Abstract
Ingestion of conjugated linoleic acid poised many health benefits; however, amount of CLA one can get through generalized diet in is inadequate in exerting the desired benefits. Therefore, presence of CLA producing lactobacilli in dairy fermented foods has a tremendous potential to increase the CLA content. Therefore, present study was focused to isolate and characterize CLA producing lactobacilli from different dairy products and human faeces. Arguably, 283 lactobacilli were isolated from various sources and tested for CLA production. Fifty-seven CLA producing (≥20 µg/ml) lactobacilli were selected from screening in de Man, Rogosa and Sharpe (MRS) broth and reconstituted with skim milk (SM), supplemented with 0.5 mg/ml of linoleic acid. Positive strains were classified into—L. plantarum (44%), L. gasseri (30%), L. fermentum (21%) and L. salivarius (5%) species. Nineteen most efficient strains (CLA ≥25 µg/ml) were further assessed in SM for CLA production. Total 08 strains produced significantly higher CLA in SM than MRS and also produced cis 9, trans 11, trans 10, cis 12 and trans 9, trans 11 isomers. Overall, L. plantarum HIF15 was reported as the best producer of CLA and other 08 lactobacilli may be utilized for the formulation of CLA-enriched functional foods to support these bacteria to synthesize CLA in the human gut.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The generic term “conjugated linoleic acids (CLA)” is defined as a set of positional and geometric (cis or trans) isomers of linoleic acid (LA, C18:2), with conjugated bonds. CLA is synthesized as an intermediate product from dietary fats in ruminants through biohydrogenation activity from two important microorganisms namely, Butyrivibrio fibrisolvens and Megasphaera elsdenii (Jenkins et al. 2008), and ∆-9 desaturase activity in ruminant’ mammary glands. Of the 24 well characterized CLA isomers; cis 9, trans 11, trans 10, cis 12 and trans 9, trans 11 are of huge significance due to their reported health benefits in humans (Kim et al. 2016).
These isomers were reported to have anti-inflammatory (c9, t11), anti-obesity (t10, c12) and anti-cancerous (t9, t11) activities when supplemented to laboratory animals. Regardless of all the recognized clinical functions of CLA the fundamental mechanism is still obscured.
The daily recommended dosage (1–3 g/day) of CLA was established for humans attain the health benefits (MacDonald 2000). However, a normal diet (36–440 mg/day) is far lower for desired beneficial effects (Nunes and Torres 2010), and human system is totally inefficient to synthesize them de novo (Chung et al. 2008).
It has been reported that ruminants derived products especially milk and meat are considered as the richest source of CLA in the human diet (Herman-Lara et al. 2012). However, the concentration of CLA present in foodstuffs is lower and depends upon feedstock and animal breed (Sosa-Castañeda et al. 2015). Besides, in a majority of these products c9, t11 isomer accounts for >70% of total CLA (Lock and Bauman 2004). Thus, looking for the safe alternative approaches to enhance the CLA content in food are desirable and is of great interest to mankind for better health.
Commercial production of CLA by alkaline isomerisation is quite expensive and yields in the production of undesirable isomers with undisclosed functions (Zheng et al. 2003). In contrary, lactic acid bacteria (LAB) fermentation produces specific CLA isomers, and some of these LABs poised other probiotic advantages due to their generally recognized as safe (GRAS) status. Previous studies have shown that strains of LAB have the abilities to synthesize CLA under in vitro and in vivo conditions (Andrade et al. 2012). Other independent studies by Taboada et al. (2015) and Özer et al. (2016) found that some strains have the ability to produce CLA in cheese products. Therefore, microbial CLA uptake in humans is visualized as the most purposeful strategy. CLA producers have the ability to crosslink with adipocytes cell lines in vitro and gut epithelial cells in human and animal model (Sosa-Castañeda et al. 2015). Moreover, in an in vitro study Dahiya and Puniya (2015) reported that CLA producing strains had good probiotic-to-functional attributes. Thus, it is significant to examine the fermented food formulations at the gut level.
There is very few reports and scanty information available on indigenous lactobacilli of Indian origin; therefore, it is utmost important to hunt for novel CLA producers. Besides those extensive studies on the production of t10, c12 and t9, t11 CLA isomers from lactobacilli are also scanty.
