Abstract
Lactobacilli have several attributes that provide health benefits to the host. The aim of this study was to screen indigenous lactobacilli from human gut and fermented foods for such attributes as production of β- and α-galactosidase and also their ability to reduce serum cholesterol. Lactobacilli were cultured on MRS broth and β-galactosidase activity was determined using o-nitrophenyl-β-D-galactopyranoside (ONPG) as a substrate. Three isolates Lactobacillus fermentum GPI-3 and L. fermentum GPI-6 and Lactobacillus salivarius GPI-1(S) showed better β-galactosidase activity than the standard strains Lactobacillus rhamnosus GG (LGG) and Lactobacillus plantarum ATCC 8014. The isolates showed variability in assimilating cholesterol during growth. Several isolates showed excellent cholesterol-lowering ability compared to standard strains LGG and L. plantarum ATCC 8014. Isolate L. rhamnosus SCB being the highest acid producer (pH 4.38) also showed the highest cholesterol reduction compared to other strains including standard strains. The ability of these isolates to produce α-galactosidase was also studied and the maximum α-galactosidase activity was found in isolate L. salivarius GPI-1(S) followed by L. fermentum FA-5 and Lactobacillus helveticus FA-7. This study therefore reports Lactobacillus isolates that have superior probiotic properties when compared to the standard strains; hence, they could be considered as potential probiotic strains, which can provide health benefits to the Indian population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid bacteria (LAB) possess various health-promoting properties useful for both humans and animals [1,2,3]. β-Galactosidase deficiency causes lactose intolerance [4, 5] and amelioration of this situation by β-galactosidase from LAB [6, 7] involves conversion of lactose into easily metabolizable glucose and galactose. The symptoms of lactose intolerance decrease the quality of life and daily activities. The addition of lactobacilli-producing β-galactosidase as probiotic in dairy products can thus be used for improving lactose digestion.
Consumption of LAB also reduces serum cholesterol levels, as suggested by human and animal studies [1, 8]. This is also corroborated by in vitro experiments using growth medium containing bile salts. Similarly, in vitro uptake of cholesterol from culture media has also been shown for many strains of lactobacilli [9,10,11]. Bile salt hydrolase plays a significant role in cholesterol removal by deconjugating the bile salts [12]. Deconjugated bile salts are less soluble and less efficiently reabsorbed from the intestinal lumen than their conjugated counterparts [13]. Additionally, free bile salts are less efficient in the solubilization and absorption of lipids in the gut [13, 14], eventually leading to less uptake of cholesterol. Lactobacilli may also remove cholesterol by bringing about co-precipitation of cholesterol with free bile salts, bacterial assimilation of cholesterol, or attachment of cholesterol to the surface of Lactobacillus cells [15, 16]. Furthermore, it was also demonstrated by Kumar et al. [17] that the amount of cholesterol removed from the broth was variable, depending on the culture and the pH, during growth.
Consumption of soybean and pulses is limited because of intestinal disturbances caused by α-D-galactosides such as melibiose, raffinose and stachyose, as well as branched polysaccharides such as galactomannans and galactoglucomannans [18, 19]. α-Galactosidase which cleaves the α-1,6-linked galactose residues from such carbohydrate complexes is therefore used for the hydrolysis and release of such oligosaccharides present in food substances. Studies have shown a reduction in gastrointestinal discomfort due to gas, after addition of probiotics to pulse and soybean meal containing diets [20]. Earlier studies have established that Lactobacillus rhamnosus GG (LGG) has cholesterol removing ability and Lactobacillus plantarum ATCC 8014 has both α-galactosidases and β-galactosidase activities [21, 22].
Due to the above attributes, lactobacilli have been used as active ingredients in probiotic food such as bio-yoghurt, dietary adjuncts, and health-related products. Therefore, in the present study, lactobacilli were assessed for these attributes and several strains were found to perform better than the standard probiotic strains L. rhamnosus GG (LGG) and L. plantarum ATCC 8014 and therefore could be considered for further studies.
Materials and Methods
Bacterial Strains and Culture Conditions
A total of 20 different lactobacilli strains from different sources were used in this study as given in Table 1. They were analyzed for their probiotic properties in an earlier study that includes bile and acid tolerance, adhesion to Caco-2 and HT-29 cells and antimicrobial activity against test pathogens [23, 24]. Prior to being used, they were serially propagated three times in the appropriate medium. Lactobacilli were cultivated in de Man, Rogosa, and Sharpe (MRS) broth (MRS; Himedia, Mumbai, India). A 1.0% inoculum was used and incubated at 37 °C for 24 h in static conditions. Seed cultures of each strain were taken at the end of the exponential phase of growth at cell densities of ca. 108 CFU/mL. Standard probiotic strain L. rhamnosus GG (LGG) and standard dairy strain L. plantarum American Type Culture Collection (ATCC) 8014 were obtained as kind gifts from Dr. Shira Doron (MD, Department of Medicine, Tufts Medical Centre, Boston, MA, USA) and Food and Drugs Laboratory (FDL; Vadodara, India), respectively.
