Abstract
In this study, the crosslinking agent citric acid (CA) is added to liquid glucose (LG)-polyvinyl alcohol (PVA) blend-based wood adhesives to improve their mechanical and thermal properties. The effect of crosslinking has been investigated using various analytical techniques, including viscosity, water contact angle, differential scanning calorimetry (DSC), and Fourier transform infrared spectroscopy (FTIR). Several variables were investigated for their effects on performance characteristics, including glass transition temperature, pencil hardness, and tensile strength. Increasing the amount of CA and the content of LG were among these factors. The crosslinked blend-based films increased in pencil hardness value and showed less hydrophilic behavior in the water contact angle test. It is expected that nucleophilic hydroxyl groups in LG and PVA will react with free carboxylic groups to form crosslinks. By analysing FTIR spectra, it has been demonstrated that CA and the LG-PVA mixture interact. After 24 h of bonding for two distinct ratios of PVA to LG (i.e., 25:5 and 20:10), the lap shear bond strength for the wood substrates bonded with the modified blend increased by 250% and 263% for 0.3 wt% CA, respectively. Blends with 0.5 and 0.7 weight% CA showed a predominately plasticizing tendency because CA that has not been reacted acts as an external plasticizer. The glass transition temperature (Tg) and increase in viscosity were indicators of the reaction’s consequences due to the acidic pH. The newly developed crosslinked LG-PVA blend-based wood adhesive showed better mechanical and water resistance properties when compared to conventional LG-PVA blend-based wood adhesives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, there has been an increasing demand for developing adhesives from natural and bio-based raw materials (Xie et al. 2021). The exploitation of bio-based adhesives has received increasing attention (Pizzi and Biofoams-a Review 2019; Pizzi 2017; Kaboorani et al. 2012; Gouveia et al. 2018). Therefore, developing an eco-friendly wood adhesive composed of renewable materials has become a key topic of wood science (Pizzi 2014; Gadhave and Vineeth 2022; Zhong et al. 2002; Cheng et al. 2013). Bio-based adhesives derived from natural resources, such as cellulose, hemicellulose, lignin, starch, tannin, chitosan, protein, and citric acid (CA), have been promoted as wood adhesives. The latter has been the cheapest bio-based adhesive with remarkable adhesion properties (Sutiawan et al. 2022).
Polyvinyl alcohol (PVA) is one of the most critical commercial water-soluble polymers. It possesses a high crystalline structure (Othman and Azahari 2011). It is prepared by partial or complete hydrolysis of polyvinyl acetate. Physical and chemical properties, including water solubility of PVA, depend on the synthesis conditions and degree of hydrolysis (Klenina et al. 1972; Gadhave et al. 2019; Gadhave 2023a, b). The approach to enhance the adhesion properties of PVA involves chemical and physical modifications. The incorporation of functional fillers, blending with other polymers, and tailoring the chemistry of PVA are the possible modifications reported (Kaboorani and Riedl 2011).
Sucrose (β-d-fructofuranosyl α-d-glucopyranoside) is a natural disaccharide that is produced from sugar beet or sugarcane, and its chemistry has attracted considerable interest (Queneau et al. 2007). Therefore, considering the chemical properties of sucrose and the reactivity of CA, there is a possibility of synthesizing a suitable eco-friendly adhesive for plywood. Glucose is a natural compound that is continuously generated by photosynthesis in plants. The introduction of Glucose to wood does not change the chemical elements of the wood (Guo et al. 2019). Previous research has already demonstrated that CA could be utilized as a green adhesive of wood-based materials [18–19]. CA water solution (Huaxu et al. 2020) was sprayed on wood particles. After or without prior drying treatment, the sprayed particles were hot-pressed at 180–200 °C to fabricate particleboards. The mechanical properties and water resistance of the resulting particleboards satisfied the JIS A 5908 standard, and the reaction mechanism was the formation of carbonyl groups between CA and wood cellulose (Umemura et al. 2013). Furthermore, sucrose was added to the CA solution to promote bond performance. However, although the previous CA-sucrose adhesive could be used for the manufacture of particleboard, it is rarely applied to plywood production due to its low viscosity and low solid content.