Current study aimed to search for CLA producing lactobacilli, from dairy products and healthy human feces. The study focused on lactobacilli for CLA production as lactobacilli are the predominant allochthonous microflora of human gut and easier to handle than other known CLA producers.
Materials and methods
Sample collection
Conventional prepared fermented dairy products (Dahi and Lassi) were collected from the local market of Karnal, Haryana, India. The human breast-milk and faecal samples were collected from civil hospital, paediatric hospitals and private nursing homes of Karnal, Panipat, Sonipat and Delhi regions. In all, the study evaluated 183 faecal samples (57 adults and 126 from infants, 0–6 months of age). All faecal samples were obtained from healthier volunteers and babies with consent from their parents. In addition, 39 dairy products, 31 breast milk, 11 National Collection of Dairy Cultures (NCDC, ICAR-NDRI, Karnal, India) and two Kimchi samples were also used for isolation of bacterial strains. Samples were homogenized before used in study.
Isolation of lactobacilli
One gram or millilitre of each sample was suspended in a modified deMan, Rogosa and Sharpe (MRS) broth, containing 0.5 mg/ml of LA substrate and incubated at 37 °C for 24 h to enrich the suspension with LA tolerable lactobacilli. From the enriched broth, 1 ml of sample was added to 9 ml of BCP–MRS broth, serially diluted in 0.1% peptone water, and subsequently plated on BCP–MRS agar, and incubated at 37 °C for 48–72 h. Characteristic yellow coloured colonies were picked up from higher dilution MRS agar plates and transferred to MRS broth. Next, the isolates were streaked on MRS agar plates for further purification. The purity of cultures was examined microscopically after performing Gram staining. Apparent lactobacilli were confirmed through PCR and pure cultures were preserved at −80 °C in glycerol stocks. For routine experiments the cultures were maintained in chalk litmus milk at 4 °C and sub-cultured twice prior to use.
Lactobacilli identification
Genomic DNA extraction
A single colony from MRS plate was suspended in 2 ml of MRS broth and incubated at 37 °C for 16–18 h to attain turbidity. Thereafter, DNA extraction method of Pospiech and Neumann (1995) was followed with minor modifications. Briefly, the bacterial suspension was transferred to a 2 ml microcentrifuge tube and centrifuged at 14,000×g for 10 min. The pellets were washed twice with Milli Q water. Cell disruption was carried out in a mini bead beater (BioSpec; impulses 5, time 30 s, 2 min incubation on ice between intermittent cycles) with the aid of glass beads (212–300 µm) in SET buffer. Subsequently, DNA was eluted, precipitated and dissolved in TE buffer (pH 8.0). The purity and concentration of DNA was assessed in a Nano drop plate reader (Tecan-Infinite Pro 200, Switzerland) and thereafter, stored at −20 °C until use.
Molecular characterization
For identification of lactobacilli species the primers and PCR conditions of Dubernet et al. (2002) and Song et al. (2000) were used (Table 1). A 25 µl PCR reaction was prepared by adding 12.5 µl of 2× master mix green (Fermentas, Lithuania), 0.3 µl of each primer, 10.9 µl of nucleases-free water and 1 µl of genomic DNA. Thermocycles were performed in Veriti thermocycler (Invitrogen Inc.) with initial hold at 95 °C for 5 min followed by 35 cycles of 95 °C for 30 s, annealing (as mentioned in Table 1) for 30 s and a 72 °C extension for 30 s. The final extension was performed at 72 °C for 7 min. The amplified PCR products were confirmed in agarose gel (1.8%, w/v, 1× TBE buffer) electrophoresis.
