Abstract
Seasonal ponds are common throughout forested regions of the north central United States. These wetlands typically flood due to snow-melt and spring precipitation, then dry by mid-summer. Periodic drying produces unique fishless habitats with robust populations of aquatic invertebrates. A basin’s physical/chemical features, the absence of vertebrate predation, and especially the duration of seasonal flooding, have long been viewed as the major structuring influences on these communities, but previous studies have shown only limited effects of environmental variables on pond invertebrates. Applying ordination methods to data from weekly collections of invertebrates during 2008–2009, we tested influences of site-level environmental gradients on the presence and relative abundance of aquatic invertebrate communities in 16 seasonal ponds in a forested region of north central Minnesota, USA. We assessed invertebrate community patterns in relation to pond size and depth, soil nutrients, canopy closure, hydroperiod, and predominant groundwater function (recharge, discharge, or flow-through). Patterns in pond invertebrate community composition were consistently related to pond depth, overhead canopy closure, and hydroperiod. Site-level hydrologic function showed weak relationships to seasonal patterns of invertebrate abundance. Although physical features of ponds had only modest influence on presence and abundance of invertebrates, weekly sampling improved models relating environmental variables to pond invertebrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seasonal ponds are shallow depressional wetlands underlain by bedrock or semi-impervious soil horizons and are common features in the north central US where late-Wisconsin glaciation left numerous small depressions in some forested landscapes (Brooks 2000; Palik et al. 2003). These wetlands are typically <1 m in depth and range from 0.1 to 0.25 ha in surface area, rarely approaching 1.0 ha (Palik et al. 2001; Brooks 2005). Small size means a high perimeter-to-area ratio which could increase sensitivity of resident communities to disturbances in adjacent uplands (Palik et al. 2001; Brooks 2005; Williams 2005).
When seasonal ponds occur in forested landscapes, their invertebrate communities comprise a large fraction of regional biodiversity (Williams 2005). The short hydroperiods that exclude most vertebrate predators are believed to be key environmental drivers of invertebrate community structure in seasonal ponds (Collinson et al. 1995; Brooks 2000; Batzer et al. 2004; Williams 2005). Nonetheless, annual hydroperiods differ considerably among pond sites and communities may vary accordingly. For example, Brooks (2000) reported increased taxon richness for aquatic invertebrate communities in wetlands having especially short and long hydroperiods. Batzer et al. (2004) described a positive relationship between taxon richness and hydroperiod length, but this resulted from rare taxa occurring only in ponds with extended hydroperiods. Hanson et al. (2009) reported hydroperiod to be related to invertebrate community patterns in seasonal ponds, but could attribute little variance to flooding duration. Patterns may also be complicated by the fact that influences of hydroperiod can transcend individual growing seasons. For example, Batzer et al. (2004) and Brooks (2000) reported that a year with unusually short hydroperiods can lead to reduced abundance of benthic invertebrates during the subsequent year.
Conservation of seasonal ponds has become an important forest management concern during the past two decades and has stimulated considerable research on these pond communities in the north central US (Minnesota Forest Resources Council 2007) and elsewhere (Colburn 2004). Resulting studies have shown that invertebrate communities in seasonal ponds are influenced by physical and chemical characteristics of sites (hydroperiod, maximum depth, pH) and adjacent uplands (overhead canopy closure, litter inputs, stand age). However, a consistent theme of this research is that even statistically significant relationships between pond communities and environmental variables usually explain little variance in invertebrate presence and abundance patterns (Palik et al. 2001; Batzer et al. 2004; Hanson et al. 2009). The inability of previous studies to identify strong relationships between environmental gradients and abundance and diversity in aquatic invertebrate communities may have resulted from failure to include factors responsible for much of the variance in communities. In addition, weak relationships between physical factors and biological communities may reflect limitations of sampling and analytical approaches. However, the resulting uncertainty is disconcerting given that adjacent vegetation management such as timber harvest may rapidly modify overhead pond canopies, litter inputs, and other features which are consistent although minor sources of pond community variance.
Earlier efforts may have underestimated two key aspects important to invertebrate community dynamics. First, within-year variability is a major source of variance in presence and abundance of aquatic invertebrates, especially in temporary habitats. Miller et al. (2008) illustrated that chronological shifts in community composition create high temporal variability. Thus, timing and frequency of sampling have potential to dramatically affect community analyses. For logistical reasons, studies of seasonal pond invertebrates are usually limited by infrequent sampling with intervals of 2 weeks to a month or more between data gathering events (Batzer et al. 2000; Brooks 2000; Batzer et al. 2004; Miller et al. 2008; Hanson et al. 2009). These approaches almost certainly influence study outcomes and may limit detection of environmental relationships or even mask community responses to disturbance. Second, despite the likely importance of site hydrology, status of groundwater dynamics and influences of groundwater are poorly known for seasonal ponds in northern forested landscapes (Kolka et al. 2011). Likewise, the extent to which groundwater exchange influences biological communities in seasonal ponds has not been described.