Citric acid (2-hydroxy-1,2,3-propanetricarboxylic acid) is an organic polycarboxylic acid containing three carboxyl groups. CA is a low-cost and readily available chemical that is used in the food, beverage, and cosmetics industries, as well as in the textile and paper industries, to improve wrinkle resistance and wet strength (Doll et al. 2006). CA has also been investigated as a cross-linking agent for wood, plant fiber, paper, starch, and biobased elastomers. Recently, it was found that CA has adhesiveness for wood (Reddy and Yang 2010).
It was revealed that increasing the molar ratio of CA/glucose (G) is more advantageous to improve the shear strength and water resistance of plywood than the reaction time. The dry and wet strengths of plywood bonded by the fully biomass-based CA-G adhesive can reach the standard requirement (≥ 0.7 MPa) when the molar ratios of CA-G were more than 0.6 and the reaction time was 1 h (Gadhave 2023a, b). Wibowo et al.22 employed zinc dichloride (ZnCl2) as a catalyst to improve the curing conditions and optimize the pressing conditions of the CA-G adhesive. As a result, ZnCl2 efficiently decreased the curing temperature of the adhesive (Genest et al. 2013). A novel eco-friendly adhesive was synthesized by combining G and CA under heating conditions for incorporation into plywood. The preliminary research result of this study shows that G and CA adhesive could be synthesized and utilized as an adhesive for plywood (Menezzi et al. 2018). Therefore, the incorporation of CA as a green additive can be a potential tool for preparing a cost-effective adhesive with enhanced physicochemical, thermomechanical, and performance properties for PVA/ microcrystalline cellulose (MCC) blend as a possible and sustainable water-based wood adhesive (Vineeth et al. 2023).
The purpose of the current work was to chemically modify LG-PVA blends with CA to enhance the performance properties of wood adhesives. The reaction structure and the thermal properties of polymer films were studied using Fourier-transform infrared (FTIR) and differential scanning calorimetry analysis (DSC). With the addition of CA to the LG-PVA blend-based adhesive, the DSC results demonstrated a significant rise in Tg, which was also confirmed by film hardness testing. In comparison with conventional LG-PVA blend-based wood adhesives, the newly developed cross-linked LG-PVA blends-based wood adhesive demonstrated better water resistance and mechanical properties.
Experimental
Materials
Cold water-soluble polyvinyl alcohol (CWS PVA) (CAS No. 9002-89-5, % degree of hydrolysis 87–89%, Molecular weight 40,000–60,000 g/mol) was obtained from Kuraray Cooperative Limited, India. Citric acid (CA, CAS No 77-92-9), liquid glucose (LG, pH (1% Solution) 3.5–5.5, % Solid content 83.5–85.5%, CAS No. 8027-56-3) were purchased from Sigma Aldrich. These raw materials were kept in a dry environment to avoid the absorption of moisture. For the whole study, deionized water (DIW) (pH – 6 to 7, total dissolved solids – 0), which was prepared and analyzed, was utilized.
Method of preparation
Liquid glucose/ PVA blends (LG/PVA) were taken in a round bottom flask with water. Initially, the temperature was kept at 60 °C with continuous overhead stirring at 170 rpm. The given amount of CA was added after 30 min, as per Table 1. The temperature of the solution was raised to 93–96 °C and kept under stirring for 2 h. Then, the solution was cooled down to 30 °C and poured into a glass container for additional research.
Group 1
Gluco-PVA-CA-11, Gluco-PVA-CA-12, Gluco-PVA-CA-13, and Gluco-PVA STD-01 were made in this group by altering the amount of citric acid from 0.3, 0.5, 0.7, and 0, respectively, as indicated in Table 1.
Group 2
As shown in Table 1, a Glucose/PVA (20:10) blend was made in this group by adjusting the amount of citric acid from 0.3, 0.5, 0.7, and 0. respectively. The results are Gluco-PVA-CA-21, Gluco-PVA-CA-22, Gluco-PVA-CA-23, and Gluco-PVA STD-02.