UV-based spectrophotometric screening for CLA production
PCR confirmed lactobacilli were further characterized for CLA biosynthesis in MRS broth using the UV-based spectrophotometric method. Stock solution of LA (30 mg/ml, 99% purity; Sigma, St. Louis, MO, USA) was prepared in sterile distilled water with 2% (w/v) Tween-80 (Hi-media, Mumbai, India) and sterilized through a 0.20 µm syringe filter. For screeningthe cultures (OD595 adjusted to ~3.0 nm) were inoculated @ 1% (v/v) to 10 ml MRS broth supplemented with 0.05% L-cys-Hcl supplemented with 0.5 mg/ml of LA as a substrate in 50 ml glass serum bottles. The samples were incubated at 37 °C for 48 h. Subsequently the lactobacilli were tested for the production of CLA in accordance to Barrett et al. (2007). Briefly, the samples were centrifuged at 13,000×g/4 °C for 5 min, the supernatant (1 ml) was vigorously mixed with 2 ml of isopropanol and left undisturbed for 3 min. To this, 1.5 ml of hexane was added for extraction of fatty acids and remained undisturbed for 3 min. An aliquot 230 µl was taken for absorbance at 233 nm in a microplate reader (Tecan-Infinite Pro 200, Switzerland). A standard curve (20–160 µg/ml) was prepared from reference t10, c12 CLA isomer to quantify total CLA. Hexane layers containing only LA were used as control. The initial selection of lactobacilli was based on CLA production and only positive strains (>20 µg/ml CLA production) were kept for further experiments. As dairy products are suitable and economical for probiotics delivery, therefore selected lactobacilli from previous experiment were tested in reconstituted skim milk (hereafter, SM) (12% w/v, containing lactose ~51%, fat ~1.0%, protein ~35%, ash ~8.20% approximately) supplemented with readily available growth promoters; 10 mg/ml yeast extracts (Hennessy et al. 2009) and 0.3% glucose (Kim and Liu 2002). Before inoculation of fresh cultures @1% to SM the medium was autoclaved at 110 °C for 10 min.
Fatty acid methyl ester (FAME) synthesis and gas chromatography (GC) analysis
Quantification of CLA isomers in LA–MRS and LA–SM were performed using gas chromatography. FAME was prepared from the fermentation medium, by following direct synthesis method of O’Fallon et al. (2007) with fewer modifications. The hexane layers containing fatty acids were dried under a stream of nitrogen and redissolved in 1 ml of hexane. The samples were stored in glass vials at −20 °C until analysed in GC.
Two microlitre sample (FAME) was injected into a fully automated GC-2010 GC machine (Shimadzu Corp, Japan) equipped with SP-2560 capillary column (100 m × 0.25 mm I.D., 0.20 µm film thickness, Supelco, USA), an automated injector (Aoc-20i) and a flame ionization detector in (1:10) split mode using hydrogen as a carrier gas. The temperatures of injector and detector were set at 270 and 280 °C, respectively. The temperature of column oven was programmed from 140 to 240 °C with step increase of 4 °C/min. The qualitative analysis of CLA isomers were performed by comparison of retention times (RTs) with methylated CLA standards (c9, t11, t10, c12 and t9, t11). For quantification, standard curves were plotted against concentrations (0–1000 µg/ml) and expressed as µg/ml.
Scanning electron microscopy (SEM)
Notably, specifically highest CLA producer was examined by SEM to analyses spore and capsule formation. The culture was first fixed with a solution of 2.5% gluteraldehyde solution, washed with phosphate buffer saline, and again re-fixed with 1.0% osmium tetraoxide. Next, the sample was serially dehydrated in ethanol series for fixed durations. Finally, the sample was placed on a stubber, gold-coated and examined under scanning electron microscope (Zeiss, UK).
Statistical analysis
All samples were analyzed in triplicates and data are presented as mean ± SD. GraphPad prism software (ver.5.0, CA, USA) was used to perform one-way ANOVA with Turkey’s test to evaluate a significance level of P < 0.05.
Result and discussion
Sample collection and lactobacilli identification
The selective media, BCP–MRS broth helped in isolation of LA tolerable lactobacilli. Consequently, 390 distinct colonies were picked from different samples. No colony was obtained from breast milk samples and that might be primarily due to the antimicrobial effect of colostrums and infusion of antibiotics during the maternity period. Microscopically, only 311 isolate were found Gram positive and of these, 283 were confirmed as lactobacilli through PCR analysis.
