Abstract
Germ cell tumors (GCT) are an intriguing group of neoplasm having myriad clinical and morphological presentation. More and more transcription factors are being evaluated for identification of same. To study the spectrum of GCTs in a tertiary care center and the use of a stem cell marker OCT4 as a diagnostic adjunct, a retrospective 5-year (2008–2013) study was carried out. Immunohistochemistry (IHC) with OCT4 was performed on all cases and IHC for α feto protein (AFP), CD30, and epithelial membrane antigen (EMA) as per requirement. Cohort included 73 cases (23 males and 50 females). Testicular and ovarian GCTs accounted for 95.83% and 35.71% respectively. In males, seminoma was the commonest (34.78%) followed by mixed GCT (26%). 17.85% of ovarian GCTs were malignant mostly constituted by dysgerminoma (18%). Benign mature cystic teratoma (MCT) constituted 50% of ovarian GCTs. OCT4 immunoexpression was seen in all cases of seminoma/dysgerminoma, embryonal carcinoma, immature teratoma, and seminomatous/embryomatous component of mixed GCTs. Pure yolk sac tumor (YST) and MCT were consistently negative. OCT4 was especially helpful in identification of mixed GCT. A panel of immunohistochemical markers would be a more ideal way to identify and clarify the components because correct identification of the components is important for therapeutic intervention and prognostication. OCT4 being a primordial germ cell marker predicts aggressive behavior and targeted therapy against this should be investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Germ cell tumors (GCTs) are a diverse and heterogeneous group of neoplasm that originate from primordial germ cells imitating the embryonic histogenesis and thereby harboring vivid morphological patterns with varied differentiation [1]. GCTs may be gonadal or extragonadal at presentation. During embryogenesis, the primitive germ cells migrate from the yolk sac to the genital ridge along midline, hence explaining the predominant midline localization of these tumors [2]. Extragonadal sites include mediastinum, sacrococcygeum, retroperitoneum, supra-sellar, and rarely nasopharynx, orbit. Overall, these tumors are amenable to surgical, chemoradiotherapeutic intervention and therefore identification of constituent elements can bear both therapeutic and prognostic implications [3, 4].
OCT4, octamer-binding transcription factor 4 (POU5F1) located on chromosome 6p21 in human genome, maintains and regulates germ cell pluripotency and is being increasingly found to be overexpressed in cancer stem cells [5, 6].
The present study was performed to study the spectrum of ovarian and testicular GCTs and explore the utility of OCT4 in reclassification and identification of GCTs. Study on magnitude and distribution of tumors along with expression of transcription factor (TF) can promote improved research and therapeutic interventions for this intriguing group of neoplasm.
Materials and Methods
A 10-year data (2003–2013) of ovarian and testicular GCTs were collected from the pathology department (King George’s Medical University) registers. In 5-year archival material, a total of 73 cases of GCT arising from either ovary or testis were retrieved. The demographic profiles were noted, and histopathological diagnosis was reviewed and subjected to OCT4 immunohistochemistry (rabbit anti mouse polyclonal antibody, BioGenex™, 100 µg, 1:40 dilution directed towards the N terminus of OCT4 protein) along with additional immunohistochemistry (IHC) panel as and when required (Fig. 1). Extragonadal GCTs included two cases of immature teratoma, one sacrococcygeal and one retroperitoneal; however, it was not included in the study. No cases were recorded in other extragonadal sites.
The percentages of cells that stained positively for OCT4 were estimated, and the staining intensity was classified as negative, weak, moderate, or strong. OCT4 is a nuclear transcription factor that is involved in gene regulation; thus, nuclear staining was considered a positive result. No staining was recorded as 0, up to 30% moderate nuclear expression was considered as 1 + , 31–60% as 2 + , 61 to 90% as 3 + , and > 90% tumor nuclear expression was considered as 4 + . Cases with staining of 1 + and above were considered as positive. The statistical analysis was done using SPSS (Statistical Package for Social Sciences) Version 15.0 statistical analysis software. The values were represented in number (%) and Mean ± SD.
