Abstract
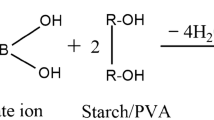
Wood adhesives with enhanced properties were prepared by incorporating either citric or boric acid. The citric acid and boric acid were added in the presence of water being the polymers and acids readily soluble in water. The reaction was carried out at a relatively lower temperature in order to prevent water evaporation and damage to the starch structure. Two series of adhesives were prepared with varying starch and polyvinyl acetate content. The adhesives were characterized by Fourier-transform infrared spectroscopy and dynamic mechanical analysis. The effects of citric and boric acid on performance properties like wet tack, tensile shear strength, rheology and viscosity stability were studied. The results showed that by addition of acids there is an increase in mechanical strength, viscosity and wet tack in the case of boric acid. Similarly, better viscosity stability was observed due to addition of citric acid. From the tests conducted, it can be concluded that the composition for optimum level performance and stability is at 0.3 wt% of citric acid and 0.1 wt% of boric acid of the total composition of adhesive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Through the years, polyvinyl alcohol (PVAl) has been used for various applications. It is very low cost and has been used for wood binding (Gu et al. 2013; Prosanov et al. 2018). It has relatively low glass transition which allows it to function as an adhesive. Due to its highly polar hydroxyl group, it also absorbs water readily and is water soluble, making it compatible with various types of polymers, such as starch (Wu et al. 2017; Moulay 2015). The PVAl adhesive solidifies by evaporating water and forming high-strength H-bonding.
Starch is one of the most readily available biopolymers. Its structure is rich in polar hydroxyl groups, which makes it highly compatible with PVAl (Wang et al. 2017). The starch mainly consists of amylose and amylopectin (Gadhave et al. 2017). Starch when heated in the presence of water undergoes gelatinization (Chen et al. 2017).
Cross-linking of hydroxyl groups can lead to better stability and adhesion properties in adhesives. For water-soluble polymers such as PVAl or starch, the use of citric and boric acid is a promising system for the modification of their structure, because both acids are easily available showing simple reaction mechanism (Reddy and Yang 2010; Widyorini et al. 2016; Liao et al. 2016; Umemura et al. 2012). Additionally, the use of citric acid has led to a decrease in browning over time of adhesives (Menzel et al. 2013; Li et al. 2015; Amirou et al. 2017). Similarly, the boric acid forms strong coordination bonds with hydroxyl groups due to the presence of vacant d-orbital in boron, which causes it to rapidly react with various nucleophiles to form complexes (Imam et al. 1999). Since PVAl and starch are rich in hydroxyl groups, boric acid can be expected to bond with them and further improve the adhesion.
There have been various studies on adhesives focused on improving the tack of the water-based adhesives. Some studies have also used alternative biopolymer to starch such as cellulose and lignin (Sanjiv Kasbe et al. 2017; Gadhave et al. 2017). But none focuses on the development of non-elastomeric tackifiers for PVAl and polyvinyl acetate (PVAc). In this study, the effect of boric and citric acid on PVAl and starch has been studied. By addition of these acids, the corresponding change in wet tack and tensile strength has been evaluated. The effect of acid addition has been confirmed by various analytical methods such as FTIR, rheology and DMA. The study aims to increase the wet tack, tensile strength and stability of the adhesive.
Materials
PVAl (solid, degree of hydrolysis of 86.5–89%, M.W. 99,000 to 105,000) was obtained from Kuraray Co. Ltd. India. Starch (corn starch amylose content 25–30%) was obtained from Sanstar Bio-polymer Ltd. These raw materials were kept in a dry environment to avoid the absorption of moisture from the environment. PVAc, di-butyl phthalate (DBP), formaldehyde, boric acid and citric acid were obtained from Sigma-Aldrich (Tables 1, 2).
Preparation method
Corn starch and PVAl were first put into a round-bottom reactor vessel. The water was added to them, and the mixture was kept for continuous stirring at 175 rpm. The temperature was slowly raised up to 60 °C after which the acids (citric acid or boric acid) were added and the final temperature was kept at 92–95 °C. The reaction was carried out for 2.5 h. Then, the solution was cooled down to 25 °C. Now, the remaining components containing PVAc, plasticizer (DBP) and preservative (formaldehyde) were added to the solution. The formaldehyde was chosen for its better antibacterial and preservative properties at lower concentrations. The new formulated solution was kept for continuous stirring at 175 rpm for 1.5 h. This final product was then filtered out to remove foreign particles in PVAc emulsion using a mesh and stored in plastic containers.