UV-based spectrophotometric screening for CLA production
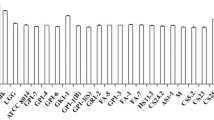
There is a concomitant interest in the industrial demand for multifaceted lactobacilli that in addition to increasing the CLA content of foods, could further improve health (Andrade et al. 2012; Gorissen et al. 2013; Lee et al. 2006). Now with the availability of rapid UV screening methods, it becomes easier to screen high CLA producer and their further CLA isomers characterization by GC analysis. Therefore, large array of 283 lactobacilli strain can be screened for CLA biosynthesis. Only 57 of the 283 lactobacilli were able to produce CLA ≥20 µg/ml. CLA production among these lactobacilli ranged from 19.5 to 71.5 µg/ml (Fig. 1). Here, strain HIF15 reported as the highest CLA producer (71.5 µg/ml) in LA-MRS broth. Our findings are in consistent with previous reports of CLA production from different LAB strains (Andrade et al. 2012; Gorissen et al. 2013). Although, the exact mechanism for CLA production is still not clear and needs a thorough investigation on mechanistic aspects. But it has been suggested that a LA detoxifying mechanism works behind it. Incorporation of LA into bacterial cell membrane changes the lipid bilayer chemistry, membrane potential and even intramembrane pathways (Sosa-Castañeda et al. 2015); therefore for survival bacteria could have to detoxify the LA.
Our major concern was to identify the high t10, c12 and t9, t11 CLA producer in addition to c9, t11 isomer. As earlier stated CLA isomer-c9, t11 accounts >70% of the total CLA produced, and these other isomers were produced in little amounts. The LA–MRS screening revealed that only 19 lactobacilli produced CLA ≥25 µg/ml (Fig. 2) and therefore, assessed in LA-SM medium. Strikingly, culturing in SM significantly (P < 0.005) enhanced CLA production abilities of 08 lactobacilli strains viz. HIF15, HIF27, HIF64, HIF70, HIF77, HIF221, HAF13 and HAF28 in comparison to LA-MRS broth with similar substrate (0.5 mg/ml) concentration (Fig. 2).
Kim and Liu (2002) and Van Nieuwenhove et al. (2007) reported similar observations for addition of non-fat dry milk powder into the fermentation medium. The proposed mechanism states that SM prevents CLA oxidation in addition to support better growth (Shantha and Decker 1993) and some of the milk proteins (α- and β-lactoglobulin) shielded the bacteria from LA toxicity effect.
The frequency distribution chart (Fig. 3) showed that more numbers of CLA producers were obtained from infant feces than adults, followed by dairy products. This variation in CLA production among LAB is well documented in literature. Barrett et al. (2007) tested 18 human feces bifidobacterial strains for CLA biosynthesis with variation (2.60–76.65%) in total CLA production. Chung et al. (2008) characterized 04 bifidobacterial strains with high (>80%) LA conversion potential from a pool of 150. Li et al. (2012) targeted 06 L. plantarum isolates with minor variations (3.85–4.90%) from traditional dairy origin. The results strongly support the facts that different strains of lactobacilli have the varying ability to produce CLA. In our case, higher production was observed for the faecal originated lactobacilli. However, it is not yet clear, how the bacteria origin determines different LA metabolism.
Lactobacilli species characterization
All 57 lactobacilli were characterized for species identification (Fig. 4). Of these, 44% correspond to L. plantarum, L. gasseri (30%), L. fermentum (21%) and L. salivarius (5%). Earlier, several different CLA producing species of lactobacilli (L. plantarum, L. acidophilus, L. casei and L. fermentum) obtained from different sources were reported by several authors (Andrade et al. 2012; Puniya et al. 2008; Ando et al. 2003; Lee et al. 2007). Our results, in respect to CLA production by L. plantarum and L. fermentum are in complete with earlier findings; however, the current study indicated a major proportion of L. gasseri and few L. salivarius strains also as CLA producers. In the present study, characterization of L. gasseri and L. fermentum species as major CLA producer in addition to L. plantarum is might be due to involvement of more faecal samples, as up to 2 months of age L. gasseri (30%) was the most common lactobacilli isolated from infant feces followed by L. fermentum (9%) (Rubio et al. 2014).