Our broad objective was to identify factors responsible for presence and abundance patterns in seasonal pond invertebrates in a way that more thoroughly accounts for within-year community variability and hydrology, along with other variables previously shown to be important. We used two strategies to build upon results of earlier studies. First, we assessed temporal and spatial variation in seasonal pond invertebrates by simultaneously sampling pond invertebrate communities in two ecoregions at 7-day intervals over periods of 7 to 8 weeks during 2008 and 2009. Second, we quantified site hydrology. We measured hyroperiod and groundwater levels in piezometers and wells beginning immediately after spring snow melt until freeze-up in the late fall to determine specific relationships between groundwater exchange (e.g. discharging or recharging) with waters in pond basins. We used gradient analysis to relate invertebrates to known hydrologic relationships (recharge, discharge, flow-through, perched) and other environmental variables. We restricted these ordinations to variables previously shown to influence these communities such as hydroperiod, canopy cover, stand-age, pond surface area, and maximum depth. We hypothesized that models of relationships between environmental gradients and patterns of invertebrates in pond sites would be improved by inclusion of weekly invertebrate and hydrologic data.
Methods
We studied 16 seasonal ponds located in two forested Ecological Subsections (Chippewa Plains and Pine Moraines/Outwash Plains in north central Minnesota, USA (Keyes et al. 1995; Almendinger et al. 2000; Fig. 1). This area is heavily forested and lies within a complex matrix of ground and stagnation moraines, along with lake and outwash plains. Soil composition here is highly variable, with loamy clay in moraines and sands on outwash plains. The entire region is overlain by thick glacial till (up to 100 m) and contains abundant wetlands and lakes. All ponds were surrounded by forest stands predominantly comprised of aspen (Populus spp.) aged from 10 to >65 years since harvest. Vegetation patterns and history of these landscapes are described in more detail by Hanson et al. (2009).
Seasonal Pond Characteristics
We delineated pond perimeters using a global positioning system. Resulting data points were uploaded to a geographic information system and surface area was calculated using an ArcGIS Measure Tool (ArcGIS 9.2, Environmental Systems Research Institute Inc. 2007).
Canopy cover above each pond was measured during peak leaf cover each year using a spherical densiometer along two random intersecting transects. Percent canopy cover values were recorded at five locations along each transect, with the third value of each transect measured at the pond center. Average % canopy cover for each pond site was calculated from these measurements.
Hydroperiod was determined as consecutive days of flooding at the deepest point in each pond. We defined hydroperiod onset as the date of the first site visit when at least 20 % of the pond basin contained standing water. Staff gauges constructed of 2.54 cm diameter PVC pipe were installed in the deepest point in each pond (Fig. 2) to measure standing water levels. When water levels receded below ground, center monitoring wells (described below) were used to measure groundwater levels. We began recording weekly water levels immediately after spring snow melt and continued through August or until ponds dried. From September until freeze-up, we recorded pond water level readings every other week (or until water levels fell beneath readable depths). Hydroperiod end dates were considered as the first date when less than 20 % of the seasonal pond basin contained standing water. In the rare event that a pond basin did not dry, we recorded the hydroperiod end date as the date when standing waters froze, usually during late October or early November.
Soil characteristics were determined from samples collected using a soil corer at the deepest point of each pond and at one randomly chosen location along the delineated boundary. These two cores were composited and were analyzed in the lab for concentrations of total phosphorous using the Bray and Kurtz Method (Bray and Kurtz 1945), total nitrogen and carbon by combustion (Yeomans and Bremner 1991), and percent clay using the hydrometer method for particle size analysis (Gee and Bauder 1986) (Fig. 3).
Invertebrate Sampling
We sampled aquatic invertebrates during spring-summer 2008 and 2009. Sampling began each year on 14 May, approximately 2 to 4 weeks after snow melt, when ponds contained standing water and remained consistently ice-free. Each year, we sampled weekly through early July when ponds began to dry. Invertebrates were collected from all ponds once a week for 8 weeks in 2008 and 7 weeks in 2009.