Two different types of adhesive series WG-1 and WG-2 containing different proportions of starch–PVAl were investigated. This was done to estimate the optimum proportion and observe the dependence of the starch quantity. Although the PVAc content was also varied to adjust the changes made in the starch–PVAl composition. Since the acids are added at the reaction stage and PVAc is added while formulation, it is assumed that the changes in properties have been caused by the addition of acids.
Characterization and testing
Fourier-transform infrared spectroscopy (FTIR)
PerkinElmer FTIR spectrometer was used to record 100 spectra. The sample films of about 200 microns were analysed by the reflectance process. The background spectra were run first and then eliminated from the obtained results of each sample.
Viscosity
The viscosities were measured by Brookfield DV1 Viscometer at 28 °C temperature and 20 rpm.
Tensile strength
Tensile strength was checked with the help of UTM Tinius Olsen H25KT, Mumbai, India. A constant amount of adhesive was applied on to 50 mm × 50 mm area of one end of the plywood (ply) and laminate (lam) wood (Fig. 1a). The adhesives were applied onto 25 mm× 25 mm area of one end of the canarium (can) wood pieces (Fig. 1b) in such a way that it properly wets the surface. The adhesive coated wood pieces were assembled in such a way that the grains of two pieces were oriented in parallel. Each end of the sample was held by grips (Fig. 1c) (total load − 5 kg cylinder + 2.5 kg arm) and pulled apart at a controlled rate (5 mm/min) and tensile strength for adhesive bond was recorded (temperature 28–30 °C, %relative humidity 65–70). To study the tensile strength development over time, the values were calculated at various time intervals of 2, 4, 6 and 24 h. For each composition, five samples were tested and standard deviation was reported.
Rheology
Rheology was checked with the help of DHA-2 Rheometer from TA Instruments, Mumbai, India. A small amount of adhesive was placed between the metallic discs of the rheometer. The discs were then closed, and the shear rate was increased from 0 to 100 s−1. The viscosity at each point was recorded, and the graph of viscosity versus shear rate was plotted. After reaching 100 s−1 shear rate, it was again reduced to 0 s−1. The graph shows the relationship between the initial zero shear rate viscosity and final zero shear rate viscosity. Three samples were analysed for each adhesive composition, and the best graph was reported.
Dynamic mechanical analysis (DMA)
DMA was performed using DMA Q800 from TA Instruments, Mumbai, India. A thin film of 200 microns was first prepared by applying it on a PTFE sheet. The film was kept for physical solidification at room temperature for 24 h. The film was peeled off from the PTFE sheet and kept in the DMA sample holder. The temperature range was from − 30 °C to 150 °C, with a ramp rate of 5 °C/min, frequency 1 Hz and strain 0.1%. Three samples were analysed for each adhesive composition.
Wet tack
Wet tack was calculated using probe tack analyser from Rohit Instruments, Mumbai, India. Few grams of the adhesive was firstly placed on the metal surface of the machine. It was taken care that the sample was still wet and no drying took place. A metal cylinder then approached the sample downward at the speed of 5 mm/min. By the subsequent movement, the adhesive is squeezed between the cylinder and the metal surface. The cylinder touches the metal surface and again lifts up at the speed of 5 mm/min. The adhesive resists the upward movement of the cylinder, and thus the force of resistance was calculated and termed as wet tack.
Stability test
Viscosities were measured by Brookfield DV1 Viscometer after keeping the samples in an incubator for 3 h at 30 °C. Change in viscosity after keeping the samples at 50 °C for 15 days and 30 days was recorded to study the viscosity stability.
Results and discussion
The results obtained by various tests are given in Table 3 (WG-1 series) and Table 4 (WG-2 series).
FTIR
Since the components responsible for changes in properties of the WG-1 and WG-2 are similar, the tests conducted for just one of WG are sufficient for establishing the desired results.
The characteristic peaks of –OH bonds present in both PVAl and starch are observed at 3340 cm-1(Fig. 2 ). A strong sharp peak is observed at 1730 cm-1 due to the presence of carbonyl (C=O) group of PVAc (Fig. 2). In the case of boric acid, there is considerable overlapping of curves due to the tertiary alcohols of boric acid and secondary alcohols of PVAl/starch.
