time rather than 5 min. Moreover, the negative coefficients of the quadratic terms of the polynomial equation indicate a synergistic interaction between the LEO and the other ingredients (AB, AC, AD) (Table 2). This indicates that the addition of a surface-active compound, such as Tween 80, or a texturizing agent, such as sodium alginate, may significantly enhance the bactericidal action of EOs. This fact could be related with the improved dispersion and stabilization of nanosized oil droplets within the continuous phase due to the action of small-molecule surfactants and biopolymers. Recently, and similarly to our results, Liang and co-workers (2012) observed an increased and prolonged antimicrobial activity against Listeria monocytogenes or Staphylococcus aureus of nanoemulsions containing peppermint EO and stabilized with starch in comparison with the bulk peppermint oil, which was attributed to the increased solubility and stability of the essential oil. There are scientific evidence to think that the smaller the droplet size, the higher the functionality of the active compounds encapsulated (Donsì et al. 2011; Ziani et al. 2011). However, Terjung and co-workers (2012) recently reported that the antimicrobial activity of EOs might not only be determined by emulsion droplet size, since the location of the active compounds in the emulsion system as well as the concentration of the active compound may also be key factors affecting their bactericidal action. Therefore, it can be stated that the overall antimicrobial activity of nanoemulsified essential oils might be determined not only by the oil droplet size but also by the oil distribution and stability within the continuous phase.
Furthermore, an optimization of the formulation was made based on a minimum droplet size and a maximum antimicrobial activity of mixtures. Thus, a formulation with 0.019 of LEO and 0.052 of Tween 80 in volume fraction (v/v) and a weight fraction of sodium alginate in the aqueous phase of 0.02 (w/v) would lead to nanoemulsions with a minimum predicted droplet size of 2 nm and a maximum predicted E. coli inactivation of −9 log-units after 30 min of contact time.
Conclusions
The pseudo-ternary phase experimental design was a useful tool to study the formulation of oil-in-water emulsions and nanoemulsions containing LEO. In all cases, quadratic or cubic models were successfully fit to experimental data to describe the behaviour of the blends. Mixtures with a LEO volume fraction and Tween 80 lower than 0.01 and higher than 0.009 (v/v) respectively led to nanoemulsions with particle sizes smaller than 100 nm. However, a fair stabilizing activity of sodium alginate was observed, since a weight fraction of sodium alginate in the aqueous phase above 0.015 (w/v) and absence of surfactant led to droplet sizes below 500 nm. The ζ-potential and viscosity of the mixtures were mainly governed by the concentration of sodium alginate in the continuous phase due to its anionic nature and texturizing properties. The colour of the emulsions was basically determined by the oil and Tween 80 concentrations, namely, the lower the LEO and the higher the Tween 80 amount, the lower the whiteness index. Regarding the functionality of the mixtures, an increase on the LEO concentration led to a greater antimicrobial activity. Moreover, a synergism between LEO, Tween 80 and sodium alginate was observed, indicating the importance of stabilizing the lipid droplets within the continuous phase to enhance its functionality. The present work reveals significant information to formulate nanoemulsions with controlled characteristics for food applications.
References
Adorjan, B., & Buchbauer, G. (2010). Biological properties of essential oils: an updated review. Flavour and Fragrance Journal, 25(6), 407–426.
Brar, S. K., & Verma, M. (2011). Measurement of nanoparticles by light-scattering techniques. Trends in Analytical Chemistry, 30(1), 4–17.
Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in foods - A review. International Journal of Food Microbiology, 94(3), 223–253.
Cornell, J. A. (2002). Experiments with mixtures: designs, models, and the analysis of mixture data. New York: John Wiley & Sons.
De Gennes, P. G. (1990). Interactions between polymers and surfactants. Journal of Physical Chemistry, 94(22), 8407–8413.
Donsì, F., Annunziata, M., Sessa, M., & Ferrari, G. (2011). Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT - Food Science and Technology, 44(9), 1908–1914.