β-Galactosidase Production
For qualitative assay, an overnight grown culture was streaked on MRS agar plate containing 0.01% X-gal (5-bromo-4-chloro-2-indolyl-β-D-galactopyranoside) and 0.1 mM IPTG (iso-propyl-thio-β-D-galactopyranoside) as an inducer. The plates were incubated for 24 h to 3 days at 37 °C in microaerobic environment and observed for the appearance of blue colonies. This was followed by quantitative assay where intracellular β-galactosidase activity in whole cells was determined according to the method of Miller [25] with slight modifications. Overnight grown cultures were harvested by centrifugation, washed twice in phosphate-buffered saline (PBS) pH 7.0, and inoculated 1% (v/v) in MRS-lac broth (containing lactose). Cultures were incubated at 37 °C for 24 h (microaerobic environment). Cells were then harvested, washed twice with PBS, and A560 was adjusted to 1.0 with the same buffer. One milliliter of the cell suspension was permeabilized with 50 μL of toluene:acetone (1:9, v/v) solution, vortexed for 7 min and immediately assayed for β-galactosidase activity. To 100 μL of the permeabilized cell suspension, 900 μL of phosphate buffer and 200 μL of o-nitrophenyl-β-D-galactopyranoside (ONPG, Sigma) (4 mg/mL) were added. Tubes were then incubated at 37 °C for 15 min, and the reaction stopped by the addition of 0.5 mL of 1 mol/L Na2CO3. Absorbance at both 420 and 560 nm was then recorded for each tube and β-galactosidase activity was calculated in Miller units (MU) as follows:
where A1560 was the absorbance just before assay and A2560 was the absorbance of the reaction mixture.
Cholesterol Removal by Different Lactobacilli and by Lactobacillus-Fermented Curd
Bacteria grown overnight in MRS broth were washed with PBS (pH 7.0) following which 1 × 108 cells were suspended in 1 mL of 0.3% ox-bile MRS broth (Himedia, Mumbai, India) containing cholesterol (150 mg/dL). Cells were allowed to grow for 24 h at 37 °C in microaerobic environment and then pelleted down and the supernatant was used for cholesterol estimation by colorimetric assay. Cholesterol reagent was added to 10 μL of supernatant and incubated for 10 min at 37 °C following which absorbance was taken at 505 nm. This assay was done with the help of cholesterol estimation kit (Reckon Diagnostics, Baroda, India). Cholesterol concentration (in mg/dL) and cholesterol reduction (%) were calculated, using the formula [(Absorbance of test)/(Absorbance of standard)] × 200 and [(150 – mean of residual cholesterol conc. in the supernatant)/150] × 100, respectively.
Cholesterol removal from broth was also checked for Lactobacillus-fermented curd (1 × 108 cells of the each culture were inoculated in to 10 mL of milk individually and incubated overnight at 37 °C under static condition (microaerobic environment)), for which, the same procedure as described above was used. Furthermore, pH and whey protein concentration of this Lactobacillus-fermented curd were also checked.
α-Galactosidase Production
α-Galactosidase activity was assessed as per method described by Donkor et al. [26] with a few modifications. To summarize it, all organisms were used following three successive propagations in sterile MRS broth at 37 °C for 24 h. Subsequently, 1 × 108 cells of the culture were inoculated into 1 mL of sterile MRS broth and incubated at 37 °C for 24 h in microaerobic environment. Following this, the cells were harvested and the cell pellet was washed twice with cold 50 mM sodium phosphate buffer (pH 5.5). Cells were finally resuspended in 1 mL of the same buffer, placed in an ice bath for 10 min followed by sonication for 10 min. The above steps of cooling and sonication were repeated twice to ensure that the bacterial cells were completely lysed. The cell debris was removed by centrifugation and the resultant supernatant was used as a crude enzyme extract. α-Galactosidase assay was carried out according to the method of Scalabrini et al. [27] with some modifications. Briefly, a 150-μL aliquot of enzyme extract was mixed with 300 μL of 5 mM p-nitrophenyl-α-D-galactopyranoside (PNPG) and incubated at 37 °C for 30 min, following which 300 μL of cold 0.2 mol/L sodium carbonate solution was added to stop the reaction. The α-galactosidase activity was determined by the rate of hydrolysis of PNPG. The amount of p-nitrophenol released was measured at 420 nm. A standard calibration curve was prepared using known concentrations of p-nitrophenol (Sigma-Aldrich, Steinheim, Germany). One unit of enzyme activity was defined as the amount of enzyme that released l.0 μM of p-nitrophenol from PNPG per milliliter per min under the assay conditions. The specific activity was expressed as units (U) of α-galactosidase activity per milligram of protein. The protein concentration of the crude enzyme extracts was determined using the method of Bradford [28].
Statistical Analysis
Values are given as mean values and standard deviations of triplicate independent experiments. Significant ANOVAs were followed by Dunnett’s test in all the assays to compare with respect to positive controls (LGG and L. plantarum ATCC 8014) (P < 0.05). All the analysis was conducted using Graph pad Prism 6.01.
