Abstract
Objective
The study aimed to explore some novel diagnostic biomarkers for papillary thyroid carcinoma (PTC) by identifying the different expression of TROP-2, SLP-2 and CD56 in benign and malignant thyroid lesions.
Methods
We evaluated the mRNA expressions of TROP-2 and SLP-2 in fine needle aspirates (FNAs) which contained 10 PTCs and 10 benign follicular adenomas (FAs) using quantitative real-time PCR (qRT-PCR). Immunohistochemical (IHC) staining of TROP-2, SLP-2 and CD56 was also performed on postoperative samples of 30 PTCs and 29 FAs. Membranous or cytoplasmic staining in > 10% of cells was considered as positive. Diagnostic sensitivity, specificity, positive predictive value, negative predictive value (NPV) and diagnostic accuracy of these three biomarkers were carried out. We further analyzed the associations between the clinical features and the expressions of markers in PTCs.
Results
The mRNA expressions of both TROP-2 and SLP-2 were increased substantially in PTCs in comparison with those in FAs (P < 0.05). Similarly, IHC for these two proteins demonstrated higher positive staining in PTCs than in FAs (96.5% vs. 12.5% for TROP-2, 83.3% vs. 20.7% for SLP-2, P < 0.05). Conversely, CD56 expression was lost with 86.7% of PTCs. In identifying malignancy, TROP-2 was the most sensitive marker and CD56 was the most specific one. When the markers were combined, the sensitivity and NPV increased to 100% and had better diagnostic accuracy. However, no association was found between biomarker expressions and clinicopathological factors in PTCs.
Conclusions
We found that TROP-2, SLP-2 and CD56 were effective diagnostic markers for PTC, especially when they were combined to use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is the most frequent tumor amongst endocrine malignancy which comprises of papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), medullary thyroid carcinoma (MTC) and anaplastic thyroid carcinoma (ATC). Approximately 80% of thyroid cancer is PTC, meaning it contributes the most as the incidence of thyroid malignancy increases drastically [1]. Generally, PTC is the most common patterns of differentiated carcinomas with low-aggression and favourable prognosis, but still some clinical evidences revealed the lymphatic metastasis, extrathyroidal extension and frequent recurrence [2]. Distinguishing malignant nodule from benign follicular adenoma (FA) is essential, since it contributes to the therapeutic approach and prognostication. Light microscopy in pathological diagnosis of thyroid tissue is widely used, we can see the typical pathological features including papillary patterns and special nuclear changes as irregular nuclear contours, nuclear overlapping, intranuclear pseudoinclusions and grooves [3]. However, regarding such features especially morphological overlap between follicular lesions and follicular variant of PTC may lead to a diagnostic dilemma at an early stage. In order to eliminate the conflict and achieve a definitive diagnosis, immunohistochemistry (IHC) and biomarkers have been studied more extensively.
Previous studies have revealed several universal immune marker for PTC such as HBME-1, CK19 and Galectin-3 [4], while none of them could reach 100% sensitivity or specificity. Ideal novel markers in the identification of PTC are worthy of being discovered. Nowadays, Stomatin-like protein-2 (SLP-2), trophoblast cell surface antigen-2 (TROP-2), and CD56 have been accepted to be indicators with better sensitivity and specificity in detecting different malignancies. SLP-2 is considered as a cancer-linked protein with diffuse and strong cytoplasmic staining. It was found that downregulation of SLP-2 could inhibit tumor cell adhesion, proliferation and motility [5]. TROP-2 is a transmembrane glycoprotein which highly expressed in various malignant tumours, whereas low expressed in normal tissues [6]. Recent studies have been reported that TROP-2 immunostaining may play an important role in discriminating PTC from other thyroid carcinomas or normal follicular tissues [7, 8]. CD56, a neural cell adhesion molecule, has been demonstrated to be over-expressed in normal thyrocytes, benign or malignant follicular epithelial cells but be usually absent in PTC [9].
The aim of this study was to evaluate the mRNA and protein levels of these three markers mentioned above by performing RT-PCR and IHC stainings, and to explore a superior diagnostic method in discriminating PTC from benign thyroid lesions.