Results
A total of 265 cases of ovarian neoplasms were reported in a 10-year period including 90 cases of GCTs and 175 other neoplasms (surface epithelial malignancies, sex cord stromal neoplasms, and Krukenberg tumors). The ovarian GCTs (OGCTs) thus accounted for 33.9% of all ovarian neoplasms and malignant GCTs (n = 35) accounted for 13.20% of all ovarian neoplasms and 38.8% of all GCTs of females. A total of 43 case of male testicular neoplasms were reported in 10-year period, of which 41 (95.34%) were testicular GCTs (TGCTs).
Paraffin blocks of 5-year period (n = 73) were available for further study. OGCTS were more (n = 50) as compared to TGCTs (n = 23). Male:female ratio was roughly 1:2. Mean age of patients was 24.52 ± 13.2 years (male and female combined) and age ranged from 2 to 60 years. Maximum number of cases was recorded in young, i.e., less than 30 years (11–20 and 21–30 years; 28.8 and 27.4% respectively). There were 14/73 (19.2%) cases in age group 31–40 years and 10/73 (13.7%) cases in age group ≤ 10 years. Age groups 41–50 years and ≥ 50 years were less represented with only 6/73 (8.2%) and 2/73 (2.7%) respectively.
The most common presenting symptoms in females were lump and pain abdomen. Ascites was present in 12% of cases. The most common presenting complaint was that of testicular enlargement (91.3%) in males. One case had ulceration and bloody discharge through the scrotal skin and one was an undescended testis.
In case of females, mature cystic teratoma (MCT) was the predominant histological subtype (n = 25, 50%) followed by dysgerminoma (DG) (n = 9, 18%), mixed GCT (n = 8, 16%), yolk sac tumor (YST) (n = 06, 12%), and immature teratoma (IT) (n = 2, 4%) (Table 1). Amongst the eight cases of mixed GCT, one of the components was YST in all cases, the other components being embryonal carcinoma (EC) in five cases, and DG in three cases.ßcatenin pathway was foun
In males, seminomatous tumor was the commonest (n = 8, 36%). Mixed GCTs (n = 6, 26%), YSTs (n = 4, 17.39%), EC (n = 2, 8.69%), MCT (n = 1, 4.3%), germ cell neoplasia in situ (GCNIS) (n = 1, 4.3%), and post-pubertal teratoma (n = 1, 4.3%) were diagnosed amongst the non-seminomatous lesions (Table 1). In mixed GCTs, teratomatous element was predominant and was in combination in four of the cases with YST, seminoma, and EC respectively. Two cases were a combination of seminoma and YST.
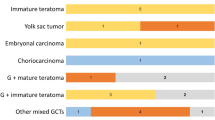
OCT4 strong nuclear positivity (3 + /4 +) was found in all cases of pure seminoma (08/08), dysgerminoma (09/09), pure embryonal carcinoma (02/02), GCNIS (01/01), and ITs (02/02) (Fig. 2b, d, f). Seminomatous and embryonal component also showed similar positivity in mixed GCTs (n = 8). Five cases of MCT of females and one case each of pre- and post-pubertal teratoma of males were taken up for OCT IHC, all of which being completely immunonegative (Fig. 2i). Initially 16 cases were diagnosed as pure YSTs based on pure morphology only, of which ten cases were completely negative for OCT4 immunoexpression (Fig. 2h) and corroborated with the morphological diagnosis. However, there was a subset of morphologically diagnosed pure YSTs (6/16) which showed strong OCT4 in some portions of tumor, suggesting that either embryonal, seminomatous/dysgerminomatous component was present in the tumor tissue (Table 2). All these cases were reviewed by senior pathologist and extended IHC panel comprising of CD30, EMA for EC, AFP for YST, and CD117 for seminoma was applied. These cases were found to have two germ cell components on IHC (CD30 + , EMA + , AFP +) which was missed earlier. Thus, these cases of morphologically diagnosed YST were reclassified as mixed GCT (YST with EC). Mixed GCTs thus accounted for a total of 14 cases.