Group 3
This group combined varying amounts of glucose and PVA with water to make a glucose/PVA blend. For all studies, the concentration of citric acid was held constant at 0.3%, as shown in Table 1.
Film casting on teflon
Each sample was coated into a film on a Teflon sheet using an applicator with a thickness of 500 μm. The cast film was then dried for a whole day at 30 °C. The cured film was used for testing purposes. Each made sample was coated into a film on a Teflon sheet. At 30 °C, the cast film had been dried for a whole day. For testing, the cured film was used.
Characterization and testing
Viscosities of the blends
The viscosities of the adhesive samples were measured using a Brookfield Viscometer RVT. The measurements were taken at a speed of 20 rpm at 30 °C. Five measurements were taken in order to reduce error.
pH of blends
A digital pH meter was used to measure the pH of the samples. The ASTM E70 standard was followed in conducting the pH test. pH measurements were taken five times in order to minimize error.
Fourier transform infra-red spectroscopy (FTIR)
Functional groups in hybrid samples were determined by IR Spectroscopy analysis using a Perkin Elmer Spectrum Express 100 spectrometer. Analysis was conducted using the ATR-FTIR method. On a Teflon sheet, a sample film was created and cured for one hour at 105˚C. For scanning, the dried film was put on a diamond crystal. 400 to 4000 cm-1 of spectrum were captured. Prior to analysis, a background scan was performed to minimize error during the reflectance technique test.
Differential scanning calorimetry (DSC)
The samples’ glass transition temperatures (Tg) were examined using DSC analysis. The test was executed with a Perkin Elmer instrument Q 100. Sample preparation involves casting adhesive over a Teflon sheet with a 200 μm applicator and drying at 105 °C for 1 h. The dried samples of about 5 mg were used directly on DSC analysis. The technique was performed from − 40 to 210 °C at a 10 °C min− 1 rate.
Dry-out period on teflon sheet
Using a notched bar film applicator, 200 μm wet films were drawn over a Teflon sheet, and at the same time, a stopwatch was started. For recording the drying times, the time from the casting step to complete drying was recorded using a stopwatch. This test was carried out at 30 °C.
Pencil hardness test
The pencil hardness test was done using a QHQ-A portable pencil hardness tester, having lead pencils of varying hardness numbers. Using an applicator, 200 µ films were cast over a glass plate. After the films were dried at 30 ± 2 °C for one day, the test was conducted following the ASTM D 3363 standard (Gadhave and Vineeth 2022).
Water contact angle measurement
The final formulations were coated over a glass slide with a notch spreader of 200 μm. and allowed to dry for one day. The next day, the water contact angle and pencil hardness were studied. A Rame-Hart contact angle goniometer was used to measure the water’s contact angle. The measurement was repeated five times to reduce the error.
Tensile strength (ply to laminate)
With the help of UTM Tinus Olsen H25KT, Mumbai, India, the tensile strength was studied.
Constant amounts of adhesive were applied to one end of the plywood (ply) and laminate (lam) wood, covering a 50 mm by 50 mm area (Fig. 1a). Figure 1b illustrates the pressure applied to the bonded area of the ply to laminate assemblies (total load − 5 kg cylinder + 2.5 kg arm). The sample was pulled apart at a controlled rate of 5 mm/min, and the adhesive bond’s tensile strength was recorded at 29 ± 2 °C and 65 ± 3% relative humidity. In order to examine the tensile strength of a plywood-laminated assembly, measurements were taken after 24 h of clamping. Each adhesive sample was analyzed using five samples, and the standard deviation was provided (Gadhave et al. 2019).