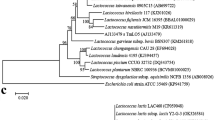
PCR-amplified products of four lactobacilli species (L, in all representative gels from a to c, represents a 100 bp molecular size marker. In gel a lanes 1–3 represent L. salivarius isolates by an amplicon of 411 bp, b lanes 1–16 represent L. plantarum by 248 bp, a–l L. fermentum by 192 bp, c lanes 1–17 indicated L. gasseri isolates by PCR product of 360 bp
CLA isomers analysis by GC
As the biological effects of CLA are isomers specific, thus biosynthesis must be considered prior to preparing a functional food. All 19 lactobacilli having CLA production ≥25 µg/ml in UV screening were analysed for specific-isomers production in LA–MRS and LA–SM. Notably, significant differences in production profiles were obtained in both the fermentation medium (Table 2). Eight lactobacilli strains (HIF15, HIF27, HIF53, HIF64, HIF128, HIF133, HIF191 and HIF221) have shown the ability to produce c9, t11, t10, c12, and t9, t11 CLA isomers in both the mediums and thus considered as potential strains (Table 2, a representative chromatograph is presented in Fig. 5a, b). In all 19 lactobacilli c9, t11 isomer were reported as the most predominant isomer in LA–MRS and LA–SM mediums. These findings are in complete agreement with previous findings of isomer variability (Sosa-Castañeda et al. 2015; Andrade et al. 2012; Puniya et al. 2008).
Strains HIF85, HIF117, HAF13, LSI04 and LSI47 did not show the production of t10, c12 and t9, t11 isomers. On the contrary, in some strains (HIF70, HIF231, HIF247 and HAF28) we only detected the presence of c9, t11 and t9, t11 isomers and no t10, c12 isomer production. From literature we understood that the biosynthesis of t9, t11 CLA isomer by lactobacilli was a further biotransformation consequence of c9, t11 CLA (Hennessy et al. 2012). Our results emphasize that production of CLA and isomer is a highly species and strain dependent phenomenon. Lin et al. (2003) detected eight different CLA isomers (t8, t10, t9, t11, t10, t12, t11, t13, t8, c10, c9, t11, t10, c12 and c11, t13) with the enzyme extract of L. acidophilus CCRC 14079 with LA. Lee et al. (2007) isolated and identified an L. plantarum PL62 from the infant faces that have c9, t11 (26.8 µg/ml) and t10, c12 (6.4 µg/ml) CLA isomers producing potential. Ando et al. (2003) optimized L. plantarum JCM 1551 that produced 2.4 mg/ml of CLA and was mainly comprised of c9, t11 (21% of total CLA) and t9, t11 (79% of total CLA) CLA isomers. Similarly, Li et al. (2012) assayed the CLA production of 06 lactobacilli by employing different substrates. Recently, Terán et al. (2015) examined 64 food-grade lactobacilli for CLA isomers production and revealed that only 04 L. plantarum strains were able to synthesize CLA isomers from LA which is lower than the 03 L. plantarum strains (HIF15, HIF64, HIF128) reported in present study. In another recent study Sosa-Castañeda et al. (2015) assessed the CLA production abilities of 13 Lactobacillus strains out of which strain L. fermentum J20 produced more c9, t11 (42.63 ± 0.91 µg/ml) and t10, c12 (8.27 ± 0.64 µg/ml) CLA isomers then reported in present investigation. By contrary, the production of c9, t11(7.73 ± 0.52 µg/ml) isomer by another L. fermentum strain J23 is lower while that of t10, c12 (11.25 ± 0.51 µg/ml) is higher than L. fermentum strains reported here.
Ogawa et al. (2001) suggested that the biotransformation of LA into CLA isomers is an isomerisation effect of linoleate isomerase (LI) enzyme. Similarly, Kishino et al. (2011) affirmed that a multi-component enzymatic system encoded in the lactobacilli genome was responsible for biohydrogenation activity. Thus, the reported lactobacilli in the present study might have produced these isomers via the LI enzymatic activity. The variability noticed in CLA production might be due to varying ability of strains to synthesize the LI enzyme (Farmani et al. 2010). Furthermore, the variation in production of CLA isomers might be due to presence of different isomeric forms of LI enzyme within the strains (Farmani et al. 2010).
Overall, strain HIF15 was found as the most efficient CLA producer in terms of bioactive isomers and total CLA production. Moreover, detailed SEM examined (Fig. 6) revealed that this strain (HIF15) was a non-spore and non-capsule forming strain. However, future studies will be required to exploit the potential health effects of these potential strains in suitable animal models.