To estimate relative invertebrate abundance and diversity, we sampled invertebrates in open water using surface activity traps (SATs, Hanson et al. 2000). Use of SATs is advantageous for sampling invertebrates in shallow wetlands because these devices collect organisms associated with the surface film (e.g., Culicidae, Corixidae, Gerridae, Gyrinidae) as well as those present in the underlying water column (e.g., cladocerans, copepods, Ostracoda) (Hanson et al. 2000). Using SATs, as opposed to sweep nets or vertical cores, also reduced the amount of organic matter and sediment inadvertently collected, resulting in cleaner samples (Hanson et al. 2000). This improved sample processing efficiency, allowing for intensive (weekly) sampling. Each week, two SATs were deployed from PVC frames permanently installed along two random transects in each pond. The SAT on the first transect was deployed 25 % of the distance from the delineated boundary to the pond center. A second SAT was placed on a second transect approximately 25 % of the distance from the center of the pond. When emergent hydrophytes were present at the sampling point, the SAT was positioned along the transect at the deep margin of the vegetation. Traps were retrieved after approximately 24 h; contents were concentrated by passage through 0.04 mm-mesh sieves and stored in 70 % ETOH. All ponds were sampled on the same day (within 12 h) during each week of sampling.
Invertebrates were sorted and identified using stereomicroscopes. Insects were typically identified to family and crustaceans were identified to genus using Merritt et al. (2008) and Thorp and Covich (2001). Numbers of invertebrates in the two SATs were summed for each pond by sampling period; resulting totals and taxon richness values were used for all analyses. Before gradient analysis and to simplify interpretation by reducing noise in our data sets (McCune and Grace 2002), we grouped invertebrates into 20 taxonomic categories based on abundance and feeding guilds following Merritt et al. (2008).
Groundwater Monitoring
We monitored hydraulic heads using piezometers and the upper limit of local water tables using wells. Piezometers and monitoring wells were installed following the methods of Sprecher (2000) in each of the 16 seasonal ponds. Nests, each containing one shallow piezometer (60 cm below ground surface), one deep piezometer (120 cm below ground surface), and one monitoring well (120 cm below ground surface), were deployed at four locations along the delineated wetland boundary (Fig. 2). A fifth nest was installed at the deepest point in each pond, adjacent to the staff gauge. Water levels in piezometers and monitoring wells were used to determine the predominant hydrologic function of each pond (i.e. discharge, recharge, flow-through, or perched).
Environmental characteristics including maximum pond depth (Zmax; cm), hydroperiod, canopy cover (%), pond surface area, phosphorous (mgkg−1), total soil nitrogen (%), total soil carbon (%), average clay (%) observed in seasonal forest ponds. Box plots depict mean and range of values during 2008–2009
Piezometers were constructed from 2.54 cm diameter PVC piping glued to prefabricated piezometer tips (purchased from Forestry Suppliers). Monitoring wells were also constructed of 2.54 cm diameter PVC piping with perforations along 120 cm of the underground portion of each pipe and the bottom capped with a perforated PVC cap. Fabric mesh was added over perforated portions of each monitoring well to exclude sand and debris. Holes for the wells and piezometers were bored using a hand auger (diameter 7.62 cm). Approximately 0.5 L of silica sand was added to the bottom of each hole. Wells or piezometers were then placed in the holes to the appropriate depth, and sand was added until piezometer tips or perforated lengths of the wells were covered, allowing free flow of groundwater. Bentonite clay was added above the sand to all piezometers and wells to form an upper seal and to prevent water seepage from above. Dredged soil was used to fill around the remainder of the piezometer, and was tamped firmly in place to eliminate air cavities. At piezometers, a second bentonite clay seal was added around the piping from about 15.25 cm below the ground surface, upward until it formed a small mound just above the soil surface to further prevent any surface water seepage. A single bentonite clay seal was also added to wells in the same manner, to prevent inflow of surface water. Pipe openings were covered with vented caps to prevent rain water or insects from entering, while allowing air equilibrations.
Elevations of piezometers, monitoring wells, and staff gauges in each wetland were surveyed. Depths to groundwater were measured from tops of pipe extensions to water levels in wells or piezometers. Surveyed elevations of piezometers and monitoring wells were then used to determine water table elevations and hydraulic heads relative to the deepest region of each seasonal pond.
Groundwater level recordings began soon after snow melt and within a few days following ground thaw. Water levels in piezometers and wells were recorded weekly, typically from early May to August. Beginning in September groundwater levels were recorded every 2 weeks until freeze up, typically in early November. Groundwater levels were determined using a thimble (open end down) attached to a pliable fabric measuring tape. The thimble was lowered into a piezometer or well until a popping sound was produced upon contact with the water surface. We used water levels in the piezometers in conjunction with levels in the monitoring wells to track and estimate the predominant hydrologic function of each pond (i.e. discharge, recharge, flow-through, perched) (Sprecher 2000).
