Abstract
This study evaluated the application of cashew gum, Arabic gum and starch on physical and thermal properties, and fatty acid profiles of spray-dried fish oil. A completely randomized design was used to evaluate the influence of the type of material on the properties of the microparticles. Hygroscopicity and solubility was higher for particles produced using cashew gum and reached 15 g/100 g and 85 g/100 g, respectively. Analyzing the thermogravimetric curves, it was found that cashew gum bulk showed two steps of degradation. For the microcapsules containing encapsulated fish oil in cashew gum, an extra degradation step at 471 °C was found. It was possible to verify the occurrence of diffused and wide peaks in the X-ray diffractograms for all three carbohydrate polymers. The particles produced presented spherical shape with cavities. The fatty acid profile for the fish oil changed only when using modified starch as wall material, where a significant loss of omega-3 fatty acids was observed. The particles produced with cashew gum had physical properties similar to those when applying materials commonly used and this biopolymer has the potential for application as a carrier in spray drying processes .
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish oil contains a high concentration of n-3 polyunsaturated fatty acids (PUFAs), which have beneficial effects on human health, such as preventing cardiac diseases and the predominant dietary sources of n-3 PUFA are fish and fish oil supplements (Jeyakumari et al. 2016). Several studies have aimed at enriching food products with fish oil due to the low intake of n-3 PUFAs and their nutritional benefits (Alemán et al. 2015; Jacobsen et al. 2008). Microencapsulation of fish oil allows the production of powder formulations, which can thus be used in many different finished food products. Additionally, fish processing facilities generate a significant amount of fish by-products that could be sources of energy, functional components, or industrial feedstock (Adeoti and Hawboldt 2014), which can be carried and protected via encapsulation.
The search for new biopolymers, as alternative encapsulation materials, has attracted tremendous attention in various fields. Cashew gum is a biopolymer extracted from the exudate of Anacardium occidentale, a common tree found in Brazil’s northeastern region (Oliveira et al. 2014). This heteropolysaccharide fundamentally consists of galactose, glucose, arabinose, raminose, and glucuronic acid (De Paula et al. 1998). Cashew gum has great potential as a carrier material in the spray drying processes. It exhibits low viscosity, is comparable in many respects to Arabic gum (De Paula and Rodrigues 1995), and is readily soluble in water with good emulsifying, adhesive and stabilizing properties (Mothé and Rao 2000). Cashew gum have been found to exhibit rheological characteristics and industrial applications similar to those of many polymers (Cunha et al. 2009). When compared to Arabic gum, a material commonly used in the encapsulation process, cashew gum showed lower viscosity and higher encapsulation efficiency (Botrel et al. 2017). Moreover, cashew gum was able to delay the fish oil oxidation probably based on the ability to avoid migration of the oil from inside the matrix and diffusion of the oxygen through the matrix (Botrel et al. 2017).
Spray drying processes based on oil-in-water emulsion involve the production of emulsions using polymeric materials followed by the atomization into a drying chamber. Microencapsulation by the spray drying technique is categorized as a glassy encapsulation system, which uses carbohydrates in the glassy amorphous state to retain bioactive compounds (Yuliani et al. 2004). Encapsulating properties, such as the glass transition temperature and physical structure, play important roles in the transformation of spray-dried powders during processing and storage. During the drying process, if the process temperature is higher than the glass transition temperature of the product, it remains in a rubbery state and is at risk of becoming sticky. In these cases, the use of high molecular weight additives can increase the glass transition temperature and reduce the risk of adhesion during drying processes (e.g., spray drying).
This study aimed to evaluate the physical and thermal properties and the fatty acid profiles of microencapsulated fish oil with cashew gum as the wall material in the spray drying process and to compare these results with those obtained using modified starch and Arabic gum.
Materials and methods
Fish oil (Sundown Naturals, Boca Raton, USA) was used as core material. Modified starch (MS) (Capsul®; National Starch Food Innovation, São Paulo, Brazil), Arabic gum (AG) (Colloides Naturels Brasil, São Paulo, Brazil) and cashew gum (CG) (isolated and purified by Federal University of Ceará, Fortaleza, Brazil) were used as wall materials. The oil had no added antioxidants as declared by the supplier.