Conclusion
The study suggests that different species of lactobacilli from human faces and dairy samples of Indian origin have the potential to produce bioactive isomers of CLA in a highly species and strain-dependent manner. Three lactobacilli species (L. plantarum, L. fermentum and L. gasseri) were reported as high CLA producer. From here strain HIF15 could be utilized for the genesis of newer CLA-enriched functional foods or as probiotics to promote the continuous synthesis of these bioactive isomers at in situ in the human gut. This will not only boost the dairy industry but simultaneously satisfying the consumers’ need for functional foods. However, further studies are required to validate these finding in suitable animal models for potential health effects in vivo. Furthermore, studies are warranted to elucidate the molecular mechanism and enzyme network of CLA biosynthesis and production.
References
Ando A, Ogawa J, Kishino S, Shimizu S (2003) CLA production from ricinoleic acid by lactic acid bacteria. J Am Oil Chem Soc 80:889–894
Andrade JC, Ascencao K, Gullon P, Henriques S, Pinto J, Rocha-Santos TA, Freitas AC, Gomes A (2012) Production of conjugated linoleic acid by food-grade bacteria: a review. Int J Dairy Technol 65:467–481
Barrett E, Ross R, Fitzgerald G, Stanton C (2007) Rapid screening method for analyzing the conjugated linoleic acid production capabilities of bacterial cultures. Appl Environ Microbiol 73:2333–2337
Chung SH, Kim IH, Park HG, Kang HS, Yoon CS, Jeong HY, Choi NJ, Kwon EG, Kim YJ (2008) Synthesis of conjugated linoleic acid by human-derived Bifidobacterium breve LMC 017: utilization as a functional starter culture for milkfermentation. J Agric Food Chem 56:3311–3316
Dahiya DK, Puniya AK (2015) Evaluation of survival, free radical scavenging and human enterocyte adherence potential of lactobacilli with anti-obesity and anti-inflammatory CLA isomer-producing attributes. J Food Process Preserv 39:2866–2877
Dubernet S, Desmasures N, Guéguen M (2002) A PCR-based method for identification of lactobacilli at the genus level. FEMS Microbiol Lett 214:271–275
Farmani J, Safari M, Roohvand F, Razavi SH, Aghasadeghi MR, Noorbazargan H (2010) Conjugated linoleic acid-producing enzymes: a bioinformatics study. Eur J Lipid Sci Technol 112:1088–1100
Gorissen L, Leroy F, De Vuyst L, De Smet S, Raes K (2013) Bacterial production of conjugated linoleic and linolenic acid in foods: a technological challenge. Crit Rev Food Sci Nutr 55:1561–1574
Hennessy A, Ross R, Devery R, Stanton C (2009) Optimization of a reconstituted skim milk based medium for enhanced CLA production by Bifidobacteria. J Appl Microbiol 106:1315–1327
Hennessy AA, Barrett E, Ross RP, Fitzgerald GF, Devery R, Stanton C (2012) The production of conjugated α-linolenic, γ-linolenic and stearidonic acids by strains of bifidobacteria and propionibacteria. Lipids 47:313–327
Herman-Lara E, Santos-Blanco V, Vivar-Vera M, García H, Ochoa-Martínez L, Martínez-Sánchez C (2012) Conjugated linoleic acid content in selected Mexican beef and dairy products. CyTA J Food 10:71–77
Jenkins T, Wallace R, Moate P, Mosley E (2008) Board-invited review: recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J Anim Sci 86:397–412
Kim Y, Liu R (2002) Increase of conjugated linoleic acid content in milk by fermentation with lactic acid bacteria. J Food Sci 67:1731–1737
Kim JH, Kim Y, Kim YJ, Park Y (2016) Conjugated linoleic acid: potential health benefits as a functional food ingredient. Annu Rev Food Sci Technol. doi:10.1146/annurev-food-041715-033028
Kishino S, Ogawa J, Yokozeki K, Shimizu S (2011) Linoleic acid isomerase in Lactobacillus plantarum AKU1009a proved to be a multi-component enzyme systemrequiring oxidoreduction cofactors. Biosci Biotechnol Biochem 75:318–322
Lee HY, Park JH, Seok SH, Baek MW, Kim DJ, Lee KE, Paek KS, Lee Y, Park JH (2006) Human originated bacteria Lactobacillus rhamnosus PL60 produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta Mol Cell Biol Lipids 1761:736–744