Morphological spectrum of various germ cell tumors with respective OCT4 status. Seminoma/dysgerminoma containing sheets of uniform cells with clear cytoplasm separated by connective tissue septae (a, H&E, × 100) and intensely stained (3 +)OCT4 (b, immunoperoxidase, × 200); GCNIS: Large atypical cells with clear cytoplasm confined within the tubules with thickened tubular basement membrane (c, H&E, × 200) positive for OCT4 (3 +) (d, immuoperoxidase, × 200), embryonal carcinoma with pleomorphic cells and prominent nucleoli (e, H&E, × 200), yolk sac tumor (glandular pattern, H&E × 200) negative for OCT4 in inset (f, immunoperoxidase, × 200).Yolk sac tumor, microcystic tumor with a Schiller Duval body (g, H&E × 200) negative for OCT4 (h, immunoperoxidase, × 200). Immature teratoma with neuroepithelial rosettes (*) (i, H&E × 200), positive for OCT4 (j, immunoperoxidase, × 200). Mixed GCT, teratoma (cartilaginous component) with yolk sac tumor (k, H&E × 100), negative for OCT4 (l, immunoperoxidase, × 200)
Discussion
GCTs have myriad morphological features derived from primitive germ cells of the embryonic gonad. GCTs are the only homologous group of neoplasm in the male and female gonads, however with a different histogenesis in both. With the advancements in molecular profiling, the testicular GCTs are considered to be either GCNIS related or unrelated. The post-pubertal GCTs are considered to be GCNIS associated and includes seminoma, EC, subset of YSTs, and post-pubertal teratomas which usually follow a malignant course [7]. The pre-pubertal GCTs on the other hand are GCNIS unrelated and include subset of YSTs, spermatocytic tumor, and pre-pubertal teratomas which include dermoid and epidermoid cyst. The ovarian GCTs on the other hand are thought to be parthenogenetically conditioned with no association of 12p isochromosome abnormalities or malignant germ cells [8]. The ovarian GCTs accounted for 33.9% of all ovarian neoplasms of which 13.20% were malignant which is slightly higher than other published literature which has reported a malignancy rate of 5 to 7% [9, 10]. The higher incidence in our study was because our hospital is a tertiary care referral hospital. On the other hand, malignant GCTs were more common in males in our study (95.7%) which is concordant with various published literature [11]. This skewness in males is because of paucity of MCTs in males.
The proportion of various morphological subtypes of malignant GCTs were almost similar in both sexes commonest being seminoma/dysgerminoma followed by mixed GCT, YST. EC is considered to be most undifferentiated of all GCTs with potential to differentiate into either teratomatous tumors or YSTs. In females, pure EC are very rare as compared to males though the reason is still unknown as was in our study where no case of pure EC was found in females; however, two cases were present in males. Also EC and YSTs were more predominantly part of mixed GCTs. Most of the cases were present in the second and third decade of life; however, in pediatric age group males, YSTs formed the predominant type both in pure and mixed GCTs. In females, the age range of YSTs was 14–34 years with an average age of presentation being 22.16 years. This is concordant with various published literature (Table 1) [12,13,14,15,16].
Morphology though is quite diagnostic in most GCTs; however, additional IHC provides an advantage for classification and identification of mixed components especially in cases of morphological overlap amongst certain patterns of a particular GCT, the identification of which has therapeutic and prognostic significance. Routine and easily available IHC markers used in identification of germ cell tumors are PLAP, CD117, EMA, CD30, and AFP. As different areas of tumor harbor different morphologies, extensive grossing to identify other components has been recommended in the literature [17].