Experimental design
The completely randomized design with three replications, was used to evaluate the influence of wall materials modified starch, Arabic gum and cashew gum in the response variables. Wall material composition varied according to the treatments (Table 1).
Microencapsulation by spray drying
Wall material solutions were prepared by dissolving modified starch, Arabic gum and cashew gum, in distilled water, for each formulation. The solutions were kept overnight at room temperature to ensure a full saturation of the polymer molecules. Oil was progressively added to the wall material solution while stirring at 3500 rpm for 10 min using a rotor–stator blender (Ultra-Turrax IKA T18 basic, Wilmington, USA. The percentage of solids (wall material) used in the feed solution was 15 g/100 g for all treatments. The oil load used was 6 g/100 g based on the total emulsion volume. These conditions were determined through previous studies (Botrel et al. 2014a, b). The emulsions were dried using a spray drier (model MSD 1.0; Labmaq do Brasil, Ribeirão Preto, Brazil) equipped with a two-fluid nozzle atomizer. The inlet air temperature used was 185 °C, outlet temperature of 98 °C, feed flow rate of 0.7 L/h and drying air flow kept at 40 L/min. The dried powders were collected and stored in opaque, air tight containers at 4 °C while waiting for further analysis.
Particle morphology
Particle morphology was evaluated by scanning electron microscopy (SEM). The powders were attached to a double-sided adhesive tape mounted on SEM stubs with a diameter of 1 cm and a height of 1 cm, and then coated with gold in a vacuum and examined with an MEV 1430 VP-LEO scanning electron microscope (Electron Microscopy Ltd., Cambridge, UK). The SEM was operated at 20 kV with magnification of 900–1200×.
Hygroscopicity
Hygroscopicity was determined according to the method proposed by Cai and Corke (2000) with some modifications. The powder samples of each treatment (approximately 1.0 g) were placed in a container with saturated NaCl solution (75.29% relative humidity) at 25 °C. After 2 weeks, the samples were weighed, and hygroscopicity was expressed as g of adsorbed moisture per 100 g dry solids (g/100 g).
Reconstitution properties
Wettability of the powders was determined using the method of Fuchs et al. (2006) with some modifications. The powder samples (0.1 g) were sprinkled over the surface of 100 mL of distilled water at 20 °C without agitation. The time it took until the last powder particles submerge was recorded and used for a relative comparison of the extent of wettability between the samples. The solubility of the powders was evaluated according to the method proposed by Eastman and Moore (1984) and Cano-Chauca et al. (2005), with some adaptations. The powders were weighed (2.5 g) and stirred into 20 mL of distilled water for 2 min using a blender. The solution was then centrifuged at 760×g for 15 min. An aliquot of 5 mL of the supernatant was transferred to a pre-weighed Petri dish and oven-dried at 110 °C for 4 h. The solubility (g/100 g) was calculated as the percentage of dried supernatant in relation to the amount of powder originally added (2.5 g).
Bulk and tapped densities
Spray-dried microparticles were added to a 100 mL graduated cylinder and weighed. The volume, read directly from the cylinder, was then used to calculate the bulk density (Jinapong et al. 2008). For tapped density, approximately 5 g of powder was freely poured into a 25 mL graduated glass cylinder, and the samples were repeatedly tapped manually by lifting and dropping the cylinder under its own weight at a vertical distance of 10 cm until a negligible difference in volume between succeeding measurements was observed. Given the mass m and the apparent (tapped) volume V of the powder, the powder tapped density was computed (Goula and Adamopoulos 2008).
Thermogravimetric analysis
Thermogravimetric (TG) and derivative thermogravimetric (DTG) curves were obtained using TGA50H thermobalance (Coorporation Shimadzu, Kyoto, Japan) under the following operating conditions: alumina pan; dynamic nitrogen atmosphere with flow of 100 mL/min; heating rate: 10 °C/min; temperature range: 50–550 °C. Approximately 5 mg of sample were used.