(a) ply to lam assembly (b) ply to lam assembly under load (Gadhave et al. 2019)
Tensile strength-wood to wood in wet and dry condition
Shear strength was evaluated at different intervals in joints prepared by gluing beech specimens in normal climates. The adhesive-coated pieces were assembled in such a way that the grains of the two pieces were oriented in parallel. A universal testing machine (UTM) Tinius Olsen H25KT instrument was employed in the analysis. Bonded assemblies as per EN 205, as shown in Fig. 2, were tested after keeping them at 29 ± 2 °C and 65 ± 3% relative humidity after 24 h and seven days. In this case, strength was calculated in MPa. Wet strength of bonded test specimens as per the standard EN 204–205, and the test was performed by keeping the specimens for seven days in a normal climate; then, the same specimens were soaked in water for an additional 24 h and then tested for water resistance. Figure 3 (a) and 2 (b) show bonded specimens as per EN 204–205 and bonded specimens in water to test the wet strength. In Fig. 3 (c), specimens after testing (bond failure) are shown. Bonding parameters such as the application rate of the adhesive, types of wood, moisture content of the wood, open time, pressing time, temperature, and pressure are described in Table 2.
Results and discussion
All the test data are combined and summarised in order to combine numerous findings from the characterization and analysis. Tables 3, 4, 5, 6 and 7, and 8 show the overall test results for the study. The next section contains thorough discussions of each analysis.
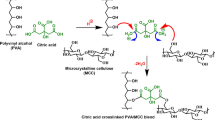
According to reports, CA interacts with hydroxyl groups to form ester bonds (Umemura et al. 2013) (Vineeth et al. 2023). Similar to PVA, LG also has hydroxyl groups that CA’s carboxy groups can readily access to form ester bonds through an esterification mechanism. Crosslinking is the process by which the formation of ester bonds connects different polymer chains. Figure 2 shows the expected mechanism of crosslinking CA with the PVA and LG hydroxyl groups. There have been reports of residual-free, unreacted CA having potential plasticizing properties (Vineeth et al. 2023).
Viscosity
Figures 4 and 5 show that the interaction of LG and PVA with the cross-linker caused the solution’s viscosity to rise. It was expected that the combination of hydroxy compounds and the carboxylic group in CA would result in group 1 esters that were simple to saponify. The pH drops as CA content rises, increasing the sample’s acidity. The esterification of LG and CA resulted in forming a fully cross-linked structure (Jin et al. 2023).
These interactions led to an increase in viscosity. The interaction of LG and PVA with CA directly influences the viscosity of the blend. Group 2 viscosities are lower than group 1 viscosities because water is an esterification process byproduct. However, as seen in Figs. 4 and 5, the tendency for group 2 is comparable to group 1 due to the decreasing order of CA content. As seen in Fig. 6, an increase in LG causes a drop in viscosity after 5% LG is added to the sample in group 3, even when the CA concentration is kept constant.
pH of solution
Figures 7 and 8 illustrate how the pH of solutions decreased as the amount of CA in the solution increased. LG has undergone oxidation when CA was present. Groups 1 and 2 exhibit a similar trend of pH decline, but because LG content varies, there is a minor variance between them, as shown in Fig. 9. Due to a rise in the amount of LG molecules in group 3 that contain hydroxyl groups, the pH remains nearly constant; as a result, the drop in hydroxyl groups has been made up for by LG molecules.
Fourier transform infrared spectroscopy (FTIR)
The CA cross-linked blend peak’s overall width and shape were the same as those of the LG-PVA non-cross-linked sample. This showed that the nature of secondary bond-like -H bonds in the cross-linked blends was identical to that in the non-cross-linked LG-PVA blend, but it also demonstrated that the cross-linking procedure resulted in a novel structure of hydrogen bonds.
According to Table 9 (Figs. 10 and 11), as the cross-linking increased, the peak of -OH for cross-linked LG-PVA blends fell to a lower wave number (for group 1 and group 2).
Differential scanning calorimetry (DSC)
Table 10 shows how the glass transition temperature of the polymer solutions increased as the CA concentration increased. Carboxylic group of CA reacts with LG and PVA to cross-link the two polymers.
The cross-linking increases the molecular weight. This reason causes an increase in the cross-linked films’ glass transition temperature. Polymer chains become inflexible as a result of high molecular weight restrictions on rotation. Due to the higher reactivity of LG’s hydroxyl groups when compared to PVA, more LG causes increased cross-linking-blends containing 0.5 and 0.7 weight%. However, CA demonstrated a primarily plasticizing tendency because unreacted CA acts as an external plasticizer, decreasing Tg. Due to the proximity of the hydroxyl groups in PVA, it is more difficult for cross-linkers to target the site and form bonds. For group 3, a similar pattern is seen.