Results
β-Galactosidase Production
Lactobacillus isolates were grown on MRS-X-gal agar plate for determining their ability to produce β-galactosidase. Most of the cultures excepting strains L. delbrueckii M, L. fermentum ASt-1, L. rhamnosus CS25, L. rhamnosus SCA, and L. rhamnosus SCB gave blue colored colonies, indicating their ability to produce β-galactosidase enzyme (Table 2). In the case of strains, L. fermentum GPI-7, L. fermentum GKI-1, L. fermentum GPI-1(B), L. fermentum IIS11.2, L. fermentum GPI-3, L. salivarius GPI-4, L. salivarius GPI-1(S), L. plantarum ATCC 8014, L. casei CS5.2, and L. plantarum CS23, blue-colored colonies appeared within 24 h while for LGG, L. fermentum GPI-6, L. fermentum FA-5, L. fermentum FA-1, L. plantarum GRI-2, L. helveticus FA-7, and L. plantarum CS24.2, colonies turned blue after 48 h of incubation. Furthermore, following enzyme assay, β-galactosidase activity was found significantly higher than both standard strains LGG and L. plantarum ATCC 8014, for most of the cultures (P < 0.05, Table 2). Excellent levels were found for L. salivarius GPI-1(S), L. fermentum GPI-6, and L. fermentum GPI-3 which were about twofold compared to LGG and L. plantarum ATCC 8014.
Cholesterol Removal by Different Lactobacilli
In the present study, lactobacilli were examined for their ability to reduce cholesterol by inoculating lactobacilli directly in MRS broth as well as MRS broth inoculated with starter culture from various lactobacilli fermented curd. The cholesterol reduction by these methods in MRS broth containing oxgall and cholesterol following 24 h for growth of various lactobacilli at 37 °C was determined (Tables 3 and 4 respectively). Uninoculated sterile broth was used as control.
Cholesterol Removal from Broth by Different Lactobacilli
Residual cholesterol concentration was determined in the supernatants from growth media and the results are given in Table 3. Most of the cultures showed good cholesterol removal in supernatant than both standard strains LGG and L. plantarum ATCC 8014, excepting L. rhamnosus CS25, L. fermentum IIS11.2 and L. fermentum GKI-1. However, strain L. rhamnosus SCB (78.76%) showed significant (P < 0.05) and best cholesterol lowering ratio among all, while strains L. plantarum CS24.2 (50.21%), L. plantarum CS23 (45.42%), L. salivarius GPI-1(S) (45.35%), and L. delbrueckii M (45.43%) were better than LGG (21.13%) and L. plantarum ATCC 8014 (30.90%).
Cholesterol Removal by Lactobacillus-Fermented Curd
Residual cholesterol concentration was also determined in the supernatant of growth media inoculated with starter culture from various lactobacilli fermented curd and the results are given in Table 4. It was observed that strain L. rhamnosus SCB (76.50%) had excellent cholesterol reducing ability from growth medium as compared to both standard strains LGG (30.54%) and L. plantarum ATCC 8014 (40.18%). The strains L. casei CS5.2 (56.34%), L. plantarum CS23 (49.57%), L. delbrueckii M (46.17%), L. salivarius GPI-1(S) (44.84%), and L. fermentum GPI-6 (45.28%) also showed significant (P < 0.05) and better cholesterol reduction ability compared to both the standard probiotic stains. The results showed that more cholesterol reduction was observed in case of Lactobacillus fermented curd as compared to use of Lactobacillus cultures directly in MRS broth except for the strains L. rhamnosus SCA, L. plantarum CS24.2, L. fermentum FA-5 and L. fermentum GPI-3 where more of cholesterol reduction was seen in case of Lactobacillus culture in broth, while the medium inoculated with L. fermentum GKI-1 (5.52%) and L. rhamnosus CS25 (4.44%) showed no significant decrease in cholesterol content.
pH of Curd Prepared with Different Lactobacilli
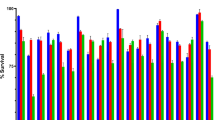
Deconjugation of bile salts by BSH takes place at acidifying and pH-controlled conditions. The pH of curd fermented by various lactobacilli was determined. It was observed (Table 4) that the strains producing more acidic curd showed better cholesterol reduction. Strain L. rhamnosus SCB (pH 4.38) being most acidic showed highest cholesterol reduction. Similarly, strains L. plantarum CS23 (pH 4.91), L. salivarius GPI-1(S) (pH 4.92), L. casei CS5.2 (pH 4.89), and L. delbrueckii M (pH 4.93) also showed acidic pH with significant reduction of cholesterol content (P < 0.05, Fig. 1).