X-ray diffraction
Samples of products were placed in a support for powder and covered with a glass sheet. Measurements were performed using a X-ray diffractometer (model XRD-6000, Coorporation Shimadzu, Kyoto, Japan) using Cu-Kα1 radiation with a wavelength of 1.54 Å at 30 kV and 30 mA. Samples were analyzed at angles from 4° to 40° in 2 h with an increment of 0.02° (1.2°/min).
Fatty acids profile
Fish oil (14 mg) was dissolved in 100 mL solution (95:5, v/v) containing ethanol (99 mL/100 mL):potassium hydroxide (1 mol/L). After shaking in vortex for 10 s, the oil was hydrolyzed in the microwave oven at 80 W for 5 min. After cooling, 400 µL of hydrochloric acid (20 mL/100 mL), 20 mg of sodium chloride, and 600 μL of ethyl acetate was added to the solution. After shaking in a vortex for 10 s followed by rest for 10 min, a 300 µL aliquot of the organic phase was taken and dried by evaporation to obtain the free fatty acids (Christie and Han 2012). The fatty acids were methylated with 100 μL boron trifluoride/methanol solution and heated for 10 min at 60 °C. The material was diluted in 400 µL methanol solution and analyzed by gas chromatography (HP7820, Agilent, Santa Clara, United States) equipped with a flame ionization detector. INNOWAX (HP) column was used (15 m × 0.25 mm × 0.20 μm) with the following temperature gradient: 120 °C, 7 °C/min to 240 °C; injector (split 1/30) at 250 °C and detector at 260 °C. Hydrogen was used as the carrier gas (2 mL/min), with an injection volume of 1 μL. Methylated fatty acid standards were used for identification of compounds by comparing the retention time. Evaluation of the fatty acid degradation in the fish oil subjected to the spray drying process was done by comparing the relative peak areas (%) of the three main components.
Statistical analysis
One way analysis of variance was performed to evaluate the effect of encapsulating composition on the parameters studied. All the measurements were conducted in triplicate. Differences among mean values were examined Tukey test at P < 0.05 significance level.
Results and discussion
Particle morphology
The particles produced using the studied wall materials showed differences in morphology and size, however, most of them presented a spherical shape with some concavities (Fig. 1). Jeyakumari et al. (2016) obtained fish oil powders contained fish gelatin, maltodextrin and they showed the microparticles with spherical shape. Cracks have a significant influence on oxidation of encapsulated oils, however, they were not evident in the capsules produced in this study. A certain degree of caking was observed on the obtained SEM images based on the hygroscopicity of the particles produced.
Physical properties of the particles
Means and standard deviations for the response variables when fish oil was encapsulated in different matrices are shown in Table 2. Physical parameters of the powders like bulk wettability, solubility, and tapped density affect flow and storage stability (Goyal et al. 2015).
The absorption of water is a critical factor for the shelf life of microencapsulated oils because water influences lipid oxidation and loss of nutritional components. The quality of preserved foods depends on the moisture content, migration, and uptake by during storage (Basu et al. 2006). The more hygroscopic particles were obtained by using the cashew gum as wall material (15.1 g/100 g). There were significant differences in hygroscopicity between treatments, indicating that the polymeric matrix has crucial effect on the ability of spray-dried particles to absorb water from the environment.
Wettability is defined as the rehydration ability of a powder in water (Fernandes et al. 2014) and the ability of microcapsules to mix with water is one of the most important properties of the reconstitution process. The powders produced with cashew gum had the highest wettability time; that is, water penetration took the longest time. The wettability is linked to the content and characteristics of lipophilic compounds in the food as well to physical factors, especially the size and shape of the particles and the reconstituting water temperature (Vissotto et al. 2006). Furthermore, the type of wall material also significantly affects this property. The results obtained for cashew gum may be associated with its higher bed density, which might hinder the dispersion and penetration of water. In the study of Fernandes et al. (2014), the time taken for the rosemary essential oil powders to become totally wet varied from 37 to 303 s when encapsulated in blends of whey protein isolate/inulin.