On evaluation of OCT4 IHC, it was found that apart from staining the routine seminoma/dysgerminoma and EC, OCT4 had a major advantage in identification of mixed GCTs which could not be picked by morphology alone. These cases were initially reported as pure YST (n = 6). Use of OCT4 IHC highlighted that 37.5% (6/16) of cases reported as pure YST harbored higher component (embryonal carcinoma- EMA + /CD 30 + areas) which was missed morphologically and would be missed even if IHC panel of only AFP had been performed to confirm YST. For centers which have limited funds and patients who cannot afford extended panel, at least addition of OCT4 may help in identification of mixed germ cell areas if one is morphologically identifying yolk sac component. The cost of OCT4 IHC is comparable with that of conventional routine IHC marker used like PLAP and AFP; however, OCT4 is much more specific to decipher a GCT lineage especially in metastatic site [18, 19]. Identification of constituent elements of GCT is of utmost importance especially embryonal and yolk sac components because these tumors are usually locally aggressive with extensive spread and metastasis. Diligent grossing and examination can also provide a clue as these components are usually associated with necrosis and hemorrhage [20] (Fig. 3). Use of IHC with specific TFs like OCT4 can serve as a diagnostic adjunct especially in cases of post-chemotherapy recurrence [21]. It is also important to distinguish embryonal carcinoma from seminoma, large cell lymphomas, or a poorly differentiated carcinoma which can mimic embryonal carcinoma especially in metastatic sites [22, 23]. Use of a single marker OCT4 in these situations may not serve the purpose because it cannot distinguish between seminoma and EC, nor can it detect the presence of YST in gonads or in metastatic sites. A panel of markers must be used so that one can identify the presence of GCTs and delineate the type of component present.
Gross photographs depicting cut surface of a seminoma demonstrating a cream-colored, multinodular neoplasm bulging from the surrounding testicular parenchyma (a), outer surface of a dysgerminoma with a lobulated external surface having a glistening smooth, gray-white fibrous capsule (b), cut surface of a mixed GCT (germ cell tumor) showing solid cystic areas with areas of necrosis (c), and cut surface of a mature cystic tumor showing hair and a fat nodule (*) (d)
OCT4 being a stem and germ cell marker, its expression is downregulated during differentiation; thus, its persistence in adult tissue is a mark of immaturity. Various studies associated increased OCT4 expression of either mRNA or protein with chemoresistance and poor prognosis in bladder, ovarian, prostate, rectal, hepatocellular, esophageal cancer, glioma, melanoma, medulloblastoma, and acute myeloid leukemia [13, 24]. However, the relationship was reverse in TGCT where it was found that cisplatin treatment decreased OCT4 levels and conferred chemoresistance. Various studies inferred that a process of post-translational modification (sumoylation) of OCT4 protein was responsible for the process [14]. Targeted therapy against this sort of TFs in future may serve to treat these tumors.
Lakshmanan et al. in their 5-year treatment and survival study of OGCT from the same institute found that 71.1% of patients received neoadjuvant chemotherapy due to advanced disease in form of ascites or large mass, and 18.5% of these patients were amenable to fertility-preserving surgery after chemotherapy. Around 36.8% patients had conservative surgery with preservation of opposite ovary and uterus. Sixty-four percent of this group of patients had return of menstrual function after a mean period of 3.5 + 0.5 months. One patient who underwent fertility-preserving surgery had delivered healthy children after treatment. Stage distribution for stage I to IV was as follows: 15.4% (n = 6), 35.9% (n = 14), 46.2% (n = 18), and 2.6% (n = 1), respectively. 17.1% (n = 6) patients had recurrence, with a median time to recurrence 16 months (range 5.5 to 37 months) and they were treated with second-line chemotherapy [15].
A study group from Tata memorial hospital (Joshi et al.) found 5% and 71% had radiologic complete response (CR) and partial response (PR), respectively, in non-seminomatous group and a 46% and 38% radiologic CR and PR, respectively, in the seminomatous group. This was attributed to a higher stage of presentation, most cases being N3, M1.The authors concluded that standard first-line CT might not suffice as a curative therapy in high nodal and high-risk diseases, and significant chemotoxicity is also a hindering factor [16].