pH of curd prepared using different lactobacilli. Values are means of three independent experiments, with standard deviations represented by vertical bars. The strains were compared with two different positive controls (L. rhamnosus GG and L. plantarum ATCC 8014) by means of two independent ANOVA tests. Significant ANOVAs were followed by Dunnett’s test for multiple comparisons vs. the positive control group. *Mean value of isolates was significantly different from that of L. rhamnosus GG (P < 0.05). ***Mean value of isolates was significantly different from that of both L. rhamnosus GG (P < 0.05) and L. plantarum ATCC 8014 (P < 0.05)
Protein Concentration in Whey
Protein levels in whey from curd fermented by various lactobacilli were determined and the results are given in Fig. 2. Whey of strain L. rhamnosus SCB fermented curd (0.63 μg/μL) showed the lowest protein concentration as compared to both standard strains LGG (0.86 μg/μL) and L. plantarum ATCC 8014 (1.28 μg/μL) fermented curd. However, L. salivarius GPI-1(S) (0.74 μg/μL), L. plantarum CS23 (0.71 μg/μL), L. plantarum CS24.2 (0.78 μg/μL), L. fermentum GPI-6 (0.75 μg/μL), L. fermentum GPI-7 (0.75 μg/μL), L. delbrueckii M (0.73 μg/μL), and L. casei CS5.2 (0.71 μg/μL) fermented curd showed significantly (P < 0.05) low protein concentration in their whey. It was observed that strains having less protein concentration in the whey are better fermenters and thus form better curd.
Protein concentration of whey. Values are means of three independent experiments, with standard deviations represented by vertical bars. The strains were compared with two different positive controls (L. rhamnosus GG and L. plantarum ATCC 8014) by means of two independent ANOVA tests. Significant ANOVAs were followed by Dunnett’s test for multiple comparisons vs. the positive control group. **Mean value of isolates was significantly different from that of L. plantarum ATCC 8014 (P < 0.05). ***Mean value of isolates was significantly different from that of both L. rhamnosus GG (P < 0.05) and L. plantarum ATCC 8014 (P < 0.05)
Based on the above data, strains were categorized as strong, moderate, and weak fermenting strains: L. rhamnosus SCB, L. plantarum strains CS24.2, CS23, L. fermentum strains GPI-7, GPI-6, L. dulbrueckii M, L. casei CS5.2, and L. salivarius GPI-1(S) were strong fermenting strains. L. rhamnosus CS25, L. fermentum strains GPI-3, GKI-1, IIS11.2, ASt-1, and FA-5 were moderately fermenting strains. The strains L. fermentum FA-1, GPI-1(B), L. rhamnosus SCA, L. helveticus FA-7, L. plantarum GRI-2 and L. salivarius GPI-4 were weakly fermenting strains. Strains L. salivarius GPI-1(S) and L. plantarum CS23 were categorized as strong fermenting strains and also showed higher β-galactosidase production. They also performed equally well in cholesterol removal when Lactobacillus culture was used directly in broth as well as when Lactobacillus fermented curd was used as inoculum.
α-Galactosidase Production
Isolates were also screened on the basis of their ability to produce α-galactosidase in order to select those with potential for digestion of complex oligosaccharides. The strains exhibited different levels of α-galactosidase activities, which are given in Table 5. Most of the cultures showed better α-galactosidase activity as compared to both standard strains LGG (0.074 U/mg protein) and L. plantarum ATCC 8014 (0.157 U/mg protein). L. salivarius GPI-1(S) (12.939 U/mg protein) showed significantly (P < 0.05) highest level of α-galactosidase activity followed by L. fermentum FA-5 (9.627 U/mg protein) and L. helveticus FA-7 (8.150 U/mg protein).
Discussion
Lactobacilli are frequently associated with health-promoting effects in human and animal intestines. Lactose intolerance, the impaired ability to digest lactose, has been recognized as a problem in many children and most adults throughout the world [29, 30]. In the present study, different lactobacilli were checked for β-galactosidase, since it is the enzyme that hydrolyses lactose into easily metabolisable glucose and galactose. Inclusion of β-galactosidase producing lactobacilli as probiotics in milk and cheese and other dairy products could help overcome lactose intolerance symptoms in humans [31]. Our study showed that most of the cultures had higher β-galactosidase activity than both standard strains L. rhamnosus GG (LGG) and L. plantarum ATCC 8014. The highest levels of this enzyme were nearly twofold in L. fermentum strain GPI-3, followed by L. salivarius GPI-1(S) and L. fermentum strain GPI-6 compared to both standard strains. The values found for the tested lactobacilli were in the range of values previously reported by Meira et al. [32] and Belicová et al. [33].