The particles produced with cashew gum and modified starch were the most soluble. The solubility parameter is used to verify the powder’s ability to remain in a homogeneous mixture with water. In general, this variable is highly influenced by the type of carrier (Yousefi et al. 2011) and the consequent presence of hydrophilic sites in the matrix structure. The cashew gum and modified starch, which had the same solubility (P > 0.05), present structures that facilitate interaction with water. Arabic gum has hydrophobic amino acid radicals in its structure that may have strongly influenced their reduced solubility. Modified starch, despite the addition of hydrophobic sites, has shorter glucose chains when compared to native starches, which facilitates the dissolution in water. Particles produced using cashew gum have higher solubility despite its longer wettability time. Oliveira et al. (2009) observed high solubility (91.3–96.4 g/100 g) for powdered cashew juice using cashew gum and maltodextrin as carriers. Kha et al. (2014) obtained solubility values varying between 84.8 and 92.3 g/100 g in a study on Gac oil microencapsulation by spray drying using a blend of whey protein and Arabic gum.
Higher densities (both bulk and tapped) were observed for particles produced with cashew gum. This may be due to the little expansion occurred in these particles during the drying process, as evidenced by the rough surface and the presence of cavities, which make these particles heavier when compared to the other treatments. Furthermore, the low density of particles produced using modified starch as a carrier can be explained by the expansion within the particles, which was maintained by the higher drying rate and faster crust formation. Aghbashlo et al. (2012) observed bed densities ranging between 0.239 and 0.274 g/mL for fish oil particles in different matrices. Goyal et al. (2015) observed bulk densities varying between 0.297 and 0.328 g/mL for flaxseed oil microencapsulated by spray drying using whey protein concentrate, sodium caseinate, and lactose matrix. Density is an important variable for packaging and transportation, indicating how much material, as a function of the mass, will fit inside a specific volume, at it varies with the geometry and size of the particles (Finney et al. 2002).
Thermogravimetric analysis
Thermogravimetry is a technique used to study the loss of sample mass as a function of temperature and to evaluate its thermal stability. The particles containing fish oil showed three or four stages of mass loss (Fig. 2). The first stage, up to 110 °C may be attributed to moisture loss from the samples. Above this range of temperature, the mass loss observed corresponded to materials decomposition. The following stages, between 200 and 350 °C, are mostly related to the wall material constituents degradation (Fritzen-Freire et al. 2012), which may be associated with carbohydrate ring dehydration, decomposition, and depolymerization of polymer units (Hosseini et al. 2013). Temperatures that provide the greatest mass loss rate in each stage are normally considered as the degradation temperature (Td) and can be observed in the DTG curves.
By analyzing the TG curves, it was found that cashew gum showed two stages of degradation, one at 256 °C and another at 310 °C. Similar results were found by Oliveira et al. (2014) for alginate-cashew gum nanoparticles, where the events due to the decomposition of the polysaccharides occurred above 257 °C. For particles containing fish oil produced using cashew gum one more step of degradation at 471 °C was added, which may be related to the thermal degradation of fish oil that remained entrapped in the matrix. This step was also observed in the particles produced with Arabic gum and modified starch, however, at different temperatures of 460 and 479 °C respectively. Differences in the temperature where the last stage occurred (encapsulated fish oil) were observed and this event may be related to greater or lesser protection of this functional component by the wall materials. Considering this argument, the highest protection was observed for modified starch, followed by cashew gum and finally Arabic gum.
X-ray diffraction
X-ray diffraction was applied to identify the degree of crystallinity. The level of crystallinity of spray-dried material depends on drying conditions and product characteristics, such as the presence of high concentrations of sugars (Bhandari et al. 1997). It was possible to verify the presence of diffused and wide peaks, which are characteristic of materials with amorphous structures (Fig. 3). The particles produced with the carbohydrate polymers in this study showed similar behaviors. The structural organization of the final products did not vary with the type of wall materials used. Similar results were observed by Cano-Chauca et al. (2005) for spray-dried mango pulp. They found that the application of 12 g/100 g maltodextrin and 12 g/100 g Arabic gum as wall materials also produced powders having amorphous characteristics. In the work of Fernandes et al. (2014), all the samples of microencapsulated rosemary essential oil in whey protein isolate matrix and inulin also had amorphous structures. Spray drying process tends to produce dry products in metastable amorphous state due to insufficient time to crystallize (Jayasundera et al. 2011).