Statistical Analysis
We assessed annual patterns in invertebrate communities using non-metric multidimensional scaling (NMS). This analysis is especially useful for depicting similarity in biological community patterns (Clarke 1993; McCune and Grace 2002). Given our weekly sampling, resulting vector plots illustrated the seasonal chronologies of taxon presence and abundance in these pond communities. We plotted vectors separately by years and Ecological Subsections to show how seasonal chronologies might differ annually and between regions. The NMS was performed using PC-ORD version 5.0 (McCune and Mefford 1999). We used Sorensen (Bray-Curtis) distance measures, and evaluated six preliminary dimensions with 250 iterations; final ordinations included two or three dimensions (axes) based on stress reduction and were adjusted with varimax rotation to allow meaningful interpretation of variance loading on axes (McCune and Mefford 1999). Stress values of final ordinations were ≤11, indicating that NMS representations may be considered reliable (McCune and Grace 2002). Final axes were tested for significance using Monte Carlo permutation tests (P for all final axes ≤0.05). Resulting plots illustrated seasonal patterns of presence and abundance of invertebrates within and among the two study years, and in each Ecological Subsection. We also examined correlations between final ordination axes and weighted average scores of invertebrate taxa to identify seasonal patterns. The gradients depicted by the axes reflect similarities and differences in invertebrate community scores (McCune and Grace 2002). Taxa that exhibited the strongest correlation with NMS axes have comparatively greater influence on community patterns based on presence and abundance.
We used a similar NMS approach to depict seasonal invertebrate presence and abundance chronologies for each hydrologic pond function (recharge, flow-through, perched). Plots again illustrated temporal changes in invertebrates, but with separate chronology vectors for each hydrologic pond function and Ecological Subsection. We again used Sorenson distance measures, evaluated six preliminary axes, applied varimax rotation, and used Monte Carlo permutation tests to identify associations between organisms and ordination axes.
Finally, we assessed relationships between patterns of invertebrate abundance and environmental variables using direct gradient analysis (redundancy analysis, RDA). All RDAs were run in Canoco 4.54 (ter Braak and Smilauer 2002). We were especially interested in testing for relationships with pond hydrologic functions (recharge, flow-through, perched) and other environmental variables. We chose RDA because preliminary detrended correspondence analysis identified relatively short gradients in our species data (<2.0 SD), indicating appropriateness of a linear model. We tested significance of environmental variables for inclusion in final models using manual forward selection procedures and randomization (Monte Carlo) tests (ter Braak and Smilauer 2002). Preliminary environmental variables included: pond hydrologic function (recharge, flow-through, perched), Ecological Subsection, % canopy cover, hydroperiod (previous year), maximum depth, stand age, pond surface area, total soil phosphorus, total soil nitrogen, total soil carbon, and average % clay for upland soil horizons. Final models included only three environmental variables (maximum depth, canopy cover, hydroperiod) shown by preliminary forward selection to be significantly associated with invertebrate community pattern (P ≤ 0.05).
Results
Hydrological data from the 16 seasonal ponds indicated high, but variable exchange with underlying groundwater and conditions ranging from recharge to flow-through, but showed that two ponds remained perched in both study years. We classified ponds as flow-through if at least one piezometer nest displayed groundwater discharge and another nest concurrently indicated groundwater recharge during the standing-water period. Based on predominant exchange conditions across study years, seven sites were classified as flow-through and seven as recharge. However, flow-patterns varied between study years, with 13 and ten sites having some groundwater discharge to their basins due to the formation of temporal groundwater mounds during 2008 and 2009, respectively. Hydrologic results are discussed in greater detail in Bischof (2011).
We collected 76 invertebrate taxa during 2008 and 2009. This taxon richness estimate is conservative because insects were usually identified to family and crustaceans to genus. Invertebrate samples were dominated by aquatic insects and crustaceans. Diptera larva were most widespread with mosquitos (Culicidae), phantom midges (Chaoboridae), and non-biting midges (Chironomidae) collected in all ponds. Insects collected in >50 % of our sites were, Anisoptera, Dixidae, Dytiscidae, Corixidae, Gerridae, Gyrinidae, Haliplidae, Hydrophilidae, Nepidae, Notonectidae, Sciomyzidae, Stratiomyidae, Tabanidae, Tipulidae, Trichoptera, Veliidae, and Zygoptera. Daphnia and Simocephalus were the most widespread crustaceans and were collected in all our sites. Other common crustaceans (collected in >50 % of our ponds) were fairy shrimp (Eubranchipus), clam shrimp (Diplostraca), seed shrimp (Ostracoda), various cladocerans, and cyclopoid copepods. Snails (Gastropoda) and fingernail clams (Sphaeridae) were also common in study ponds. Invertebrate communities included members of all general feeding groups described by Merritt et al. (2008).