Ferreira, J. P., Alves, D., Neves, O., Silva, J., Gibbs, P. A., & Teixeira, P. C. (2010). Effects of the components of two antimicrobial emulsions on food-borne pathogens. Food Control, 21(3), 227–230.
Fisher, K., & Phillips, C. (2008). Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends in Food Science and Technology, 19(3), 156–164.
Friedman, M., Henika, P. R., Levin, C. E., & Mandrell, R. E. (2004). Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. Journal of Agricultural and Food Chemistry, 52(19), 6042–6048.
Goddard, E. D. (2002). Polymer/surfactant interaction: interfacial aspects. Journal of Colloid and Interface Science, 256(1), 228–235.
Gómez-Díaz, D., & Navaza, J. M. (2003). Rheology of aqueous soluions of food additives: Effect of concentration, temperature and blending. Journal of Food Engineering, 56(4), 387–392.
Hammer, K. A., Carson, C. F., & Riley, T. V. (1999). Antimicrobial activity of essential oils and other plant extracts. Journal of Applied Microbiology, 86(6), 985–990.
Hessien, M., Singh, N., Kim, C., & Prouzet, E. (2011). Stability and tunability of O/W nanoemulsions prepared by phase inversion composition. Langmuir, 27(6), 2299–2307.
Heurtault, B., Saulnier, P., Pech, B., Proust, J., & Benoit, J. (2003). Physico-chemical stability of colloidal lipid particles. Biomaterials, 24(23), 4283–4300.
Holley, R. A., & Patel, D. (2005). Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiology, 22(4), 273–292.
Hsu, J., & Nacu, A. (2003). Behaviour of soybean oil-in-water emulsion stabilized by nonionic surfactant. Journal of Colloid and Interface Science, 259(2), 374–381.
King, K. (1994). Changes in the functional properties and molecular weight of sodium alginate following γ irradiation. Food Hydrocolloids, 8(2), 83–96.
Kralova, I., & Sjöblom, J. (2009). Surfactants used in food industry: A review. Journal of Dispersion Science and Technology, 30(9), 1363–1383.
Li, P., & Chiang, B. (2012). Process optimization and stability of d-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrasonics Sonochemistry, 19(1), 192–197.
Liang, R., Xu, S., Shoemaker, C. F., Li, Y., Zhong, F., & Huang, Q. (2012). Physical and antimicrobial properties of peppermint oil nanoemulsions. Journal of Agricultural and Food Chemistry, 60(30), 7548–7555.
Maher, P. G., Fenelon, M. A., Zhou, Y., Kamrul Haque, M., & Roos, Y. H. (2011). Optimization of β-casein stabilized nanoemulsions using experimental mixture design. Journal of Food Science, 76(8), C1108–C1117.
Mancini, M., Moresi, M., & Sappino, F. (1996). Rheological behaviour of aqueous dispersions of algal sodium alginates. Journal of Food Engineering, 28(3–4), 283–295.
Mason, T. G., Wilking, J. N., Meleson, K., Chang, C. B., & Graves, S. M. (2006). Nanoemulsions: formation, structure, and physical properties. Journal of Physics Condensed Matter, 18(41), R635–R666.
McClements, D. J. (2011). Edible nanoemulsions: fabrication, properties, and functional performance. Soft Matter, 7(6), 2297–2316.
McClements, D. J. (2005). Food emulsions. Principles, Practices and Techniques. Boca Raton, FL: CRC Press.
McClements, D. J. (2002a). Colloidal basis of emulsion colour. Current Opinion in Colloid and Interface Science, 7(5–6), 451–455.
McClements, D. J. (2002b). Theoretical prediction of emulsion colour. Advances in Colloid and Interface Science, 97(1–3), 63–89.
McClements, D. J., & Rao, J. (2011). Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Critical Reviews in Food Science and Nutrition, 51(4), 285–330.
Neumann, M. G., Schmitt, C. C., & Iamazaki, E. T. (2003). A fluorescence study of the interactions between sodium alginate and surfactants. Carbohydrate Research, 338(10), 1109–1113.