Fatty acid profile
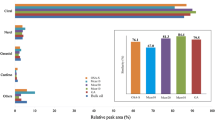
The fatty acid profile for bulk fish oil and the oil extracted from the microcapsules was investigated to evaluate the degree of alteration of these components after spray drying (Table 3). Regarding the omega-3 fatty acid content, it was found that there was significant loss of these functional compounds only when modified starch was used as the wall material. The losses reached 32.0, 34.6, and 32.2% for linolenic acid, EPA, and DHA, respectively. Simultaneously, an increase in the amount of SFA and MUFA was observed. For the treatments using cashew and Arabic gum, similar fatty acids profile was observed after the drying process, with no significant losses when compared to the bulk oil. Only a relatively small reduction in the amount of C20:5 and C22:6 fatty acids was observed when cashew gum was used. The level of saturated fatty acids remained virtually unchanged in the three treatments.
Czerniak et al. (2015) found no difference between the composition of fatty acids from menhaden fish oil encapsulated in yeast cells and bulk oil. The authors found significant loss of PUFAs, but the total amount of MUFA did not change significantly. Vasile et al. (2016) found no significant changes in the relative amount of fatty acid C18:3 between the bulk oil and microencapsulated oil in alginate matrix-chitosan and alginate-chitosan-Prosopis alba exudate gum. The importance of evaluation and definition of the best type or mix of encapsulants to protect the core material is reinforced by our results. The greatest loss of components with modified starch can be explained by the occurrence of foaming during the homogenization process compared to other treatments, which may have increased the contact of the solution with oxygen and accelerated the oxidation process during the emulsification process, which impacts on the quality of the oil microencapsulated. Results also suggest that PUFAs present in the encapsulated oil undergo oxidative damage to some extent during the spray drying process. Such damage could possibly be minimized by applying an oxygen free atmosphere (Czerniak et al. 2015) or adding antioxidants to the spray drying feed solution.
Conclusion
The study of nonconventional biopolymers which can be used in encapsulation processes using spray drying is increasing and cashew gum may be an alternative. It was found that particles obtained by using cashew gum as wall material showed solubility close to that found for modified starch. On the other hand, the use of cashew gum resulted in particles presenting high hygroscopicity and longer wettability time. The spray-dried fish oil produced using the tested wall materials were amorphous with relatively spherical shape particles without cracks or holes. The fatty acid profile of the encapsulated fish oil underwent changes only when using modified starch as the wall material, where the loss of omega-3 was observed. Cashew gum presented physical characteristics of interest that indicate its potential use as a carrier in the drying processes of food products and in different carrier formulations. The use of new sources aims to expand the possibilities and to provide new and better properties.