Several studies have shown a direct relationship between consumption of cultured dairy products and a reduction of serum cholesterol levels in humans and animals [34,35,36], although the exact mechanism of cholesterol reduction by lactobacilli is unclear. Several mechanisms have been proposed, which include assimilation of cholesterol into bacterial cell membranes [16, 37], co-precipitation of cholesterol with deconjugated bile [38], cholesterol binding to the bacterial cell walls [39], incorporation of cholesterol into the cellular membranes of lactobacilli during growth [40], conversion of cholesterol into coprostanol [41], production of short-chain fatty acids (SCFAs) during the growth of bacteria [42], and enzymatic deconjugation of bile acids by bile-salt hydrolase (BSH) of lactobacilli [11, 43]. Moreover, deconjugated bile salts being less soluble are efficiently reabsorbed from the intestinal lumen than their conjugated counterparts, resulting in excretion of larger amount of free bile acids in feces. Therefore, the deconjugation of bile acids by lactobacilli could lead towards a reduction in serum cholesterol either by increasing the demand of cholesterol for formation of new bile acids to replace those lost in feces or by reducing cholesterol solubility and thereby absorption of cholesterol throughout the intestinal lumen [13, 44]. In addition, Gilliland et al. [45] reported that cholesterol was partially removed from the medium after culturing of Lactobacillus acidophilus RP32 in the presence of oxbile as the source of bile salts. Liong and Shah [46] reported that the precipitation of cholesterol in culture fluids appears to be related to deconjugation of bile salts due to BSH activity of lactobacilli and their subsequent precipitation at low pH. In our study, the extent of cholesterol removal was from 2.40 to 78.76% in case of Lactobacillus directly used in 0.3% oxbile containing MRS broth and ranged from 4.44 to 76.50% when Lactobacillus was used from fermented curd. Among the strains tested, L. rhamnosus SCB achieved the highest removal in both types of cholesterol removal studies, using lactobacilli directly in MRS broth and as inoculation from fermented curd, compared to both standard strains. In the present study, our isolated LAB showed excellent cholesterol removal (up to 78.76%) similar to earlier reports by Kuda et al. [47] (up to 61%) and Miremadi et al. [48] (up to 65%). Kumar et al. [17] revealed that the amount of cholesterol that was removed from the growth media was variable, depending on the culture and the pH, during the growth of lactobacilli. pH is an important parameter for the assimilation and reduction of cholesterol. Although some studies have shown that the optimal pH for bile salt deconjugation by lactobacilli is lower than 6.0 [49, 50], others have suggested that the high BSH activity of some Lactobacillus species can be partially attributed to the low pH of the medium. In our study, it was also seen that strains which produced more acidic curd showed better cholesterol reduction. L. rhamnosus SCB being most acidic (pH 4.38) showed the highest cholesterol removal compared to other strains including standard strains. Isolates L. plantarum CS23 (pH 4.91), L. salivarius GPI-1(S) (pH 4.92), and L. casei CS5.2 (pH 4.89) also showed acidic pH with significant removal of cholesterol in MRS broth.
Protein concentration in whey of curd produced by different lactobacilli was also determined. It was observed that strains having less whey protein concentration had better curd fermenting ability (data not shown). Result showed that curd containing strains L. rhamnosus SCB, L. plantarum CS24.2, L. plantarum CS23, L. fermentum GPI-7, L. fermentum GPI-6, L. delbrueckii M, L. casei CS5.2, and L. salivarius GPI-1(S) showed less protein concentration in their whey. Hence, we conclude that these isolates were good fermenting strains.
α-Galactosidase hydrolyses α-D-galactosidic bonds present in oligosaccharides like raffinose and stachyose. It is not synthesized by humans and thus the presence of these oligosaccharides could hinder digestion and cause flatulence, since these sugars are then utilized by the gas generating intestinal microorganisms. These enzymes can be used to digest these oligosaccharides and upgrade the nutrition of legume food [51, 52]. In the past, α-galactosidase was considered as an effective food additive to remove these anti-nutrient oligosaccharides, which occurred in soybean meal containing diets [19, 53]. Hence, in the present study, α-galactosidase activity of these isolates was also checked and the specific activity for each was calculated. It is seen that most of the cultures showed better α-galactosidase activity as compared to both the standard strains LGG (0.074 U/mg protein) and L. plantarum ATCC 8014 (0.157 U/mg protein). L. salivarius GPI-1(S) (12.939 U/mg protein) showed the highest value of α-galactosidase activity compared to other isolates including both standard strains followed by L. fermentum FA-5 (9.627 U/mg protein) and L. helveticus FA-7 (8.150 U/mg protein). Some of the isolates showed better α-galactosidase activity than that reported by Liu et al. [20] in case of L. rhamnosus and L. casei.
Conclusions
This study has therefore been able to select several lactobacilli with better health promoting attributes than standard probiotic strains LGG and L. plantarum ATCC 8014 in terms of production of β-galactosidase and α-galactosidase, in addition to ability to reduce cholesterol levels.
References
Wu, Y., Zhang, Q., Ren, Y., & Ruan, Z. (2017). Effect of probiotic Lactobacillus on lipid profile: a systematic review and meta-analysis of randomized, controlled trials. PLoS One, 12(6), e0178868. https://doi.org/10.1371/journal.pone.0178868
van den Elsen, L. W. J., Poyntz, H. C., Weyrich, L. S., Young, W., & Forbes-Blom, E. E. (2017). Embracing the gut microbiota: the new frontier for inflammatory and infectious diseases. Clin. Transl. Immunology, 6(1), e125. https://doi.org/10.1038/cti.2016.91
Jariwala, R., Mandal, H., & Bagchi, T. (2017). Indigenous lactobacilli strains of food and human sources reverse enteropathogenic E. coli O26:H11-induced damage in intestinal epithelial cell lines: effect on redistribution of tight junction proteins. Microbiology, 163(9), 1263–1272. https://doi.org/10.1099/mic.0.000507
Sánchez, B., Delgado, S., Blanco-Míguez, A., Lourenço, A., Gueimonde, M., & Margolles, A. (2017). Probiotics, gut microbiota, and their influence on host health and disease. Molecular Nutrition & Food Research, 61(1). https://doi.org/10.1002/mnfr.201600240
Habtamu, L. D., Ashenafi, M., Taddese, K., Birhanu, K., & Getaw, T. (2015). Occurrence of lactose intolerance among Ethiopians. J. Food Process. Technol., 6, 1.