Materials and methods
Subjects selection
This is a retrospective case controlled study. Thyroid nodules with fine needle aspiration (FNA) were retrospectively collected from patients admitted to the first affiliated hospital of Nanjing Medical University. According to the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) and postoperative pathological examination confirmed, the specimens were divided into classic PTC and FA. A total of 20 cytological cases are used for RNA detection of TROP-2 and SLP-2 by PCR test. Meanwhile, IHC staining was performed to investigate SLP-2, TROP-2 and CD56 protein expressions on surgical specimens that included 30 PTCs and 29 FAs. Detailed clinicopathological information concerning age, gender, tumor size, lymph node invasion and distant metastases were recorded by two researchers and no conflicts existed. Patients with PTC were staged based on tumor-node-metastasis (TNM) classification system of American Joint Committee on Cancer (AJCC).
RNA extraction and quantitative RT-PCR
The total RNA was strictly extracted from each FNA case in accordance with the instruction of the TRIzol kit (Invitrogen Life Technologies, New York, NY, USA). The concentration and the purity of the generating RNA samples were determined by ultraviolet spectrophotometer, OD260/OD280 ratios were between 1.8 and 2.6, indicating that the RNA was of good quality with less impurities and could be utilized for reverse transcription PCR. Then, cDNA was gained with a reverse transcription kit (Takara Bio, Primescript™ RT Mix, Japan), TROP-2 and SLP-2 mRNA expression levels were detected by quantitative RT-PCR using the SYBR® Premix Ex TaqTM kit on the StepOnePlusRT-PCR system (Applied biosystems, CA, USA). The primer sequences for specific gene and β-actin (internal reference) were designed and synthesized by Sangon Biotech (Shanghai, China), as summarized in Table 1. The steps of qRT-PCR reaction consisted of 40 cycles: 95 °C for 10 s for degeneration, 60 °C for 60 s for annealing, and 95 °C for 15 s for extension. Each PCR assay was repeated at least three times. At last, the results were calculated by the data analysis system equipped with the formula of 2-ΔΔCT.
TROP-2, SLP-2 and CD56 immunohistochemistry
IHC staining was performed following the instruction of Histostain-SP kit (MXB Biotechnologies, Fuzhou, China). All formalin-fixed, paraffin-embedded thyroid tissues was sectioned into 4um thickness and then let dry. Briefly, the sections were deparaffinized in xylene, rehydrated by gradient ethanol elution and antigen retrieval at 98 °C for 20 min, immersed in 3% H2O2 to block endogenous peroxidase for 10 min, then treated in normal goat serum as blocking reagent for 20 min. The slides were sequentially incubated with the primary antibodies of goat TROP-2 (clone: AF560, Dilution 1:200, R & D, USA), rabbit SLP-2 (clone: 10348-1-AP, dilution 1:1000, Proteintech, China), and rat CD56 (clone: 123c3, dilution 1:100, Zymed, USA) at 4 °C overnight and followed by secondary antibodies conjugated with horseradish peroxidase (HRP) for 30 min at 37 °C. After washing with phosphate-buffered saline (PBS), the streptavidin-peroxidase (SP) for another 30 min. Finally, they were observed by two independent pathologists after using 3,3-diaminobenzidine (DAB) as the chromogen, hematoxylin as the counterstain, then dehydrating, clearing and drying. Negative control was conducted in parallel with PBS buffer in place of primary antibodies, while known IHC staining for PTC was seen as positive control. The expression of TROP-2, SLP-2 or CD56 was considered positive when staining in at least 10% of the cells.
Statistical analysis
The continuous data were expressed by mean ± SD and analysed using t test, while comparisons between groups of categorical variables such as the protein expression and pathology feature were analysed by the χ2 test or Fisher’s exact test. Additionally, sensitivity, specificity, PPV, NPV, and diagnostic accuracy were calculated by traditional formulas for diagnostic test. All statistical data were analysed using software SPSS 23.0 and Graphpad Prism 5.0, P < 0.05 was considered as statistically significant.
Results
Patient characteristics
A total of 20 thyroid FNA cases (10 PTC, 10 FA) and 59 surgical specimens (30 PTC, 29 FA) were included in this study. The patients with FNA were aged between 18 and 61 years with 9 males and 11 females. There was no statistical difference in gender and age between PTC (5 males and 5 females; mean age 30.4 ± 10.3) and FA group (4 males and 6 females; mean age 29.1 ± 14.4). As for patients who underwent surgery and subjected to IHC stainings, the mean age was 39.5 ± 15.3 and women make up 50% for PTC, while the age of FA patients was 37.4 ± 10.7 and the female was about 62%. Similarly, no significant difference was found between the two groups in term of gender and age. Moreover, we observed 11 cases of Lymph node metastasis (LNM), 14 cases of tumor > 1 cm and 4 cases of stage III or IV thyroid cancer, but no case of distant metastasis in PTC group.