Qian, C., & McClements, D. J. (2011). Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocolloids, 25(5), 1000–1008.
Rao, J., & McClements, D. J. (2012). Lemon oil solubilization in mixed surfactant solutions: rationalizing microemulsion & nanoemulsion formation. Food Hydrocolloids, 26(1), 268–276.
Raybaudi-Massilia, R. M., Mosqueda-Melgar, J., & Martín-Belloso, O. (2008a). Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. International Journal of Food Microbiology, 121(3), 313–327.
Raybaudi-Massilia, R. M., Rojas-Graü, M. A., Mosqueda-Melgar, J., & Martín-Belloso, O. (2008b). Comparative study on essential oils incorporated into an alginate-based edible coating to assure the safety and quality of fresh-cut Fuji apples. Journal of Food Protection, 71(6), 1150–1161.
Ren, S., Mu, H., Alchaer, F., Chtatou, A., & Müllertz, A. (2013). Optimization of self nanoemulsifying drug delivery system for poorly water-soluble drug using response surface methodology. Drug Development and Industrial Pharmacy, 39(5), 799–806.
Rinaudo, M. (2008). Main properties and current applications of some polysaccharides as biomaterials. Polymer International, 57(3), 397–430.
Rojas-Graü, M. A., Raybaudi-Massilia, R. M., Soliva-Fortuny, R. C., Avena-Bustillos, R. J., McHugh, T. H., & Martín-Belloso, O. (2007). Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biology and Technology, 45(2), 254–264.
Sánchez-González, L., Vargas, M., González-Martínez, C., Chiralt, A., & Cháfer, M. (2011). Use of essential oils in bioactive edible coatings: a review. Food Engineering Reviews, 3(1), 1–16.
Tajkarimi, M. M., Ibrahim, S. A., & Cliver, D. O. (2010). Antimicrobial herb and spice compounds in food. Food Control, 21(9), 1199–1218.
Terjung, N., Löffler, M., Gibis, M., Hinrichs, J., & Weiss, J. (2012). Influence of droplet size on the efficacy of oil-in-water emulsions loaded with phenolic antimicrobials. Food and Function, 3(3), 290–301.
Tiwari, B. K., Valdramidis, V. P., O'Donnell, C. P., Muthukumarappan, K., Bourke, P., & Cullen, P. J. (2009). Application of natural antimicrobials for food preservation. Journal of Agricultural and Food Chemistry, 57(14), 5987–6000.
Tønnesen, H. H., & Karlsen, J. (2002). Alginate in drug delivery systems. Drug Development and Industrial Pharmacy, 28(6), 621–630.
Vargas, M., Cháfer, M., Albors, A., Chiralt, A., & González-Martínez, C. (2008). Physicochemical and sensory characteristics of yoghurt produced from mixtures of cows’ and goats’ milk. International Dairy Journal, 18(12), 1146–1152.
Ziani, K., Chang, Y., McLandsborough, L., & McClements, D. J. (2011). Influence of surfactant charge on antimicrobial efficacy of surfactant-stabilized thyme oil nanoemulsions. Journal of Agricultural and Food Chemistry, 59(11), 6247–6255.
Acknowledgments
This study was supported by the Ministerio de Ciencia e Innovación (Spain) throughout the project AGL2009-11475. Laura Salvia-Trujillo thanks the Ministry of Science and Education (Spain) for the predoctoral grant. Prof. Olga Martín-Belloso thanks the Institució Catalana de Recerca i Estudis Avançats (ICREA) for the Academia 2008 Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salvia-Trujillo, L., Rojas-Graü, M.A., Soliva-Fortuny, R. et al. Formulation of Antimicrobial Edible Nanoemulsions with Pseudo-Ternary Phase Experimental Design. Food Bioprocess Technol 7, 3022–3032 (2014). https://doi.org/10.1007/s11947-014-1314-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-014-1314-x