Drying time on glass plate
A drying time test was conducted on a Teflon sheet at controlled conditions (% RH − 50–55% Temp- 29–31 °C). The drying time for Gluco-PVA-CA-13 and Gluco-PVA-CA-23 was more than that of Gluco-PVA-CA-11 and Gluco-PVA-CA-21. The high tendency of hydrogen bonding in PVA with LG as compared to LG is because water loss from adhesive film gets enhanced, as shown in Tables 3 and 5, and 7. The unreacted -OH group in LG acts as a humectant, which leads to an increase in the dry-out time on the Teflon sheet. Also, unreacted CA acts as an external plasticizer, which reduces water, leaving a tendency to blend samples.
Pencil hardness of film
The flexibility of polymer can be measured by pencil hardness. According to Tables 3 and 5, the hardness of the film increased as the CA content increased. The effect of CA content in the blends increased the hardness. In contrast to the LG, which has bulky rings in its chain, the PVA features flexible chains that are easily bent. The rings stiffen the final LG-based compounds. The same pattern is seen as more CA promotes improved cross-linking, which brings the chains together to form a dense structure. Cross-linking increased slip from a sharp pencil. But as CA concentration increased to 0.3 wt%, the film lowered hardness. As observed from viscosity analysis and DSC, the unreacted CA penetrates between polymeric chains and acts as a plasticizer [32], decreasing the hardness of the film. According to Table 7, the hardness increased as the LG concentration increased up to 0.3%. Cross-linking resulted in an increase in hardness that is proportional to the LG content.
Water contact angle
Generally, the relationship between the water contact angle and an adhesive’s resistance to humidity is linear. The contact angle of the sample film with the water droplet correlates with the hydrophobic or hydrophilic nature of the material. The contact angle of the blends with water is shown in Figs. 12 and 13, and 14, and the values are addressed in Tables 3 and 5, and 7. Since PVA-LG blends are rich in hydroxyl groups, the blank sample showed maximum wetting and lowest contact angle with water. As explained, citric acid acts as a cross-linker by connecting polymeric chains by the esterification reaction with the carboxylic acid group of citric acid with hydroxyl groups of PVA-LG (Shi et al. 2008). Since cross-linking uses hydroxyl groups of the blend, the observation from contact angle measurement justifies the decrease in wetting nature with an increment in the contact angle of cross-linked samples.
In group 1, it has been found that Gluco-PVA-CA-21 is significantly more hydrophobic than Gluco-PVA-CA-23, and a similar finding was achieved in group 2.
This is caused by crosslinking between the pendent side functional group of PVA and LG with CA and the formation of a dense network structure, as shown in Fig. 2. The extra hydroxyl group in the LG samples causes a reduction in hydrophobicity as it works as a water-loving group, which is also confirmed through a dry-out time study. CA cross 0.5% showed a slight decrease in contact angle compared to CA cross 0.3, possibly due to the unreacted citric acid.
Tensile strength (ply to lam)
The purpose of this test is to determine the tensile strength of plywood to laminate assemblies and to investigate their effects. In comparison to the laminate, the plywood did not wet as thoroughly during the adhesive application. In this instance, the wetting was simple due to the laminate’s numerous pores on the surface. After putting the blends’ samples to plywood and laminate, an analysis of the blends’ bonds was carried out. The bond strength increased as the CA content increased, as seen in Figs. 15 and 16 for groups 1 and 2. Cohesion within the blend is brought about by the cross-linking of CA with LG and PVA molecules. Better bond strength is imparted with the wood surface by cohesive force, which also minimizes free volume and creates chain interaction (Gadhave et al. 2020). More densely packed chains interact with the wood surface because the closer packing and enhanced cohesion have reduced the free volume. After a day, adhesive-applied assemblies were tested to determine the extent of bond strength. It is clear that using 0.3 weight% of CA in the formulation, a cross-linker strengthens the bonds. For 24 h bonded substrates, the blend containing 0.3 weight% CA shows an increment of 213% enhancement in tensile strength. The viscosity and adhesiveness have decreased as a result of the plasticizing behaviour at 0.5 weight%. The bond strength has increased as a result of better contact between the adhesive and the wood surface. Since an increase in CA causes an increase in cross-linking, it is possible to draw the conclusion that the concentration of CA directly correlates with bond strength. Figure 17 illustrates how increasing LG results in stronger adhesion, which is consistent with the cellulose and glucose interacting well.