Study of pediatric OGCTs (Rajeshwari et al.) and extracranial pediatric male GCTs (Kumar et al.) showed event-free survival (EFS) and overall survival (OS) rates were 80.8% and 92.7% at a median follow-up of 80 months in OGCTs and 73.3% and 87.9% in the testicular GCT group at the end of 4 years. The first-line chemotherapy administered was bleomycin, etoposide, and cisplatin (BEP). Recurrence was seen in (19.2%) patients (4 cases of grade 3 IT and one case of mixed GCT) in pediatric OGCTs. Morphology of pediatric OGCTs cases (post-surgical resection) revealed transformation to that of mature teratoma in 3 out of 4 cases of IT. One case showed persistence of immature teratomatous morphology and mixed GCT retained the same morphology. Second-line chemotherapy using vincristine, Adriamycin, cyclophosphamide and vinblastine, ifosfamide, and carboplatin was given to the two patients with metastatic malignant recurrence. Post-chemotherapy resection of residual retroperitoneal mass in 8/16 cases of pediatric male showed GCTs mature (n = 4) and immature teratomatous (n = 1) element and 3 cases showed no residual tumor [25, 26]. Thus, it can be inferred that ITs and mixed GCTs are usually prone to recur and may require second-line chemotherapy.
Shen et al. studied the genomic and epigenomic characteristics of tumor and also corroborated the same with tumor morphology. They found distinctive molecular patterns in TGCTs [27]. As a whole, the tumors were highly aneuploid with paucity of somatic mutations. Driver mutations were identified only in seminoma/seminomatous components, one of the most important being KIT mutation. It was seen that KIT-mutated seminomas had a higher lymphocytic infiltration, the absence of global DNA methylation, reduced KRAS mutation frequency and copy number alterations, reduced i(12p) events, and a more prevalence of cryptorchidism. Also KIT mutation may predispose to bilaterality in TGCTs; however, no such association has been found in OGCTs [26,27,28,29]. Polypoidization is considered an early event in the genesis of both TGCTs and OGCTs; however, the ovarian ITs are found to be diploid indicating that they may develop through a different pathway. DNA copy number alterations are similar in both sexes; however, 12p gain are more prevalent in TGCTs with i(12p) being pathognomic [30]. Two circulating microRNAs miR-371 (highly expressed in seminomas and EC) and miR-375 (highly expressed in teratomas and YST) are purported to be predictive markers for identifying residual tumor masses and avoidance of unnecessary chemotherapy; however, validation is still required [31].
Also strategies for targeted therapy were suggested like DNA methyltransferase inhibitors in NSGCT. Guadecitabine was found to be effective in cells of refractory TGCTs by Albany et al. [32]. PARP inhibitors have been suggested in a subset of non-EC non-seminomatous GCTs harboring BRCA1 and RAD51C promoter methylation [33]. Candidate predictive markers like DNAJC15/MCJ genes are being identified in non-seminomatous GCTs similar to breast and uterine cancer as recurrent epigenetic silencing these genes are associated with drug resistance [34].
Microsatellite instability (MSI) has been reported in various proportions in different types of GCTs being more frequent in DG and YST than IT while one author observed the reverse. MSI was supposed to be more significant in OGCTs than TGCTs [35, 36]. WNT/ßcatenin pathway was found to have a role in YST/IT which showed nuclear accumulation of ßcatenin. Whereas post-pubertal TGCTs are proposed to originate from the precursor lesion GCNIS, no such lesion is found in OGCTS except for in dysgenetic gonads where gonadoblastoma (GB) is considered to be a precursor lesion with a step-wise progression from GB to DG is proposed. Such cases are phenotypic females with male karyotype or at least parts of the Y-chromosome present in their genome [37, 38].