Temporal and Spatial Patterns in Invertebrates
Non-metric multidimensional scaling (NMS) reflected differences in seasonal patterns of invertebrate presence and abundance between 2008 and 2009, and between Ecological Subsections. A two-dimensional solution (Table 1) was found using NMS, with axes 1 and 2 explaining 65 and 24 % of the variance, respectively (cumulative R 2 = 0.90; Table 2). Resulting patterns show considerable dissimilarity in invertebrate communities during early-mid-season, especially between Ecological Subsections during 2008 (Fig. 4a). Seasonal weighted average scores in 2008 and 2009 for Eubranchipus, Culicidae, and Trichoptera confirm that these were early season residents in our study ponds. Scores for other taxa clustered centrally along both axes, suggesting that these groups were collected from all ponds throughout the mid to later portions of each season (Fig. 4a).
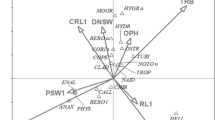
Invertebrate community scores (symbols) from non-metric multidimensional scaling. Lines are connected forming chronological vectors depicting time, hence show seasonal pattern of invertebrate community composition. Grey circles represent weighted averages of the combined 2008 and 2009 invertebrate taxon scores as labeled. Panel a–separate lines illustrate chronosequence for each year (2008 [solid] and 2009 [dashed]) and ecological subsection. Panel b–separate lines represent years and ponds with similar hydrologic function including recharge (light dash), flow-through (heavy dash) and perched (solid). Culicidae and Eubranchipus are highlighted because they shown most separation from other taxa. Only the first two axes are shown
Invertebrate groups showing at least moderate (R 2 > 0.30) correlations with NMS axes were Culicidae, Diptera (mixed or non-predacious), Trichoptera, Hemiptera (predacious), Coleoptera (predacious), Coleoptera (mixed or non-predacious), crustaceans (Eubranchipus, and Copepoda), Mollusca (Sphaeridae), and Hirudinea (Table 2). Highest NMS axis correlations were for Culicidae (R 2 = 0.91, axis 1), Eubranchipus (R 2 = 0.73, axis 1), non-predacious Coleoptera (R 2 = 0.78, axis 1), predacious Coleoptera (R 2 = 0.60, axis 2), predacious Hemiptera (R 2 = 0.58, axis 2) and Trichoptera (R 2 = 0.53, axis 2). Higher R2 values suggested that presence and abundance of these taxa had relatively greater influence on chronological community patterns in our sites.
Invertebrate Community Patterns and Hydrologic Context
We also used NMS to relate invertebrate community composition to hydrologic pond function (recharge, flow-through and perched). Our final NMS model identified a three-dimensional solution (Table 3) with three axes explaining approximately 46, 33, and 15 % of the variance with a cumulative R 2 = 0.94 (Table 4). Our ordinations showed little consistent separation between recharge and flow-through ponds. However, perched sites diverged somewhat from those with other hydrologic functions and this relationship is evident based on the first two axes in both 2008 and 2009 (Fig. 4b). Several invertebrate groups showed at least moderate (R 2 > 0.30) association with the NMS axes including, aquatic insects (Culicidae, Trichoptera, predacious Hemiptera), Hydracarina, all crustaceans (Eubranchipus, Diplostraca, Ostracoda, cladocerans, Copepoda), Sphaeridae, and Hirudinea. Weighted averages of Culicidae and Hirudinea showing strongest associations with axes in the final hydrologic NMS model (R 2 = 0.62, axis 2, and R 2 = 0.55, axis 2, respectively) (Table 4).
General Relationships with Environmental Variables
Several environmental variables indicated by our RDA were significantly associated with invertebrate presence and abundance patterns during 2008 and 2009. Maximum pond depth, percent canopy cover, and hydroperiod were all significant sources of variance in 2008 and 2009, (Fig. 5a, b Table 5). Our RDA did not identify hydrologic function (recharge, flow-through, discharge) as a significant source of variance in invertebrate patterns during 2008 or 2009 (P > 0.05), so it was not included in our final RDA models.
Plots of final repeated measures redundancy analysis (rRDA) models of pond invertebrate communities using the three environmental variables that explained the most variance. Length of dashed vectors indicates strength of relationships between axes and environmental variables. Solid arrows indicate direction of sharpest increase in abundance of aquatic invertebrate groups. Panel (a) displays 2008 results; panel (b) displays 2009 results
Still, invertebrate taxa showed relatively distinct yet variable associations with several other environmental variables. For example, Pulmonata, Culicidae, Eubranchipus, and Diplostraca were positively associated with increasing pond maximum depth during 2008, but only Diplostraca and cladocerans showed similar associations during 2009. Non-predatory Coleoptera, predatory Diptera, predatory Hemiptera, and Odonata showed increased abundance in ponds with longer hydroperiods during 2008, but none of these relationships were similarly strong during 2009. Several taxa (Chaoboridae, non-predatory Diptera, Ostracoda, and pulmonate snails) showed opposite patterns, achieving higher abundance in ponds with shorter hydroperiods, at least during 2008 (Fig. 5a, b). No taxa showed strong positive associations with increasing canopy cover (%); however, several taxa (Chironomidae, non-predatory Hemiptera, and Hydracarina) did show negative associations with canopy cover in 2008 and perhaps 2009.