References
Adeoti IA, Hawboldt K (2014) A review of lipid extraction from fish processing by-product for use as a biofuel. Biomass Bioenergy 63:330–340. doi:10.1016/j.biombioe.2014.02.011
Aghbashlo M, Mobli H, Madadlou A, Rafiee S (2012) The correlation of wall material composition with flow characteristics and encapsulation behavior of fish oil emulsion. Food Res Int 49:379–388. doi:10.1016/j.foodres.2012.07.031
Alemán M, Bou R, Guardiola F, Durand E, Villeneuve P, Jacobsen C, Sørensen AD (2015) Antioxidative effect of lipophilized caffeic acid in fish oil enriched mayonnaise and milk. Food Chem 167:236–244. doi:10.1016/j.foodchem.2014.06.083
Basu S, Shivhare US, Mujumdar AS (2006) Models for sorption isotherms for foods: a review. Dry Technol 24:917–930. doi:10.1080/07373930600775979
Bhandari BR, Datta N, Howes T (1997) Problems associated with the spray drying of sugar-rich foods. Dry Technol 15:671–684. doi:10.1080/07373939708917253
Botrel DA, Borges SV, Fernandes RVB, Carmo EL (2014a) Optimization of fish oil spray drying using a protein:inulin system. Dry Technol 32:279–290. doi:10.1080/07373937.2013.823621
Botrel DA, Fernandes RVB, Borges SV, Yoshida MI (2014b) Influence of wall matrix systems on the properties of spray-dried microparticles containing fish oil. Food Res Int 62:344–352. doi:10.1016/j.foodres.2014.02.003
Botrel DA, Borges SV, Fernandes RVB, Antoniassi R, Faria-Machado AF, Feitosa JPA, Paula RCM (2017) Application of cashew tree gum on the production and stability of spray-dried fish oil. Food Chem 221:1522–1529. doi:10.1016/j.foodchem.2016.10.141
Cai YZ, Corke H (2000) Production and properties of spray-dried Amaranthus betacyanin pigments. J Food Sci 65:1248–1252. doi:10.1111/j.1365-621.2000.tb10273.x
Cano-Chauca M, Stringheta PC, Ramos AM, Cal-Vidal J (2005) Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov Food Sci Emerg Technol 6:420–428. doi:10.1016/j.ifset.2005.05.003
Christie WW, Han X (2012) Chromatographic and spectroscopic analysis of lipids: general principles. In: Christie WW, Han X (eds) Lipid analysis: isolation, separation, identification and lipidomic analysis, 4th edn. Woodhead Publishing Limited, Cambridge, pp 21–54
Cunha PLR, De Paula RCM, Feitosa JPA (2009) Polysaccharides from Brazilian biodiversity: an opportunity to change knowledge into economic value. Quím Nova 32:649–660. doi:10.1590/S0100-40422009000300009
Czerniak A, Kubiak P, Białas W, Jankowski T (2015) Improvement of oxidative stability of menhaden fish oil by microencapsulation within biocapsules formed of yeast cells. J Food Eng 167:2–11. doi:10.1016/j.jfoodeng.2015.01.002
De Paula RCM, Rodrigues JF (1995) Composition and rheological properties of cashew tree gum, the exudate polysaccharide from Anacardium occidentale L. Carbohydr Polym 26:177–181. doi:10.1016/0144-8617(95)00006-S
De Paula RCM, Heatley F, Budd PM (1998) Characterization of Anacardium occidentale exsudate polysaccharide. Polym Int 45:27–35. doi:10.1002/(SICI)1097-0126(199801)45:1<27:AID-PI900>3.0.CO;2-9
Eastman JE, Moore CO (1984) Cold water soluble granular starch for gelled food composition. U.S. Patent 4465702
Fernandes RVB, Borges SV, Botrel DA, Oliveira CR (2014) Physical and chemical properties of encapsulated rosemary essential oil by spray drying using whey protein–inulin blends as carriers. Int J Food Sci Technol 49:1522–1529. doi:10.1111/ijfs.12449
Finney J, Buffo R, Reineccius GA (2002) Effects of type of atomization and processing temperatures on the physical properties and stability of spray-dried flavors. J Food Sci 67:1108–1114. doi:10.1111/j.1365-2621.2002.tb09461.x
Fritzen-Freire CB, Prudêncio ES, Amboni RDMC, Pinto SS, Negrão-Murakami AN, Murakami FS (2012) Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res Int 45:306–312. doi:10.1016/j.foodres.2011.09.020
Fuchs M, Turchiuli C, Bohin M, Cuvelier ME, Ordonnaud C, Peyrat-Maillard MN, Dumoulin E (2006) Encapsulation of oil in powder using spray drying and fluidised bed agglomeration. J Food Eng 75:27–35. doi:10.1016/j.jfoodeng.2005.03.047