TROP-2 and SLP-2 mRNA expression in PTC and FA
The results demonstrated that TROP-2 mRNA expression in PTC (8.1 ± 0.84) was greatly higher (8.1-fold increase) than that in FA group (1.0 ± 0.39). In addition, we also discovered that the mRNA level of SLP-2 was significantly up-regulated about 2.7-fold in PTC in comparison with FA (2.7 ± 0.75 vs. 1.0 ± 0.20, P < 0.05), as shown in Fig. 1.
TROP-2, SLP-2 and CD56 immunohistochemistry in PTC and FA
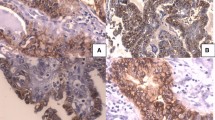
Immunohistochemical results indicated that TROP-2 and CD56 expression was mainly present in plasma membrane, while SLP-2 expression in the cytoplasm, as seen in Fig. 2. In PTC cases, percentages of positive staining for TROP-2, SLP-2 and CD56 were 96, 83.3, and 13.3%, respectively. It is obvious that a high level of TROP-2 and SLP-2 expression was more frequent in carcinoma tissues when compared with those in benign adenomas. Evaluation of CD56 staining showed strong expression in 27 cases (93.1%) of the FA group, whereas 26 PTC cases (86.7%) got varying degrees of loss in CD56 expression. There were significant differences regarding staining patterns of these three markers between groups (P < 0.05). The detailed data were presented in Table 2.
The diagnostic value of markers in distinguishing between PTC and FA
In discriminating diagnosis of PTC from FA, the diagnostic value of TROP-2, SLP-2 and CD56 immunostainings and their different combinations were presented in a form of sensitivity, specificity, PPV, NPV, and diagnostic accuracy (Table 3). Consequently, CD56 had the highest value of specificity of 93.1% and PPV of 92.9% for PTC. With the combined use of TROP-2 and CD56, the sensitivity, as well as NPV, reached 100%, and diagnostic accuracy increased to 91.8%. When assessed for triple markers, sensitivity and NPV improved to 100% as well, while specificity and PPV decreased. We further investigated whether there were possible associations between immunohistochemical expression of all investigated markers and clinicopathological characteristics (gender, age, tumor size, LNM, TNM) of PTC patients, but no any correlation was found as shown in Table 4.
Discussion
PTC is the most frequent well-differentiated thyroid carcinoma (DTC) with favourable prognosis and the 10-year survival rate is more than 90%, but still approximately 5 ~ 20% PTC might suffer the risk of recurrence and aggression. Therefore, the diagnostic methods in detecting property of thyroid nodule have been increasingly reported, such as thyroid ultrasonography, radionuclide imaging, fine-needle aspiration biopsy (FNAB), ultrasonic elastography (UE), BRAFV600E and RAS mutation detection and so on. It is widely accepted that histopathological examination is the golden standard for the diagnosis of PTC. Nevertheless, the pathological morphology sometimes cannot discriminate PTC from benign lesions which mimic the traits of PTC, we urgently need to find a more accurate method to assist diagnosis. In recent years, immunohistochemical staining has been widely applied to diagnose thyroid cancer and the well-known biomarkers [10, 11] included Glactin-3, HBME-1, FN1, cytokeratine-19, et al. However, it is not enough to provide a dependable diagnosis of malignancy because no single marker has superior sensitivity and specificity. In our study, we focus on investigating the expression of CD56, TROP-2, SLP-2 and various combinations to improve diagnostic ability for PTC.