Tensile shear strength testing- wet and dry
Tensile lap shear test is a key analysis to evaluate the performance of adhesive over the wood substrate. The analysis involved testing of single lap adhesive joint over wood specimen.
The analysis involved testing at intervals; values are denoted in Tables 4 and 6, and 8. Time-wise test correlates the bond development time in the wooden specimens. The samples were conditioned as per EN 204–205 standard for the water resistance test.
The main observation was that incorporating CA showed better water resistance over the wood substrate. Cohesion within the blend is brought about by the crosslinking of CA with LG and PVA molecules (Gadhave et al. 2020). Better bond strength is imparted with the wood surface by cohesive force, which also minimizes free volume and creates chain interaction. More tightly bound chains interact with the wood surface because the closer packing and enhanced cohesion have reduced the free volume. When compared to pristine blends, the addition of CA results in a higher number of crosslinking sites and improved water resistance. After 24 h immersion in water, Gluco-PVA STD-01 has lower strength than Gluco-PVA- CA-11 by 500%, as shown in Fig. 18. Group 2 also demonstrated similar tread, as shown in Fig. 19. As seen in Fig. 20, an increase in LG causes a drop in water resistance properties, even when the CA concentration is kept constant.
Applying the blends to specimens of steamed beech wood allowed for an analysis of the blends’ adhesion strength. The bond strength increased as the CA content increased after 24 h and seven days, as shown in Figs. 21, 22 and 23, and 24 for groups 1 and 2. The cohesiveness of the blend is achieved by the cross-linking of CA with LG and PVA molecules. Cohesive force increases the bonding strength with the wood surface by reducing free volume and generating chain interaction (Gadhave et al. 2020). More tightly packed chains interact with the wood surface because the closer packing and enhanced cohesion have reduced the free volume. To study the extent of bond strength, adhesive-applied wood substrates were tested after 24 h and 7 days. It can be seen that the addition of a cross-linker increases the bond strength, with 0.3 wt% of CA in the formulation the blend with 0.3 wt% CA shows an increment of 250% enhancement in tensile strength for 24 h and seven days bonded substrates. The plasticizing effect at 0.5 wt% has contributed to a decrease in viscosity and bond strength. As a result of improved interaction between the wood surface and the adhesive, the bond strength has increased. It may be concluded that the concentration of CA is directly related to bond strength since increases in CA lead to increased cross-linking; in the case of Figs. 25 and 26, an increase in LG results in improved adhesion strength, which is consistent with excellent interaction between the cellulose and glucose.
Conclusion
A combined approach of physical and chemical modification of PVA and LG blend-based adhesive has been investigated in the study. It was observed that by blending PVA with LG and crosslinking with a green cross-linker, CA has prominently enhanced the physicochemical and mechano-thermal properties of the adhesive sample. Due to its multi-carboxylic structure, esterification could occur between the carboxyl and hydroxyl groups on the PVA-LG blend at 0.3 wt%, CA acted as a crosslinking agent, as evidenced by increased Tg, viscosity, contact angle, and mechanical properties. However, the higher concentrations of CA limited the mobility of the blend, which resulted in lowering the Tg. The decrease in mechanical properties was further evidenced by a drop in pencil hardness and tensile strength with 0.5 and 0.7 wt% CA. With these observations, the residual-free CA in the blends acted as a plasticizer. The carboxyl groups on CA can improve the interactions and form stronger bonds with the PVA-LG blend and wood substrate. The films that have been crosslinked showed diminished hydrophilicity. Therefore, incorporating CA as a green additive can prepare a cost-effective adhesive with enhanced physicochemical, thermomechanical, and performance properties for the PVA-LG blend as a potential and sustainable water-based wood adhesive.