Viscosity
Due to the addition of boric and citric acid, it was expected that the formation of coordination complex and ester linkage would happen, respectively (Kusumah et al. 2016). But the findings were interesting: as the citric acid content increased, a decrease in viscosity was observed at all compositions (Fig. 3a, c). This was attributed to the function of citric acid as the plasticizer, since the citric acid forms ester linkages at temperatures well above 95–97 °C (Reddy and Yang 2010; Jiugao et al. 2005). In this study, the citric acid is unable to function as a cross-linker, so it acts as a plasticizer by being present between the chains and making them move easily. This explains the decrease in viscosity as the amount of citric acid is increased.
The cross-linking reaction between WG 1 with boric acid caused an increase in the viscosity of the adhesive (Fig. 3c). The similar trend was observed for WG 2 with boric acid (Fig. 3d). The boric acid reacts with the PVAl and forms a coordination complex (Awada et al. 2014; Choi et al. 2017). The complex makes it harder for chains to move, which causes the viscosity to increase (Fig. 3b, d). The closer packing of the chains due to complex formation has led to greater stiffness and modulus (as observed in DMA studies in the following section); chain entanglement is one of the reasons for an increase in modulus which proves the gradual increase in viscosity.
Wet tack
Wet tack force provides the information about the internal cohesion in an adhesive. It shows the similar trend as observed in the case of viscosity. Addition of citric acid has led to a decrease in wet tack (Fig. 4a, c). The presence of citric acid which acts as a plasticizer has weakened the cohesion of the adhesive. In the case of boric acid, the coordination complex helps in packing of chains together, thereby increasing the wet tack (Fig. 4b, d).
Tensile strength (ply to lam)
The plywood to laminate tensile strength test has been conducted to study the effect on hardwood (plywood) to softwood (laminate) assemblies. While applying the adhesive, the plywood did not wet well enough as compared to laminate. Since the laminate has many pores on its surface, the wetting was easy in this case. The tensile strength shows the similar trend to that of the wet tack, since the presence of citric acid in the polymer chains has led to an increase in the plasticizing effect. The presence of these molecules in the adhesive has led to a decrease in the tensile strength (Fig. 5a, c). The weak cohesion between chains and a decrease in surface area in contact with the wood substrate due to citric acid has played a significant role in the weakening of the adhesive.
The boric acid consequently has formed a strong complex with the starch and PVAl hydroxyl groups, which have resulted in the increase in tensile strength (Fig. 5b, d) (Gadhave et al. 2018). The tensile strength values at different time intervals help in understanding the drying time of the adhesive. For 2 and 4 h, the tensile strength values are too low and the assemblies have not developed enough strength, so care has to be taken while handling these wood assemblies. Consequently, after 6 h, the wood assemblies developed enough strength in all adhesive compositions.
Tensile strength (can to can)
The additional can-to-can wood tensile strength study was done to observe the hardwood behaviour of the adhesives. In this case, both the wood surfaces bonded are of the same hardwood (canarium wood). The trends of the tensile strength of can-to-can are similar to that of ply-to-lam tests, which is a decrease due to citric acid addition and an increase due to boric acid addition. A major difference is observed while comparing their values. The tensile strength of can to can (of WG 1, after 24 h, at 0% of boric and citric acid) was found to be 27 and that of ply to lam (of WG 1, after 24 h, at 0% of boric and citric acid) was 16 (Fig. 6). This huge difference is observed due to the difference in the wood substrate as stated earlier. The hardwood to hardwood (can to can) is strongly bonded as compared to the hardwood to softwood (ply to lam).
Stability
The incorporation of citric acid caused a decrease in the viscosity of the adhesive as shown in Fig. 7a, c. The viscosity build-up over time is observed in all the concentration of citric acid; this is mainly due to the increase in secondary forces. Over time, the internal movement of chains causes the polar hydroxyl groups to achieve better orientation. This orientation has low energy state, and thus the low energy configuration is achieved through increased H-bonding. The increase in H-bonding has led to the increase in viscosity over time. An important point to note is that, due to an increase in cross-linking the viscosity is decreased. So, addition of optimal amount of citric acid can help to overcome the problem of viscosity build-up over time. As shown in Fig. 7a, c, the viscosity curve is converging towards 1% concentration. The citric acid even shows almost equal zero shear viscosity (as confirmed by rheology study in the following section), which implies the stability imparted due to cross-linking with citric acid.