Trophoblast cell surface protein 2 (TROP-2), also known as tumor cell-associated calcium signal transducer 2 (TACSTD2), is a transmembrane glycoprotein with 323 amino acids of about 35 kDa, which is encoded by the gene located on the human 1p32 chromosome. Trop-2 has been regarded as an oncogene which is over-expressed in various human cancers such as lung, gastric, liver, ovarian, and bladder cancers, while low expressed in normal tissues [6, 12,13,14,15]. In the early stage of 21th century, over-expression of TROP-2 could aid in distinguishing PTC from benign thyroid nodules according to molecular profiling study [16]. Subsequently, researchers [7, 8, 17] increasingly evaluated the value of TROP-2 expression in both cytological and histological thyroid specimens, and the results suggested that there was a high sensitivity and specificity for the diagnosis of PTC, especially in morphologically equivocal cases. Further analysis revealed that TROP-2 was associated with tumor-node-metastasis (TNM) staging and N classification [18]. In our study, we investigated the expression levels of TROP-2 using qRT-PCR and IHC staining. As expected, we observed that TROP-2 mRNA and protein expression in PTC tissue was significantly elevated as compared to that in FA group. It was also noteworthy that TROP-2 had the highest sensitivity (96%) and diagnostic accuracy (91.8%) among three markers we detected. Therefore, TROP-2 may be a key indicator to provide an effective evidence in differentiating malignant from benign thyroid nodules. However, be similar to two previous studies [8, 19], none of age, gender, tumor size, LNM or TNM staging was related to TROP-2 expression. We postulate that the relatively small samples lead to such results and TROP-2 may confer poor PTC prognosis like previously mentioned tumors.
Stomatin like protein 2 (SLP-2), a new member of stomatin super family with 356 amino acid residues, is identified as a mitochondrial inner membrane protein coded by the gene STOML2 localized on 9p13 chromosome. During the last several years, an increasing number of studies have claimed that SLP-2 was strongly associated with multiple human malignancies, including lung cancer, oophoroma, cervical carcinoma, gallbladder cancer, et al. [5, 20, 21]. However, very little was known with regard to thyroid tumor. Liu et al. [22] was first to report that SLP-2 was overexpressed in PTC tissues at both mRNA and protein levels. Particularly, they found that the increased SLP-2 expression was correlated with tumor > 1 cm in size, late tumor stage and LNM, but not relevant to age and gender. In addition, a recent study by Bartolome et al. [23] indicated that SLP-2 up-regulation in the aggressiveness of PTC had a close relationship with BRAF mutation, and SLP-2 might be clinically useful for the identification of high-risk PTC. Our results supported that the mRNA levels and positive stainings of SLP-2 in PTC were greater than those in FA group, but no association with the clinicopathological factors. Further studies are warranted to identify whether SLP-2 is a prognostic molecule and novel therapeutic target for PTC.
CD56 is a neural cell adhesion molecule, a transmembrane protein expressed in activated T lymphocytes, NK cells, neural and endocrine tissue. Its expression could regulate the cellular motility and migratory capacity, as well as reduce the invasion and metastasis of tumor [24]. It has been reported that CD56 expression is low or even negative in PTC tissue, whereas expressed at high levels in normal thyroid tissue or benign thyroid lesion [25, 26]. Scarpino et al. [27] investigated CD56 expression using IHC staining and PCR methods, 18 out of 61 PTC cases revealed negative immunostaining and 43 cases exhibited focal weak staining. Similarly, reduced RNA levels in PTC cases were observed in contrast to FA group. A later study performed by EI Demellawy et al. [28] showed a sensitivity of 100% and a specificity of 100% by evaluating CD56 diagnostic performance for PTC. Furthermore, CD56 not only applies to the diagnosis of classical PTC, but also to follicular variant PTC (FVPTC). In a recent study [29], 88.9% FAs displayed CD56 positive staining while only 14.3% FVPTCs had such staining, the sensitivity and specificity were around 86% and 89%, respectively. Consistent with these studies, we obtained a diffuse CD56 staining in 93.1% of FA cases, and a negative expression in 86.7% of PTCs. The high specificity and PPV of CD56 in our study (93.1 and 92.9%, respectively) exceeds the diagnostic utility of TROP-2 or SLP-2, which further pointing out that CD56 could be a relatively reliable marker in identification of PTC.
Many biomarkers are now being combined to diagnose thyroid carcinoma [30,31,32]. In our study, given that the expression of TROP-2, SLP-2 and CD56 was highly related to PTC, we subsequently assessed the value of diagnosis based on integrated staining patterns of the three antibodies. The combined results of TROP-2 positivity and CD56 negativity can optimize the diagnosis of PTC, since the sensitivity, as well as NPV, reached 100% and the diagnostic accuracy increased to 91.8%. We simultaneously discovered that the triple panels of CD56, TROP-2 and SLP-2 expression could be very helpful in identification of PTC. Therefore, a combination of two or more markers could draw a more credible and accurate conclusion, especially when a single marker confused us.