Pithva, S., Shekh, S., Dave, J., & Vyas, B. R. (2014). Probiotic attributes of autochthonous Lactobacillus rhamnosus strains of human origin. Applied Biochemistry and Biotechnology, 173(1), 259–277. https://doi.org/10.1007/s12010-014-0839-9
Carević, M. B., Vukašinović-Sekulić, M. S., Banjanac, K. M., Milivojević, A. D., Ćorović, M. M., & Bezbradica, D. I. (2017). Characterization of β-galactosidase from Lactobacillus acidophilus: stability and kinetic study. Adv. Technol., 6(1), 5–13. https://doi.org/10.5937/savteh1701005C
Michael, D. R., Davies, T. S., Moss, J. W. E., Calvente, D. L., Ramji, D. P., Marchesi, J. R., Pechlivanis, A., Plummer, S. F., & Hughes, T. R. (2017). The anti-cholesterolaemic effect of a consortium of probiotics: an acute study in C57BL/6J mice. Scientific Reports, 7(1), 2883. https://doi.org/10.1038/s41598-017-02889-5
Ghahremani, E., Mardani, M., & Rezapour, S. (2015). Phenotypic and genotypic characterization of lactic acid bacteria from traditional cheese in Khorramabad city of Iran with probiotic potential. Applied Biochemistry and Biotechnology, 175(5), 2516–2527. https://doi.org/10.1007/s12010-014-1434-9
Shehata, M. G., El Sohaimy, S. A., El-Sahn, M. A., & Youssef, M. M. (2016). Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Annals of Agricultural Science, 61(1), 65–75. https://doi.org/10.1016/j.aoas.2016.03.001
Mahmoudi, I., Moussa, O., & Hassouna, M. (2017). Symbiotic, hypocholesterolemic and antioxidant effects of potential probiotic lactobacilli strains isolated from Tunisian camel milk. Advances in Microbiology, 7(04), 328–342. https://doi.org/10.4236/aim.2017.74027
Jayashree, S., Pooja, S., Pushpanathan, M., Rajendhran, J., & Gunasekaran, P. (2014). Identification and characterization of bile salt hydrolase genes from the genome of Lactobacillus fermentum MTCC 8711. Applied Biochemistry and Biotechnology, 174(2), 855–866. https://doi.org/10.1007/s12010-014-1118-5
Costabile, A., Buttarazzi, I., Kolida, S., Quercia, S., Baldini, J., Swann, J. R., Brigidi, P., & Gibson, G. R. (2017). An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PLoS One, 12(12), e0187964. https://doi.org/10.1371/journal.pone.0187964
Werner, A., Kuipers, F., & Verkade, H. J. (2004). Fat absorption and lipid metabolism in cholestasis. In Molecular pathogenesis of cholestasis, vol. 1: (Trauner, M. and Jansen, Peter L.M., eds.), Landes Bioscience (pp. 314–328). USA. https://doi.org/10.1007/978-1-4419-9034-1_23
Kumar, M., Nagpal, R., Kumar, R., Hemalatha, R., Verma, V., Kumar, A., Chakraborty, C., Singh, B., Marotta, F., Jain, S., & Yadav, H. (2012). Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Experimental Diabetes Research, 2012, 1–14. https://doi.org/10.1155/2012/902917
Aquino, M. G. B., Ebuen, B. U., Widwidan, C., & Soriano, G. P. (2017). Cholesterol lowering potential of Lactobacillus brevis. International Journal of Research Studies in Microbiology and Biotechnology, 3, 7–10.
Kumar, A., Kumar, M., Ghosh, M., & Ganguli, A. (2013). Modeling in vitro cholesterol reduction in relation to growth of probiotic Lactobacillus casei. Microbiology and Immunology, 57(2), 100–110. https://doi.org/10.1111/1348-0421.12008
LeBlanc, J. G., Ledue-Clier, F., Bensaada, M., de Giori, G. S., Guerekobaya, T., Sesma, F., Juillard, V., Rabot, S., & Piard, J. C. (2008). Ability of Lactobacillus fermentum to overcome host α-galactosidase deficiency, as evidenced by reduction of hydrogen excretion in rats consuming soya α-galacto-oligosaccharides. BMC Microbiology, 8(1), 22–30. https://doi.org/10.1186/1471-2180-8-22
Mishra, B. K., Hati, S., Das, S., Mishra, S., & Mandal, S. (2017). α-galactosidase and β-glucosidase enzyme activity of lactic strains isolated from traditional fermented foods of West Garo Hills, Meghalaya. International Journal of Current Microbiology and Applied Sciences, 6, 1193–1201.