Goula AM, Adamopoulos KG (2008) Effect of maltodextrin addition during spray drying of tomato pulp in dehumidified air: II. Powder properties. Dry Technol 26:726–737. doi:10.1080/07373930802046377
Goyal A, Sharma V, Sihag MK, Tomar SK, Arora S, Sabikhi L, Singh AK (2015) Development and physico-chemical characterization of microencapsulated flaxseed oil powder: a functional ingredient for omega-3 fortification. Powder Technol 286:527–537. doi:10.1016/j.powtec.2015.08.050
Hosseini SF, Zandi M, Rezaei M, Farahmandghavi F (2013) Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: preparation, characterization and in vitro release study. Carbohydr Polym 95:50–56. doi:10.1016/j.carbpol.2013.02.031
Jacobsen C, Let MB, Nielsen NS, Meyer AS (2008) Antioxidant strategies for preventing oxidative flavour deterioration of foods enriched with n-3 polyunsaturated lipids: A comparative evaluation. Trends Food Sci Technol 19:76–93. doi:10.1016/j.tifs.2007.08.001
Jayasundera M, Adhikari B, Adhikari R, Aldred P (2011) The effects of proteins and low molecular weight surfactants on spray drying of model sugar-rich foods: powder production and characterisation. J Food Eng 104:259–271. doi:10.1016/j.jfoodeng.2010.12.017
Jeyakumari A, Janarthanan G, Chouksey MK, Venkateshwarlu G (2016) Effect of fish oil encapsulates incorporation on the physico-chemical and sensory properties of cookies. J Food Sci Technol 53:856–863. doi:10.1007/s13197-015-1981-2
Jinapong N, Suphantharika M, Jamnong P (2008) Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J Food Eng 84:194–205. doi:10.1016/j.jfoodeng.2007.04.032
Kha TC, Nguyen MH, Roach PD, Stathopoulos CE (2014) Microencapsulation of gac oil by spray drying: optimization of wall material concentration and oil load using response surface methodology. Dry Technol 32:385–397. doi:10.1080/07373937.2013.829854
Mothé CG, Rao MA (2000) Thermal behavior of gum Arabic in comparison with cashew gum. Thermochim Acta 357–358:9–13. doi:10.1016/S0040-6031(00)00358-0
Oliveira MA, Maia GA, Figueiredo RW, Souza ACR, Brito ES, Azeredo HMC (2009) Addition of cashew tree gum to maltodextrin-based carriers for spray drying of cashew apple juice. Int J Food Sci Technol 44:641–645. doi:10.1111/j.1365-2621.2008.01888.x
Oliveira EF, Paula HCB, De Paula RCM (2014) Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloid Surf B Biointerfaces 113:146–151. doi:10.1016/j.colsurfb.2013.08.038
Vasile FE, Romero AM, Judis MA, Mazzobre MF (2016) Prosopis alba exudate gum as excipient for improving fish oil stability in alginate-chitosan beads. Food Chem 190:1093–1101. doi:10.1016/j.foodchem.2015.06.071
Vissotto FA, Montenegro FM, Santos JM, Oliveira SJR (2006) Evaluation of the influence of lecithination and agglomeration on the physical properties of a cocoa powder beverage (cocoa powder beverage lecithination and agglomeration). Ciênc Tecnol Aliment 26:666–671. doi:10.1590/S0101-20612006000300028
Yousefi S, Emam-Djomeh Z, Mousavi SM (2011) Effect of carrier type and spray drying on the physicochemical properties of powdered and reconstituted pomegranate juice (Punica Granatum L.). J Food Sci Technol 48:677–684. doi:10.1007/s13197-010-0195-x
Yuliani S, Bhandari B, Rutgers R, D’Arcy B (2004) Application of microencapsulated flavor to extrusion product. Food Rev Int 20:163–185. doi:10.1081/FRI-120037159
Acknowledgements
The authors thank FAPEMIG (Minas Gerais State Foundation for Research Development, Brazil) (CAG-PPM-00318-11) and CNPq (National Counsel of Technological and Scientific Development, Brazil) (Grant No. 448530/2014-7) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Botrel, D.A., Borges, S.V., Yoshida, M.I. et al. Properties of spray-dried fish oil with different carbohydrates as carriers. J Food Sci Technol 54, 4181–4188 (2017). https://doi.org/10.1007/s13197-017-2877-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2877-0