However, there are still some deficiencies that need to be further improved. Primarily, there was a lack of knowledge about comparison between follicular variant PTC and other thyroid neoplastic lesions, such as anaplastic thyroid carcinoma (ATC), medullary carcinoma (MC) and follicular neoplasms (FN), on account of the rarity of above diseases. In addition, we did not find any significant relation between the expression of TROP-2, SLP-2 or CD56 and clinicopathological factors of PTCs, supposedly because of the limited samples and lack of long-term follow-up. Further, the mechanisms of markers and their impacts on the prognosis of PTC are still not clear. In this regard, prospective studies with larger samples are needed to be performed.
In conclusion, we found that increased mRNA and protein levels of TROP-2, SLP-2, and negative staining of CD56 support the diagnosis of PTC. CD56 is the most specific but less sensitive marker, while TROP-2 is more sensitive. It is worth noting that the combined use of TROP-2, SLP-2 and CD56 may increase the diagnostic value for PTC and should be recommended.
References
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L (2016) 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26(1):1–133
Choi H, Kasaian K, Melck A, Ong K, Jones SJ, White A, Wiseman SM (2015) Papillary thyroid carcinoma: prognostic significance of cancer presentation. Am J Surg 210(2):298–301
DeLellis RA (2006) Pathology and genetics of thyroid carcinoma. J Surg Oncol 94(8):662–669
Liu H, Lin F (2015) Application of immunohistochemistry in thyroid pathology. Arch Pathol Lab Med 139(1):67–82
Wang Y, Cao W, Yu Z, Liu Z (2009) Downregulation of a mitochondria associated protein SLP-2 inhibits tumor cell motility, proliferation and enhances cell sensitivity to chemotherapeutic reagents. Cancer Biol Ther 8(17):1651–1658
Shvartsur A, Bonavida B (2015) Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer 6(3–4):84–105
Liu H, Shi J, Lin F (2017) The potential diagnostic utility of TROP-2 in thyroid neoplasms. Appl Immunohistochem Mol Morphol 25(8):525–533
Bychkov A, Sampatanukul P, Shuangshoti S, Keelawat S (2016) TROP-2 immunohistochemistry: a highly accurate method in the differential diagnosis of papillary thyroid carcinoma. Pathology 48(5):425–433
Ceyran AB, Şenol S, Şimşek BC, Sağıroğlu A (2015) Role of cd56 and e-cadherin expression in the differential diagnosis of papillary thyroid carcinoma and suspected follicular-patterned lesions of the thyroid: the prognostic importance of e-cadherin. Int J Clin Exp Pathol 8(4):3670–3680
Toy H, Etli O, Celik ZE, Sezgin AA (2017) Associations between nucleus size, and immunohistochemical Galectin-3, Cytokeratine-19 and Hbme-1 markers in thyroid papillary carcinoma: a morphometric analyze. Pathol Oncol Res
Fischer S, Asa SL (2008) Application of immunohistochemistry to thyroid neoplasms. Arch Pathol Lab Med 132(3):359–372
Stepan LP, Trueblood ES, Hale K, Babcook J, Borges L, Sutherland CL (2011) Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: potential implications as a cancer therapeutic target. J Histochem Cytochem 59(7):701–710
Guan GF, Zhang DJ, Wen LJ, Yu DJ, Zhao Y, Zhu L, Guo YY, Zheng Y (2015) Prognostic value of TROP2 in human nasopharyngeal carcinoma. Int J Clin Exp Pathol 8(9):10995–11004
Zhao W, Zhu H, Zhang S, Yong H, Wang W, Zhou Y, Wang B, Wen J, Qiu Z, Ding G, Feng Z, Zhu J (2016) Trop2 is overexpressed in gastric cancer and predicts poor prognosis. Oncotarget 7(5):6136–6145
Ambrogi F, Fornili M, Boracchi P, Trerotola M, Relli V, Simeone P, La Sorda R, Lattanzio R, Querzoli P, Pedriali M, Piantelli M, Biganzoli E, Alberti S (2014) Trop-2 is a determinant of breast cancer survival. PLoS One 9(5):e96993
Finley DJ, Arora N, Zhu B, Gallagher L, Fahey TJ (2004) Molecular profiling distinguishes papillary carcinoma from benign thyroid nodules. J Clin Endocrinol Metab 89(7):3214–3223