Liu, X., Champagne, C. P., Lee, B. H., Boye, J. I., & Casgrain, M. (2014). Thermostability of probiotics and their α-galactosidases and the potential for bean products. Biotechnology Research International, 2014, 472723.
Silvestroni, A., Connes, C., Sesma, F., de Giori, G. S., & Piard, J. C. (2002). Characterization of the melA locus for alpha-galactosidase in Lactobacillus plantarum. Applied and Environmental Microbiology, 68(11), 5464–5471. https://doi.org/10.1128/AEM.68.11.5464-5471.2002
Kumar, M., Rakesh, S., Nagpal, R., Hemalatha, R., Ramakrishna, A., Sudarshan, V., Ramagoni, R., Shujauddin, M., Verma, V., Kumar, A., Tiwari, A., Singh, B., & Kumar, R. (2013). Probiotic Lactobacillus rhamnosus GG and Aloe Vera gel improve lipid profiles in hypercholesterolemic rats. Nutrition, 29(3), 574–579. https://doi.org/10.1016/j.nut.2012.09.006
Dhanani, A.S. (2014) The interaction of Lactobacillus strains with intestinal epithelial cell lines. PhD thesis, The Maharaja Sayajirao University of Baroda, Vadodara.
Mandal, H., Jariwala, R., & Bagchi, T. (2015). Isolation and characterization of lactobacilli from human faeces and indigenous fermented foods for their potential application as probiotics. Canadian Journal of Microbiology, 62, 349–359.
Miller, J. H. (1972). Assay of β-galactosidase. In Experiments in molecular genetics, Cold Spring Harbor Laboratory Press (pp. 352–355). New York.
Donkor, O. N., Henriksson, A., Vasiljevic, T., & Shah, N. P. (2007). α-Galactosidase and proteolytic activities of selected probiotic and dairy cultures in fermented soymilk. Food Chemistry, 104(1), 10–20. https://doi.org/10.1016/j.foodchem.2006.10.065
Scalabrini, P., Rossi, M., Spettoli, P., & Matteuzzi, D. (1998). Characterization of Bifidobacterium strains for use in soymilk fermentation. International Journal of Food Microbiology, 39(3), 213–219. https://doi.org/10.1016/S0168-1605(98)00005-1
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Heyman, M. B. (2006). Lactose intolerance in infants, children, and adolescents. Pediatrics, 118(3), 1279–1286. https://doi.org/10.1542/peds.2006-1721
Azcarate-Peril, M. A., Ritter, A. J., Savaiano, D., Monteagudo-Mera, A., Anderson, C., Magness, S., & Klaenhammer, T. R. (2017). Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proceedings of the National Academy of Sciences, 114(3), E367–E375. https://doi.org/10.1073/pnas.1606722113
Gheytanchi, E., Heshmati, F., Shargh, B. K., Nowroozi, J., & Movahedzadeh, F. (2010). Study on b-galactosidase enzyme produced by isolated lactobacilli from milk and cheese. African Journal of Microbiology Research, 4, 454–458.
Meira, S. M. M., Helfer, V. E., Velho, R. V., Lopes, F. C., & Brandelli, A. (2012). Probiotic potential of Lactobacillus spp. isolated from Brazilian regional ovine cheese. The Journal of Dairy Research, 79(01), 119–127. https://doi.org/10.1017/S0022029911000884
Belicová, A., Mikulášová, M., & Dušinský, R. (2013). Probiotic potential and safety properties of Lactobacillus plantarum from Slovakbryndza cheese. BioMed Research International, 2013, 760298.
Fabian, E., & Elmadfa, I. (2006). Influence of daily consumption of probiotic and conventional yoghurt on the plasma lipid profile in young healthy women. Annals of Nutrition & Metabolism, 50(4), 387–393. https://doi.org/10.1159/000094304
Homayouni, A., Payahoo, L., & Azizi, A. (2012). Effects of probiotics on lipid profile: A review. American Journal of Food Technology, 7, 251–265.
Guan, X., Xu, Q., Zheng, Y., Qian, L., & Lin, B. (2017). Screening and characterization of lactic acid bacterial strains that produce fermented milk and reduce cholesterol levels. Brazilian Journal of Microbiology, 48(4), 730–739. https://doi.org/10.1016/j.bjm.2017.02.011
Tomaro-Duchesneau, C., Jones, M. L., Shah, D., Jain, P., Saha, S., & Prakash, S. (2014). Cholesterol assimilation by Lactobacillus probiotic bacteria: an in vitro investigation. BioMed Research International, 2014, 9.