Discussion
Because our present analyses more thoroughly integrated influences of seasonal chronology of taxa and pond hydrology relative to earlier work, our results provide two points of clarification regarding constraints on aquatic invertebrate communities in seasonal ponds. First, ordinations indicated that hydrologic pond function (and related site-level soil characteristics) had only a weak influence on presence and abundance patterns of invertebrates in our 16 pond sites. General similarity between invertebrate communities of recharge and flow-through ponds was found using NMS (although dissimilarity was apparent between two perched sites and the remaining 14 ponds with some groundwater exchange). Associations between invertebrate community patterns and environmental gradients were identified by our RDA models (maximum pond depth, overhead canopy cover, and hydroperiod), but did not indicate that groundwater function was a significant source of invertebrate community variance. Among significant environmental variables identified by RDA, maximum depth had the most influence on invertebrates and explained 14 and 19 % of the variance during 2008 and 2009. Percent canopy cover and hydroperiod explained 11–12 % and 9–11 % of the variance respectively. Pond hydrologic function apparently had little influence on these patterns, although two ponds without groundwater exchange (perched) showed relatively less similarity to recharge and flow-through sites. This may indicate that hydrologic function was not a primary cause of high unexplained variance described by similar studies (Williams 1996; Batzer et al. 2004; Hanson et al. 2009).
Second, our NMS models showed strong patterns of seasonal (weekly) variability in both Ecological Subsections during both study years. During late May, invertebrate communities were dominated by detritivores and herbivores (Eubranchipus, Culicidae and Trichoptera), with predators and scavengers such as Hemiptera becoming more prevalent later in each season (similar to patterns suggested by Wiggins et al. 1980). Our results are in line with Miller et al. (2008) who reported that Culicidae, Chaoboridae, and Dixidae along with Eubranchipus sp., Trichoptera and Hydracarina were early-season residents of seasonal ponds near Remer, Minnesota, within 150 km of our sites. These findings have profound implications for sampling and data interpretation and point to a need for careful consideration of this temporal variance component (as suggested by Williams 1996 and Miller et al. 2008).
More broadly, weekly sampling may have better captured patterns of temporal variability in invertebrates and improved our RDA models of community structure. For example, Batzer et al. (2004) described a similar direct gradient analysis (CCA) of seasonal pond invertebrate communities and reported that environmental variables (tree basal area, hydroperiods, wetland nutrients, and others) cumulatively explained approximately 25 % of invertebrate variance in their first two ordination axes. Using a similar approach, Hanson et al. (2009) reported that environmental variables (canopy openness, wetland total phosphorus, alkalinity) explained only about 10 % of the variance in invertebrate communities in pond sites (first two axes following RDA). In the present analysis, our first two ordination axes (based on three environmental variables: maximum depth, overhead canopy cover, and hydroperiod) explained approximately 38 % of the variance in presence and abundance of pond invertebrates.
Finally, we noted high variability in relationships between taxon scores and environmental vectors in RDA plots. For example, Eubranchipus was positively associated with maximum pond depth during 2008, but showed an opposite trend in 2009. This variability is puzzling and may indicate that important pond characteristics were not measured, or that ranges of values along gradients were simply within limits tolerated by these organisms. We do know that extreme seasonal variability is common in seasonal ponds and, along with multiple causal mechanisms, this complicates data interpretation and comparisons among studies. It is also likely that time required to process samples gathered using sweep nets or benthic cores makes application of these more quantitative methods less practical if frequent sampling is necessary.
We believe our study may be the first reporting results of community patterns in seasonal pond invertebrates in relation to groundwater function. Previous authors emphasized more general indices of hydrology (often consecutive days and seasonal patterns of ponding) on aquatic communities in seasonally flooded habitats (Wiggins et al. 1980; Wellborn et al. 1996; Williams 1996; Schneider 1999). Hydroperiod often reflects groundwater exchange with ponds, yet is still a vague metric of pond hydrology. Groundwater exchange with ponds may have more complex effects on the chemistry of pond waters, therefore possible influences on invertebrate communities, in ways not depicted by flooding duration. Some work suggests formation of spring groundwater mounds adjacent to seasonal ponds, creating hydraulic gradients toward ponds, and seasonal groundwater discharge to these sites (Phillips and Shedlock 1993; Hanes and Stromberg 1998). Alternatively, during summer dry periods, groundwater mounds typically disappear, (Anderson and Munter 1981; Winter 1981), hydraulic gradients shift towards adjacent uplands, and ponds recharge to groundwater. Water levels sometimes decrease rapidly from June to July when adjacent forest stands maximize leaf area, and thus evapotranspiration (Huntington 2003; Brooks 2004, 2005). Rapid changes in water chemistry or physical properties of ponds may influence invertebrate communities in ways that are not discernable when data are limited to hydroperiod length.