The cross-linking reaction between WG 1 with boric acid caused an increase in the viscosity of the glue (shown in Fig. 7b). The similar stability trend was observed for WG 2 series cross-linked with boric acid (shown in Fig. 7d). The viscosity build-up over time is observed for the WG 1; this is mainly due to the increase in secondary forces over time. Due to an increase in cross-linker percentage, the viscosity build-up is increased. However, at 0.1% of boric acid there is the least change in viscosity after 15 and 30 days for both the adhesives (Fig. 7b, d).
Rheology
The rheology measures the viscosity of a compound at varying shear rate, with the so-called zero shear viscosity as the viscosity extrapolated for zero shear rates.
In the case of the WG-1, a difference in the zero shear viscosities is given, indicating instability (Fig. 8a). The zero shear viscosities of WG-13 (with addition of citric acid) before and after the shear rate cycle are almost identical. This phenomenon can be attributed to the plasticizing mechanism of citric acid with the starch/PVAl, leading to a certain stabilization effect (Fig. 8b).
In the case of boric acid in WG-16, the stabilization effect is very low. The applied shear rate has damaged the existing H-bonding which was developed upon complex formation. The chains move far apart beyond recovery as the shear rate is applied. As a consequence, the major drop in the viscosity is observed (Fig. 8c).
Dynamic mechanical analysis
Higher storage modulus value in rubbery region and shift of tan delta second peak to the higher temperature with decreased peak height and peak broadening indicate the formation of the cross-linked network in boric acid-incorporated systems more profoundly than citric acid-incorporated systems (Fig. 9a).
The tan delta curves show two major peaks in each series: one near 50 °C (first tan delta peak) which corresponds to PVAc glass transition; the other near 90 °C (second tan delta peak) associated with PVAl glass transition (Fig. 9b). The first tan delta peak is identical in each adhesive composition, since the PVAc undergoes no such modification. The films of citric acid modification show minor changes in the storage modulus and tan delta curves, confirming the hypothesis of citric acid acting as a plasticizer. The second tan delta peak shows a major change by an increase in area under the curve in the case of boric acid and also the peak temperature shift (Fig. 9b). The mechanism of complex formation by boric acid with hydroxyl groups is shown in Fig. 10. These changes confirm an increase in glass transition temperature of the boric acid-modified adhesive. The observations provide conclusive evidence to the increase in viscosity, tensile strength and wet tack as discussed in the previous sections.
Conclusion
The purpose of this study was to achieve better handling strength, tack development and better stability over the existing white glues. The approach employed to achieve the above properties was the addition of citric and boric acid into the PVAl/starch system. By increasing the content of boric acid, it was found that the wet tack and tensile strength increased. Similarly, the addition of citric acid imparted better viscosity stability and rheological properties. The maximum tensile strength and wet tack were observed at 0.1 wt% of citric acid and 1 wt% of boric acid. While the citric acid showed better stability, the better tensile strength and wet tack were achieved by boric acid.
References
Amirou S, Pizzi A, Delmotte L (2017) Citric acid as waterproofing additive in butt joints linear wood welding. Eur J Wood Wood Prod 75:651–654. https://doi.org/10.1007/s00107-017-1167-x
Awada H, Montplaisir D, Daneault C (2014) The development of a composite based on cellulose fibres and polyvinyl alcohol in the presence of boric acid. BioResources 9:3439–3448. https://doi.org/10.15376/biores.9.2.3439-3448
Chen L, Wang Y, Zia-ud-Din, Fei P, Jin W, Xiong H et al (2017) Enhancing the performance of starch-based wood adhesive by silane coupling agent(KH570). Int J Biol Macromol 104:137–144. https://doi.org/10.1016/j.ijbiomac.2017.05.182
Choi B-K, Park S-J, Seo M-K (2017) Effect of graphene oxide on thermal, optical, and gas permeability of graphene oxide/poly(vinyl alcohol) hybrid films using the boric acid. J Nanosci Nanotechnol 17:7368–7372. https://doi.org/10.1166/jnn.2017.14784