Liong, M. T., & Shah, N. P. (2006). Effects of a Lactobacillus casei synbiotic on serum lipoprotein, intestinal microflora, and organic acids in rats. Journal of Dairy Science, 89(5), 1390–1399. https://doi.org/10.3168/jds.S0022-0302(06)72207-X
Liong, M. T., & Shah, N. P. (2005). Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. Journal of Dairy Science, 88(1), 55–66. https://doi.org/10.3168/jds.S0022-0302(05)72662-X
Lye, H. S., Rusul, G., & Liong, M. T. (2010). Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. International Dairy Journal, 20(3), 169–175. https://doi.org/10.1016/j.idairyj.2009.10.003
Lye, H. S., Rusul, G., & Liong, M. T. (2010). Removal of cholesterol by lactobacilli via incorporation of and conversion to coprostanol. Journal of Dairy Science, 93(4), 1383–1392. https://doi.org/10.3168/jds.2009-2574
de Preter, V., Vanhoutte, T., Huys, G., Swings, J., de Vuyst, L., Rutgeerts, P., & Verbeke, K. (2007). Effects of Lactobacillus casei Shirota, Bifidobacterium breve, and oligofructose-enriched inulin on colonic nitrogen-protein metabolism in healthy humans. American Journal of Physiology. Gastrointestinal and Liver Physiology, 292, 358–368.
Lambert, J. M., Bongers, R. S., de Vos, W. M., & Kleerebezem, M. (2008). Functional analysis of four bile salt hydrolase and penicillin acylase family members in Lactobacillus plantarum WCFS1. Applied and Environmental Microbiology, 74(15), 4719–4726. https://doi.org/10.1128/AEM.00137-08
Pereira, D. I., McCartney, A. L., & Gibson, G. R. (2003). An in vitro study of the probiotic potential of a bile salt hydrolyzing Lactobacillus fermentum strain, and determination of its cholesterol-lowering properties. Applied and Environmental Microbiology, 69(8), 4743–4752. https://doi.org/10.1128/AEM.69.8.4743-4752.2003
Gilliland, S. E., Nelson, C. R., & Maxwell, C. (1985). Assimilation of cholesterol by Lactobacillus acidophilus. Applied and Environmental Microbiology, 49(2), 377–381.
Liong, M. T., & Shah, N. P. (2005). Bile salt deconjugation ability, bile salt hydrolase activity and cholesterol co-precipitation ability of lactobacilli strains. International Dairy Journal, 15(4), 391–398. https://doi.org/10.1016/j.idairyj.2004.08.007
Kuda, T., Yazaki, T., Ono, M., Takahashi, H., & Kimura, B. (2013). In vitro cholesterol-lowering properties of Lactobacillus plantarum AN6 isolated from aji-narezushi. Letters in Applied Microbiology, 57(3), 187–192. https://doi.org/10.1111/lam.12094
Miremadi, F., Ayyash, M., Sherkat, F., & Stojanovska, L. (2014). Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic lactobacilli and bifidobacteria. Journal of Functional Foods, 9, 295–305. https://doi.org/10.1016/j.jff.2014.05.002
Klaver, F. A., & van der Meer, R. (1993). The assumed assimilation of cholesterol by lactobacilli and Bifidobacterium bifidum is due to their bile salt-deconjugating activity. Applied and Environmental Microbiology, 59(4), 1120–1124.
Brashears, M. M., Gilliland, S. E., & Buck, L. M. (1998). Bile salt deconjugation and cholesterol removal from media by Lactobacillus casei. Journal of Dairy Science, 81(8), 2103–2110. https://doi.org/10.3168/jds.S0022-0302(98)75785-6
Adeyemo, S. M., & Onilude, A. A. (2014). Reduction of oligosaccharide content of soybeans by the action of L. plantarum isolated from fermented cereals. African Journal of Biotechnology, 13(37), 3790–3796. https://doi.org/10.5897/AJB2013.13398
Yang, D., Tian, G., Du, F., Zhao, Y., Zhao, L., Wang, H., & Ng, T. B. (2015). A fungal alpha-galactosidase from Pseudobalsamia microspora capable of degrading raffinose family oligosaccharides. Applied Biochemistry and Biotechnology, 176(8), 2157–2169. https://doi.org/10.1007/s12010-015-1705-0
Kidd, M. T., Morgan Jr., G. W., Zumwalt, C. D., Price, C. J., Welch, P. A., Brinkhaus, F. L., & Fontana, E. A. (2001). α-Galactosidase enzyme supplementation to corn and soybean meal broiler diets. Journal of Applied Poultry Research, 10(2), 186–193. https://doi.org/10.1093/japr/10.2.186
Acknowledgements
The authors thank the Department of Biotechnology, New Delhi, India, for research support to Prof. Tamishraha Bagchi (grant number BT/PR14954/FNS/20/496/2010).
Funding
Hemanti Mandal is supported by a fellowship from the Department of Biotechnology, New Delhi, India (DBT-JRF/2012-13/146).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mandal, H., Bagchi, T. In Vitro Screening of Indigenous Lactobacillus Isolates for Selecting Organisms with Better Health-Promoting Attributes. Appl Biochem Biotechnol 185, 1060–1074 (2018). https://doi.org/10.1007/s12010-018-2709-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2709-3