Conclusion
Benign MCT was the commonest GCT in females and seminoma in males. YST and EC are mostly components of mixed GCT in adults, judicious grossing is advocated for identification of same. In males, post-pubertal teratomas are usually found as a component of mixed GCT. OCT4 can serve as a valuable additional marker for routine immunohistochemical panel for GCTs especially mixed GCTs. OCT4 being a primordial germ cell marker predicts aggressive behavior and hence targeted therapy against this should be investigated. More and more predictive markers and target molecules are coming up with the advent of molecular profiling of these tumors which might prevent the unpleasant effects of standard chemotherapeutic regimen.
Code Availability
Not applicable.
References
Pierce GB (1967) Teratocarcinoma: model for a developmental concept of cancer. Curr Top Dev Biol 2:223–246. https://doi.org/10.1016/s0070-2153(08)60289-6
Anderson R, Copeland TK, Schöler H, Heasman J, Wylie C (2000) The onset of germ cell migration in the mouse embryo. Mech Dev 91(1–2):61–68. https://doi.org/10.1016/s0925-4773(99)00271-3
Cheng L, Sung MT, Cossu-Rocca P, Jones TD, MacLennan GT, De Jong J, Lopez-Beltran A, Montironi R, Looijenga LH (2007) OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. J Pathol 211(1):1–9. https://doi.org/10.1002/path.2105
Gershenson DM (2007) Management of ovarian germ cell tumors. J Clin Oncol 25(20):2938–2943. https://doi.org/10.1200/JCO.2007.10.8738
Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H, Honecker F, Bokemeyer C, Perlman EJ, Schneider DT, Kononen J, Sauter G, Oosterhuis JW (2003) POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res 63(9):2244–2250
Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Schöler H, Smith A (1998) Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 95(3):379–91. https://doi.org/10.1016/s0092-8674(00)81769-9
Berney DM, Looijenga LH, Idrees M, Oosterhuis JW, Rajpert-De Meyts E, Ulbright TM, Skakkebaek NE (2016) Germ cell neoplasia in situ (GCNIS): evolution of the current nomenclature for testicular pre-invasive germ cell malignancy. Histopathology 69(1):7–10. https://doi.org/10.1111/his.12958
Nogales FF, Dulcey I, Preda O (2014) Germ cell tumors of the ovary: an update. Arch Pathol Lab Med 138(3):351–362. https://doi.org/10.5858/arpa.2012-0547-RA
Sankaranarayanan R, Ferlay J (2006) Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol 20(2):207–225. https://doi.org/10.1016/j.bpobgyn.2005.10.007
Mankar DV, Jain GK (2015) Histopathological profile of ovarian tumours: a twelve year institutional experience. Muller J Med Sci Res 6:107–111
Bosl GJ, Motzer RJ (1997) Testicular germ-cell cancer. N Engl J Med 337(4):242–253. https://doi.org/10.1056/NEJM199707243370406
Bhattacharyya NK, De A, Bera P, Sristidhar M, Chakraborty S, Bandopadhyay R (2010) Ovarian tumors in pediatric age group - a clinicopathologic study of 10 years’ cases in West Bengal. India Indian J Med Paediatr Oncol 31(2):54–57. https://doi.org/10.4103/0971-5851.71656
Atlasi Y, Mowla SJ, Ziaee SAM, Bahrami A-R (2007) OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int J Cancer 120:1598–1602
Wu YC, Ling TY, Lu SH, Kuo HC, Ho HN, Yeh SD, Shen CN, Huang YH (2012) Chemotherapeutic sensitivity of testicular germ cell tumors under hypoxic conditions is negatively regulated by SENP1-controlled sumoylation of OCT4. Cancer Res 72(19):4963–4973. https://doi.org/10.1158/0008-5472.CAN-12-0673