Gadhave RV, Mahanwar PA, Gadekar PT (2017a) Starch-based adhesives for wood/wood composite bonding: review. Open J Polym Chem 07:19–32. https://doi.org/10.4236/ojpchem.2017.72002
Gadhave RV, Mahanwar PA, Gadekar PT (2017b) Bio-renewable sources for synthesis of eco-friendly polyurethane adhesives—review. Open J Polym Chem 07:57–75. https://doi.org/10.4236/ojpchem.2017.74005
Gadhave RV, Kasbe PS, Mahanwar PA, Gadekar PT (2018) To study the effect of boric acid modification on starch–polyvinyl alcohol blend wood adhesive. J Indian Acad Wood Sci 15:190–198. https://doi.org/10.1007/s13196-018-0225-2
Gu R, Mu B, Guo K (2013) Structural characterization of Konjak powder-chitosan-PVA blending adhesive. Nongye Gongcheng Xuebao/Trans Chin Soc Agric Eng 29:277–283. https://doi.org/10.3969/j.issn.1002-6819.2013.07.035
Imam SH, Mao L, Chen L, Greene RV (1999) Wood adhesive from crosslinked poly(vinyl alcohol) and partially gelatinized starch: preparation and properties. Starch - Stärke. https://doi.org/10.1002/(sici)1521-379x(199906)51:6<225::aid-star225>3.0.co;2-f
Jiugao Y, Ning W, Xiaofei M (2005) The effects of citric acid on the properties of thermoplastic starch plasticized by glycerol. Starch - Stärke 57:494–504. https://doi.org/10.1002/star.200500423
Kusumah SS, Umemura K, Yoshioka K, Miyafuji H, Kanayama K (2016) Utilization of sweet sorghum bagasse and citric acid for manufacturing of particleboard I: effects of pre-drying treatment and citric acid content on the board properties. Ind Crops Prod 84:34–42. https://doi.org/10.1016/j.indcrop.2016.01.042
Li TX, Li K, Wang YM, Su HB, Wang Q, Cui HZ (2015) Effects of gelatinization characteristics on starch-based superabsorbent polymer. Mater Res Innov 19:817–821. https://doi.org/10.1179/1432891714Z.0000000001199
Liao R, Xu J, Umemura K (2016) Low density sugarcane bagasse particleboard bonded with citric acid and sucrose: effect of board density and additive content. BioResources. https://doi.org/10.15376/biores.11.1.2174-2185
Menzel C, Olsson E, Plivelic TS, Andersson R, Johansson C, Kuktaite R et al (2013) Molecular structure of citric acid cross-linked starch films. Carbohydr Polym 96:270–276. https://doi.org/10.1016/j.carbpol.2013.03.044
Moulay S (2015) Review: poly(vinyl alcohol) Functionalizations and Applications. Polym—Plast Technol Eng 54:1289–1319. https://doi.org/10.1080/03602559.2015.1021487
Prosanov IY, Abdulrahman ST, Thomas S, Bulina NV, Gerasimov KB (2018) Complex of polyvinyl alcohol with boric acid: structure and use. Mater Today Commun 14:77–81. https://doi.org/10.1016/j.mtcomm.2017.12.012
Reddy N, Yang Y (2010) Citric acid cross-linking of starch films. Food Chem 118:702–711. https://doi.org/10.1016/j.foodchem.2009.05.050
Sanjiv Kasbe P, Kumar N, Manik G (2017) A molecular simulation analysis of influence of lignosulphonate addition on properties of modified 2-ethyl hexyl acrylate/methyl methacrylate/acrylic acid based pressure sensitive adhesive. Int J Adhes Adhes 78:45–54. https://doi.org/10.1016/j.ijadhadh.2017.06.014
Umemura K, Ueda T, Munawar SS, Kawai S (2012) Application of citric acid as natural adhesive for wood. J Appl Polym Sci 123:1991–1996. https://doi.org/10.1002/app.34708
Wang Y, Xiong H, Wang Z, Zia-ud-Din, Chen L (2017) Effects of different durations of acid hydrolysis on the properties of starch-based wood adhesive. Int J Biol Macromol 103:819–828. https://doi.org/10.1016/j.ijbiomac.2017.05.102
Widyorini R, Nugraha PA, Rahman MZA, Prayitno TA (2016) Bonding ability of a new adhesive composed of citric acid-sucrose for particleboard. BioResources. https://doi.org/10.15376/biores.11.2.4526-4535
Wu Z, Wu J, Peng T, Li Y, Lin D, Xing B et al (2017) Preparation and application of starch/polyvinyl alcohol/citric acid ternary blend antimicrobial functional food packaging films. Polymers (Basel). https://doi.org/10.3390/polym9030102
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gadhave, R.V., Mahanwar, P.A., Gadekar, P.T. et al. A study on the effect of starch–polyvinyl alcohol blends by addition of citric acid and boric acid for enhancement in performance properties of polyvinyl acetate-based wood adhesive. J Indian Acad Wood Sci 17, 9–20 (2020). https://doi.org/10.1007/s13196-019-00249-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13196-019-00249-6