Aquatic invertebrates tolerate broad ranges of environmental conditions (Batzer et al. 2004; Hanson et al. 2009). Weak, even non-significant relationships between invertebrates in seasonal ponds and site-level pond or upland characteristics are commonly reported for studies of these communities (Batzer et al. 2000; Palik et al. 2001; Batzer et al. 2004; Hanson et al. 2009). Alternatively, Williams (1996, p. 646) suggested that descriptions “of temporary waters constraining their faunas is based more on human perception than on fact”. This may result from high tolerance of the invertebrates to extreme and variable environmental conditions as long as there is some period of ponding (Wissinger et al. 1999; Batzer et al. 2000, 2004; Hanson et al. 2009).
Groundwater function and climate likely do influence biological communities of seasonal forest ponds as they do wetlands in the Prairie Pothole Region and elsewhere (Euliss et al. 2004). We speculate that relationships among seasonal pond communities, groundwater function, and other environmental gradients (chemical and physical features) are probably more subtle in forested regions. Simplistic measures of hydroperiod and groundwater inflow or outflow to ponds appear to be weakly associated with faunal patterns. Our results indicate that knowledge of even specific hydrological metrics does not explain much variance in the composition of invertebrate communities in seasonal ponds. Future efforts to understand and conserve seasonal pond communities may benefit more from new approaches linking landscape patterns such as geographical isolation with details of habitat requirements, and perhaps analytical innovations to improve models for communities whose populations fluctuate dramatically over short time periods. We suggest that such approaches may be more fruitful than additional attempts to clarify mechanistic links between pond communities and environmental factors at small geographic scales. Nonetheless, our results may inform modelers and land managers who consider how these invertebrate communities may respond to future climatic and wetness regimes that are projected for the region.
References
Almendinger JC, Hanson DS, Jordan JK (2000) Landtype associations of the Lake States. Minnesota Department of Natural Resources, Saint Paul
Anderson MP, Munter JA (1981) Seasonal reversals of groundwater flow around lakes and the relevance to stagnation points and lake budgets. Water Resour Res 17:1139–1150
Batzer DP, Jackson R, Mosner M (2000) Influences of riparian logging on plants and invertebrates in small, depressional wetlands of Georgia. Hydrobiologia 441:123–132
Batzer DP, Palik BJ, Buech R (2004) Relationships between environmental characteristics and macroinvertebrate communities in seasonal woodland ponds of Minnesota. J N Am Bentholl Soc 23:50–68
Bischof MM (2011) Influence of adjacent uplands and groundwater on the hydrology and invertebrate community composition of seasonal ponds in north central Minnesota. MS Thesis, North Dakota State University
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–45
Brooks RT (2000) Annual and seasonal variation and the effect of hydroperiod on benthic macroinvertebrates of seasonal forest (“vernal”) ponds in central Massachusetts. Wetlands 20:707–715
Brooks RT (2004) Weather-related effects on woodland vernal pool hydrology and hydroperiod. Wetlands 24:104–114
Brooks RT (2005) A review of basin morphology and pool hydrology of isolated ponded wetlands: implications for seasonal forest pools of the northeastern United States. Wetl Ecol Manag 13:335–348
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Colburn E (2004) Vernal pools: ecology and conservation. McDonald and Woodward Publishing Company, Granville
Collinson NH, Biggs J, Corfield A, Hodson MJ, Walker D, Whitfield M, Williams PJ (1995) Temporary and permanent ponds: an assessment of the effects of drying out on the conservation value of aquatic macroinvertebrate communities. Biol Conserv 74:125–133
Environmental Systems Research Institute Inc. (2007) What’s new in ArcGIS 9.2. Environmental Systems Research Institute. http://www.esri.com
Euliss NH Jr, LaBaugh JW, Fredrickson LH, Mushet DM, Laubhan MK, Swanson GA, Winter TC, Rosenberry DO, Nelson RD (2004) The wetland continuum: a conceptual framework for interpreting biological studies. Wetlands 24:448–458
Gee GW, Bauder JW (1986) Particle size analysis. In: Klute A (ed) Methods of soil analysis. Part 1. Physical and mineralogical methods. Agronomy Monograph No. 9, 2nd edn. ASA and SSSA, Madison, pp 825–844