References
Cheng HN, Dowd MK, He Z (2013) Investigation of modified cottonseed protein adhesives for wood composites. Ind Crops Prod 46:399–403. https://doi.org/10.1016/j.indcrop.2013.02.021
Del Menezzi C, Amirou S, Pizzi A, Xi X, Delmotte L (2018) Reactions with Wood Carbohydrates and Lignin of Citric Acid as a bond promoter of Wood Veneer panels, polymers (Basel). 10:833. https://doi.org/10.3390/polym10080833
Doll KM, Shogren RL, Willett JL, Swift G (2006) Solvent-free polymerization of citric acid andd-sorbitol. J Polym Sci Part Polym Chem 44:4259–4267. https://doi.org/10.1002/pola.21535
Gadhave RV (2023a) Comparative Study of Effect of Addition of Calcium Carbonate and Clay on the Performance Properties of Polyvinyl Acetate Wood Glue. Open J Polym Chem 13:1–13. https://doi.org/10.4236/ojpchem.2023.131001
Gadhave RV (2023b) Synthesis and characterization of starch-stabilized polyvinyl acetate-N-methylol acrylamide polymer-based wood adhesive. J Indian Acad Wood Sci 20:51–61. https://doi.org/10.1007/s13196-023-00312-3
Gadhave RV, Vineeth SK (2022) Synthesis and characterization of starch stabilized polyvinyl acetate-acrylic acid copolymer-based wood adhesive. Polym Bull. https://doi.org/10.1007/s00289-022-04558-8
Gadhave RV, Mahanwar PA, Gadekar PT, Kasbe PS (2019) A study on the effect of starch–polyvinyl alcohol blends by addition of citric acid and boric acid for enhancement in performance properties of polyvinyl acetate-based wood adhesive. J Indian Acad Wood Sci. https://doi.org/10.1007/s13196-019-00249-6
Gadhave RV, Dhawale VSKPV, Gadekar PT (2020) Effect of boric acid on poly vinyl alcohol- tannin blend and its application as water-based wood adhesive. Des Monomers Polym 23:188–196. https://doi.org/10.1080/15685551.2020.1826124
Genest S, Salzer R, Steiner G (2013) Molecular imaging of paper cross sections by FT-IR spectroscopy and principal component analysis. Anal Bioanal Chem 405:5421–5430. https://doi.org/10.1007/s00216-013-6967-1
Gouveia S, Otero L, Fernández-Costas C, Filgueira D, Sanromán Á, Moldes D (2018) Green Binder Based on Enzymatically Polymerized Eucalypt Kraft Lignin for Fiberboard Manufacturing: a preliminary study, polymers (Basel). 10:642. https://doi.org/10.3390/polym10060642
Guo W, Xiao Z, Wentzel M, Emmerich L, Xie Y, Militz H (2019) Modification of scots pine with activated glucose and citric acid: physical and mechanical properties, BioResources. 14:3445–3458. https://doi.org/10.15376/biores.14.2.3445-3458
Huaxu Z, Hua LS, Tahir PM, Ashaari Z, Al-Edrus SSO, Ibrahim NA, Abdullah LC, Mohamad SF (2020) Physico-Mechanical and Biological durability of Citric Acid-Bonded Rubberwood Particleboard, polymers (Basel). 13:98. https://doi.org/10.3390/polym13010098
Jin T, Zeng H, Huang Y, Liu L, Yao W, Lei H, Shi S, Du G, Zhang L (2023) Bio-hyperbranched polyesters synthesized from citric acid and bio-polyols and applications on wood adhesives. Polym Test 120:107974. https://doi.org/10.1016/j.polymertesting.2023.107974
Kaboorani A, Riedl B (2011) Improving performance of polyvinyl acetate (PVA) as a binder for wood by combination with melamine based adhesives. Int J Adhes Adhes 31:605–611. https://doi.org/10.1016/j.ijadhadh.2011.06.007
Kaboorani A, Riedl B, Blanchet P, Fellin M, Hosseinaei O, Wang S (2012) Nanocrystalline cellulose (NCC): a renewable nano-material for polyvinyl acetate (PVA) adhesive. Eur Polym J 48:1829–1837. https://doi.org/10.1016/j.eurpolymj.2012.08.008