Simms A, Jacob RP, Cohen C, Siddiqui MT (2016) TROP-2 expression in papillary thyroid carcinoma: potential diagnostic utility. Diagn Cytopathol 44(1):26–31
Guan H, Guo Z, Liang W, Li H, Wei G, Xu L, Xiao H, Li Y (2017) Trop2 enhances invasion of thyroid cancer by inducing MMP2 through ERK and JNK pathways. BMC Cancer 17(1):486
Kong JS, Kim HJ, Kim MJ, Kim A, Lee D, Han K, Park S, Koh JS, Myung JK (2018) The significance of TROP2 expression in predicting BRAF mutations in papillary thyroid carcinoma. J Pathol Transl Med 52(1):14–20
Xiao B, Xie Z, Guo L, Wu J, Zhang H (2015) Stomatin-like protein 2 expression is associated with clinical survival in patients with cervical cancer. Int J Clin Exp Pathol 8(2):1804–1809
Wang WX, Lin QF, Shen D, Liu SP, Mao WD, Ma G, Qi WD (2014) Clinicopathological significance of SLP-2 overexpression in human gallbladder cancer. Tumour Biol 35(1):419–423
Liu Z, Yang Y, Zhang Y, Ye X, Wang L, Xu G (2014) Stomatin-like protein 2 is associated with the clinicopathological features of human papillary thyroid cancer and is regulated by TGF-β in thyroid cancer cells. Oncol Rep 31(1):153–160
Bartolome A, Boskovic S, Paunovic I, Bozic V, Cvejic D (2016) Stomatin-like protein 2 overexpression in papillary thyroid carcinoma is significantly associated with high-risk clinicopathological parameters and BRAFV600E mutation. APMIS 124(4):271–277
Prag S, Lepekhin EA, Kolkova K, Hartmann-Petersen R, Kawa A, Walmod PS, Belman V, Gallagher HC, Berezin V, Bock E, Pedersen N (2002) NCAM regulates cell motility. J Cell Sci 115(Pt 2):283–292
Nechifor-Boila A, Borda A, Sassolas G, Hafdi-Nejjari Z, Borson-Chazot F, Lifante JC, Sturm N, Lavérriere MH, Berger N, Decaussin-Petrucci M (2013) Immunohistochemical markers in the diagnosis of papillary thyroid carcinomas: The promising role of combined immunostaining using HBME-1 and CD56. Pathol Res Pract 209(9):585–592
Ozolins A, Narbuts Z, Strumfa I, Volanska G, Stepanovs K, Gardovskis J (2012) Immunohistochemical expression of HBME-1, E-cadherin, and CD56 in the differential diagnosis of thyroid nodules. Medicina (Kaunas) 48(10):507–514
Scarpino S, Di NA, Melotti F, Talerico C, Cancrini A, Ruco L (2007) Papillary carcinoma of the thyroid: low expression of NCAM (CD56) is associated with downregulation of VEGF-D production by tumour cells. J Pathol 212(4):411–419
El DD, Nasr AL, Babay S, Alowami S (2009) Diagnostic utility of CD56 immunohistochemistry in papillary carcinoma of the thyroid. Pathol Res Pract 205:303–309
Abouhashem NS, Talaat SM (2017) Diagnostic utility of CK19 and CD56 in the differentiation of thyroid papillary carcinoma from its mimics. Pathol Res Pract 213(5):509–517
Erdogan-Durmus S, Ozcan D, Yarikkaya E, Kurt A, Arslan A (2016) CD56, HBME-1 and cytokeratin 19 expressions in papillary thyroid carcinoma and nodular thyroid lesions. J Res Med Sci 21:49
Murtezaoglu AR, Gucer H (2017) Diagnostic value of TROP-2 expression in papillary thyroid carcinoma and comparison with HBME-1, galectin-3 and cytokeratin 19. Pol J Pathol 68(1):1–10
Pyo JY, Choi SE, Shin E, Koo J, Hong S (2017) The Intraoperative Immunohistochemical Staining of CD56 and CK19 Improves Surgical Decision for Thyroid Follicular Lesions. J Pathol Transl Med 51(5):463–470
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This work was economically supported by grants from the Project of the peak of Six Personnel in Jiangsu Province. The authors declare that there is no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Yang, X., Hu, Y., Shi, H. et al. The diagnostic value of TROP-2, SLP-2 and CD56 expression in papillary thyroid carcinoma. Eur Arch Otorhinolaryngol 275, 2127–2134 (2018). https://doi.org/10.1007/s00405-018-5045-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-018-5045-x