Hanes T, Stromberg L (1998) Hydrology of vernal pools on non-volcanic soils in the Sacramento Valley. In: Witham CW, Bauder ET, Belk D, Ferren WR, Ornduff R (eds) Ecology, conservation and management of vernal pool ecosystems-proceedings of the 1996 conference. California Native Plant Society, Sacramento, pp 38–40
Hanson MA, Roy CC, Euliss NH Jr, Zimmer KD, Riggs MR, Butler MG (2000) A surface associated activity trap for capturing water-surface and aquatic invertebrates in wetlands. Wetlands 20:205–212
Hanson MA, Bowe SE, Ossman FG, Fieberg J, Butler MG, Koch R (2009) Influences of forest harvest and environmental gradients on aquatic invertebrate communities of seasonal ponds. Wetlands 29:884–895
Huntington TG (2003) Climate warming could reduce runoff significantly in New England. Agric For Meteorol 117:193–201
Keyes J Jr, Carpenter C, Hooks S, Koenig F, McNab WH, Russell W, Smith ML (1995) Ecological unit of the Eastern United States-first approximation (map and booklet of map unit tables). U.S. Forest Service, Atlanta
Kolka RK, Palik BJ, Tersteeg DP, Bell JC (2011) Effects of riparian buffers on hydrology of northern seasonal ponds. Trans Am Soc Agric Biol Eng (ASABE) 54:2111–2116
McCune B, Grace JB (2002) Analysis of ecological communities. MJM Software Design, Gleneden Beach
McCune B, Mefford MJ (1999) PC-ORD. Multivariate analysis of ecological data, version 4. MjM Software Design, Gleneden Beach
Merritt RW, Cummins KW, Berg MB (2008) An introduction to the aquatic insects of North America. Kendall Hunt, Dubuque
Miller TM, Hanson MA, Church JO, Palik B, Bowe SE, Butler MG (2008) Invertebrate community variation in seasonal forest wetlands: implications for sampling and analyses. Wetlands 28:874–881
Minnesota Forest Resources Council (2007) Analysis of the current science behind riparian issues: report to the Minnesota forest resources council, St. Paul, Minnesota
Palik BJ, Batzer DP, Buech R, Nichols D, Cease K, Egeland L, Streblow DE (2001) Seasonal pond characteristics across a chronosequence of adjacent forest ages in Northern Minnesota. Wetlands 21:532–542
Palik BJ, Buech R, Egeland L (2003) Using an ecological land hierarchy to predict seasonal-wetland abundance in upland forests. Ecol Appl 13:1153–1163
Phillips PJ, Shedlock RJ (1993) Hydrology and chemistry of groundwater and seasonal ponds in the Atlantic coastal plain in Delaware. J Hydrol 141:157–178
Schneider DW (1999) Snowmelt ponds in Wisconsin: influence of hydroperiod on invertebrate community structure. In: Batzer DP, Rader RB, Wissinger SA (eds) Invertebrates in freshwater Wetlands of North America: ecology and management. Wiley, New York, pp 299–318
Sprecher SW (2000) Installing monitoring wells/piezometers in wetlands. WRAP Technical Notes Collection (ERDC TN-WRAP-00-02). US Army Engineer Research and Development Center, Vicksburg
ter Braak CJF, Smilauer P (2002) CANOCO: reference manual and CanocoDraw for Windows User’s guide: software for canonical community ordination (version 4.5). Microcomputer Power, Ithaca
Thorp JH, Covich AP (2001) Ecology and classification of North American freshwater invertebrates. Academic, San Diego
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27:337–363
Wiggins GB, Mackay RJ, Smith IM (1980) Evolutionary and ecological strategies of animals in annual temporary pools. Arch Hydrobiol Suppl 58:97–207
Williams DD (1996) Environmental constraints in temporary fresh waters and their consequences for the insect fauna. J N Am Bentholl Soc 15:634–650
Williams DD (2005) Temporary forest pools: can we see the water for the trees? Wetl Ecol Manag 13:213–233
Winter TC (1981) Effects of water-table configuration on seepage through lakebeds. Limnol Oceanogr 26:925–934
Wissinger SA, Bohonak AJ, Whiteman HH, Brown WS (1999) Subalphine wetlands in Colorado: habitat permanence, salamander predation and invertebrate communities. In: Batzer DP, Rader RB, Wissinger SA (eds) Invertebrates in freshwater Wetlands of North America: ecology and management. Wiley, New York, pp 757–790
Yeomans JC, Bremner JM (1991) Carbon and nitrogen analysis of soils by automated combustion techniques. Commun Soil Sci Plant Anal 22:843–850
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bischof, M.M., Hanson, M.A., Fulton, M.R. et al. Invertebrate Community Patterns in Seasonal Ponds in Minnesota, USA: Response to Hydrologic and Environmental Variability. Wetlands 33, 245–256 (2013). https://doi.org/10.1007/s13157-012-0374-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-012-0374-9