Klenina OV, Klenin VI, Polubarinova LI, Frenkel’ SY (1972) The formation of supermolecular order in aqueous polyvinyl alcohol solutions as a crystallization process. Polym Sci U S S R 14:2567–2576. https://doi.org/10.1016/0032-3950(72)90416-9
Othman N, Azahari N (2011) Thermal properties of Polyvinyl Alcohol (PVOH)/Corn Starch Blend Film. Malaysian Polym J 6
Pizzi A (2014) Types, processing and properties of bioadhesives for wood and fibers, in: Adv. Biorefineries, Elsevier, : pp. 736–770. https://doi.org/10.1533/9780857097385.2.736
Pizzi A (2017) Renewable polymeric adhesives, polymers (Basel). 9. https://doi.org/10.3390/polym9040126
Pizzi A, Biofoams-a Review T-B (2019) J Renew Mater 7:477–492. https://doi.org/10.32604/jrm.2019.06511
Queneau Y, Jarosz S, Lewandowski B, Fitremann J (2007) Sucrose Chemistry and Applications of Sucrochemicals, in: : pp. 217–292. https://doi.org/10.1016/S0065-2318(07)61005-1
Reddy N, Yang Y (2010) Citric acid cross-linking of starch films. Food Chem 118:702–711. https://doi.org/10.1016/j.foodchem.2009.05.050
Shi R, Bi J, Zhang Z, Zhu A, Chen D, Zhou X, Zhang L, Tian W (2008) The effect of citric acid on the structural properties and cytotoxicity of the polyvinyl alcohol/starch films when molding at high temperature. Carbohydr Polym 74:763–770. https://doi.org/10.1016/j.carbpol.2008.04.045
Sutiawan J, Hermawan D, Massijaya MY, Kusumah SS, Lubis MAR, Marlina R, Purnomo D, Sulastiningsih IM (2022) Influence of different hot-pressing conditions on the performance of eco-friendly jabon plywood bonded with citric acid adhesive. Wood Mater Sci Eng 17:400–409. https://doi.org/10.1080/17480272.2021.1884898
Umemura K, Ueda T, Munawar SS, Kawai S (2012a) Application of citric acid as natural adhesive for wood. J Appl Polym Sci 123:1991–1996. https://doi.org/10.1002/app.34708
Umemura K, Ueda T, Kawai S (2012b) Characterization of wood-based molding bonded with citric acid. J Wood Sci 58:38–45. https://doi.org/10.1007/s10086-011-1214-x
Umemura K, Sugihara O, Kawai S (2013) Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard. J Wood Sci 59:203–208. https://doi.org/10.1007/s10086-013-1326-6
Vineeth SK, Gadhave RV, Gadekar PT (2023) Polyvinyl alcohol–cellulose blend wood adhesive modified by citric acid and its effect on physical, thermal, mechanical and performance properties. Polym Bull 80:8013–8030. https://doi.org/10.1007/s00289-022-04439-0
Xie F, Zhang H, Nie C, Zhao T, Xia Y, Ai L (2021) Structural characteristics of tamarind seed polysaccharides treated by high-pressure homogenization and their effects on physicochemical properties of corn starch. Carbohydr Polym 262:117661. https://doi.org/10.1016/j.carbpol.2021.117661
Zhong Z, Susan Sun X, Fang X, Ratto JA (2002) Adhesive strength of guanidine hydrochloride—modified soy protein for fiberboard application. Int J Adhes Adhes 22:267–272. https://doi.org/10.1016/S0143-7496(02)00003-9
Acknowledgements
We would like to thank Institute of Chemical Technology, Mumbai, India, for the support throughout this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhawale, P., Gadhave, S. & Gadhave, R.V. Liquid glucose-polyvinyl alcohol blend wood adhesive modified by citric acid and its effect on physical, thermal, mechanical and performance properties. J Indian Acad Wood Sci (2024). https://doi.org/10.1007/s13196-024-00345-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13196-024-00345-2