Lakshmanan M, Gupta S, Kumar V, Akhtar N, Chaturvedi A, Misra S, Jain K, Garg S (2018) Germ cell tumor ovary: an institutional experience of treatment and survival outcomes. Indian J Surg Oncol 9(2):215–219. https://doi.org/10.1007/s13193-018-0742-x
Joshi A, Zanwar S, Shetty N, Patil V, Noronha V, Bakshi G et al (2016) Epidemiology of male seminomatous and nonseminomatous germ cell tumors and response to first-line chemotherapy from a tertiary cancer center in India. Indian J Cancer 53:313–316. https://doi.org/10.4103/0019-509X.197741
Ulbright TM (2005) Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod Pathol 18(Suppl 2):S61-79. https://doi.org/10.1038/modpathol.3800310
Cheng L (2004) Establishing a germ cell origin for metastatic tumors using OCT4 immunohistochemistry. Cancer 101(9):2006–2010. https://doi.org/10.1002/cncr.20566
Sung MT, Jones TD, Beck SD, Foster RS, Cheng L (2006) OCT4 is superior to CD30 in the diagnosis of metastatic embryonal carcinomas after chemotherapy. Hum Pathol 37(6):662–667. https://doi.org/10.1016/j.humpath.2006.01.019
Talerman, Aleksander, and Russell Vang. Blaustein’s pathology of the female genital tract. 6th ed., Springer, 2011.Chapter 16 ,Germ cell tumor. P 848–897. https://doi.org/10.1007/978-1-4419-0489-8
Sung MT, Jones TD, Beck SD Foster RR, Cheng L ( 2006) OCT3/4 is superior to CD30 in the diagnosis of metastatic EC after chemotherapy. Hum Pathol 37(6) 662–7
McKenney JK, Heerema-McKenney A, Rouse RV (2007) Extragonadal germ cell tumors: a review with emphasis on pathologic features, clinical prognostic variables, and differential diagnostic considerations. Adv AnatPathol 14(2):69–92. https://doi.org/10.1097/PAP.0b013e31803240e6
Baker PM, Oliva E (2005) Immunohistochemistry as a tool in the differential diagnosis of ovarian tumors: an update. Int J GynecolPathol 24(1):39–55
Ismail S. Mohiuddin, Sung-Jen Wei, Min H. Kang (2020) Role of OCT4 in cancer stem-like cells and chemotherapy resistance. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1866(4) 165432 https://doi.org/10.1016/j.bbadis.2019.03.005.
Rajeswari B, Nair M, Ninan A, Parukuttyamma K (2016) Ovarian tumors in children: 10-year experience from a tertiary care center in South India. Indian J Cancer 53:292–295. https://doi.org/10.4103/0019-509X.197726
Hemanth Kumar, S.V. Saju, Venkatraman Radhakrishnan, Anand Raja, Trivadi S ( 2020) Ganesan, Manikandan Dhanushkodi et al. Analysis of extra-cranial germ cell tumors in male children: experience from a single centre in India. Pediatr Hematol Oncol J 5(2):37–42. https://doi.org/10.1016/j.phoj.2020.03.012
Shen H, Shih J, Hollern DP, Wang L, Bowlby R, Tickoo SK et al (2018) Integrated molecular characterization of testicular germ cell tumors. Cell Rep 23(11):3392–3406. https://doi.org/10.1016/j.celrep.2018.05.039
Biermann K, Göke F, Nettersheim D, Eckert D, Zhou H, Kahl P, Gashaw I, Schorle H, Büttner R (2007) c-KIT is frequently mutated in bilateral germ cell tumours and down-regulated during progression from intratubular germ cell neoplasia to seminoma. J Pathol 213(3):311–318. https://doi.org/10.1002/path.2225
Looijenga LH, de Leeuw H, van Oorschot M, van Gurp RJ, Stoop H, Gillis AJ et al (2003) Stem cell factor receptor (c-KIT) codon 816 mutations predict development of bilateral testicular germ-cell tumors. Cancer Res 63(22):7674–7678
Gilbert D, Rapley E, Shipley J (2011) Testicular germ cell tumours: predisposition genes and the male germ cell niche. Nat Rev Cancer 11(4):278–288. https://doi.org/10.1038/nrc3021