Lee K, Paek K, Lee H, Park JH, Lee Y (2007) Antiobesity effect of trans-10, cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J Appl Microbiol 103:1140–1146
Li H, Liu Y, Liu X, Zhang H (2012) Conjugated linoleic acid conversion by six Lactobacillus plantarumstrains cultured in MRS broth supplemented with sunflower oil and soymilk. J Food Sci 77:M330–M336
Lin TY, Lin CW, Wang YJ (2003) Production of conjugated linoleic acid by enzyme extract of Lactobacillus acidophilus CCRC14079. Food Chem 83:27–31
Lock AL, Bauman DE (2004) Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids 39:1197–1206
Macdonald HB (2000) Conjugated linoleic acid and disease prevention: a review of current knowledge. J Am Coll Nutr 19:111S–118S
Nunes JC, Torres AG (2010) Fatty acid and CLA composition of Brazilian dairy products, and contribution to daily intake of CLA. J Food Compos Anal 23:782–789
O’Fallon J, Busboom J, Nelson M, Gaskins C (2007) A direct method for fatty acid methyl ester synthesis: application to wet meat tissues, oils, and feedstuffs. J Anim Sci 85:1511–1521
Ogawa J, Matsumura K, Kishino S, Omura Y, Shimizu S (2001) Conjugated linoleic acid accumulation via 10-hydroxy-12-octadecaenoic acid during microaerobic transformation of linoleic acid by Lactobacillus acidophilus. Appl Environ Microbiol 67:1246–1252
Özer CO, Kılıç B, Kılıç GB (2016) In vitro microbial production of conjugated linoleic acid by probiotic L. plantarum strains: utilization as a functional starter culture in sucuk fermentation. Meat Sci 114:24–31
Pospiech A, Neumann B (1995) A versatile quick-prep of genomic DNA from Gram-positive bacteria. TIG 11:217–218
Puniya AK, Chaitanya S, Tyagi AK, De S, Singh K (2008) Conjugated linoleic acid producing potential of lactobacilli isolated from the rumen of cattle. J Ind Microbiol Biotechnol 35:1223–1228
Rubio R, Jofre A, Martín B, Aymerich T, Garriga M (2014) Characterization of lactic acid bacteria isolated from infant feces as potential probiotic starter cultures for fermented sausages. Food Microbiol 38:303–311
Shantha N, Decker E (1993) Conjugated linoleic acid concentrations in processed cheese containing hydrogen donors, iron and dairy-based additives. Food Chem 47:257–261
Song YL, Kato N, Liu CX, Matsumiya Y, Kato H, Watanabe K (2000) Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group-and species-specific primers derived from the 16S–23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol Lett 187:167–173
Sosa-Castañeda J, Hernández-Mendoza A, Astiazarán-García H, Garcia H, Estrada-Montoya M, González-Córdova A, Vallejo-Cordoba B (2015) Screening of Lactobacillus strains for their ability to produce conjugated linoleic acid in milk and to adhere to the intestinal tract. J Dairy Sci 98:6651–6659
Taboada N, Van Nieuwenhove C, Alzogaray SL, Medina R (2015) Influence of autochthonous cultures on fatty acid composition, esterase activity and sensory profile of Argentinean goat cheeses. J Food Compos Anal 40:86–94
Terán V, Pizarro PL, Zacarías M, Vinderola G, Medina R, Van Nieuwenhove C (2015) Production of conjugated dienoic and trienoic fatty acids by lactic acid bacteria and bifidobacteria. J Funct Foods 19:417–425
Van Nieuwenhove C, Oliszewski R, Gonzalez S, ChaiaA Perez (2007) Conjugated linoleic acid conversion by dairy bacteria cultured in MRS broth and buffalo milk. Lett Appl Microbiol 44:467–474
Zheng G, Genwang Z, Yan S (2003) Research development of the preparation methods of conjugated linoleic acid. Chem Peking 66:592
Acknowledgements
We gratefully acknowledge ICAR-NDRI, Karnal for the financial support in form of fellowship to DKD. We express our sincere thanks to Dr. Ajai Kumar, AIRF (JNU), New Delhi for providing technical assistance for GC facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dahiya, D.K., Puniya, A.K. Isolation, molecular characterization and screening of indigenous lactobacilli for their abilities to produce bioactive conjugated linoleic acid (CLA). J Food Sci Technol 54, 792–801 (2017). https://doi.org/10.1007/s13197-017-2523-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2523-x