Isabella Syring, Joanna Bartels, Stefan Holdenrieder, Glen Kristiansen, Stefan C. Müller, Jörg Ellinger (2015) Circulating serum miRNA (miR-367-3p, miR-371a-3p, miR-372-3p and miR-373-3p) as biomarkers in patients with testicular germ cell cancer, The Journal of Urology 193(1) 331-7, ISSN 0022-5347. https://doi.org/10.1016/j.juro.2014.07.010
Albany C, Hever-Jardine M. P, von Herrmann K. M, Yim C. Y, Tam J, Warzecha J. M et al. (2017) Refractory testicular germ cell tumors are highly sensitive to the second generation DNA methylation inhibitor guadecitabine. Oncotarget. 8: 2949–59.https://doi.org/10.18632/oncotarget.13811
Cavallo F, Graziani G, Antinozzi C, Feldman DR, Houldsworth J, Bosl GJ et al (2012) Reduced proficiency in homologous recombination underlies the high sensitivity of embryonal carcinoma testicular germ cell tumors to cisplatin and poly (ADP-ribose) polymerase inhibition. PLoS ONE 7(12):e51563. https://doi.org/10.1371/journal.pone.0051563
Fernández-Cabezudo MJ, Faour I, Jones K, Champagne DP, Jaloudi MA, Mohamed YA, Bashir G et al (2016) Deficiency of mitochondrial modulator MCJ promotes chemoresistance in breast cancer. JCI Insight 1(7):e86873. https://doi.org/10.1172/jci.insight.86873
Faulkner SW, Friedlander ML (2000) Microsatellite instability in germ cell tumors of the testis and ovary. Gynecol Oncol 79(1):38–43. https://doi.org/10.1006/gyno.2000.5906.
King BL, Carcangiu ML, Carter D, et al (1995) Microsatellite instability in ovarian neoplasms. Br J Cancer. 1995;72: 376 –382 King, B., Carcangiu, ML., Carter, D. et al. Microsatellite instability in ovarian neoplasms. Br J Cancer 72 376–382. https://doi.org/10.1038/bjc.1995.341
JorgensenN,Muller J, Jaubert F, Clausen OP, Skakkebaek NE (1997) Heterogeneity of gonadoblastoma germ cells: similarities with immature germ cells, spermatogonia and testicular carcinoma in situ cells. Histopathology. 30: 177–86. https://doi.org/10.1046/j.1365-2559.1997.d01-580.x
Cools M, Looijenga LH, Wolffenbuttel KP, Drop SL (2009) Disorders of sex development: update on the genetic background, terminology and risk for the development of germ cell tumors. World J Pediatr 5(2):93–102. https://doi.org/10.1007/s12519-009-0020-7
Acknowledgements
Authors are thankful to King Georges Medical University, Lucknow, for providing necessary resources. We would like to acknowledge Prof Raj Mehrotra, former Professor, dept of Pathology and Dean, KGMU, who had guided us in our study and is no longer with us. We want to thank our technical team members especially Mr. Kamlesh Sharma and Miss Nidhi Varma for their support. The authors have no other relevant affiliations or financial involvements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This is a retrospective study on the paraffin blocks stored in the Department of Pathology, KGMU, Lucknow, and consent for use of the stored tissue is taken from the patients at the time of surgery. Institute ethics committee has a waiver for these retrospective studies on paraffin blocks.
Conflict of Interest
The authors declare no competing interests.
Transparency Declaration
The authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paramita, P., Preeti, A., Mili, J. et al. Spectrum of Germ Cell Tumor (GCT): 5 Years’ Experience in a Tertiary Care Center and Utility of OCT4 as a Diagnostic Adjunct. Indian J Surg Oncol 13, 533–541 (2022). https://doi.org/10.1007/s13193-022-01522-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-022-01522-w