Abstract
After rainfall and soils, fire and herbivory are two of the main determinants of savanna ecosystems. Although the interactive effects of fire and herbivores on soil and vegetation are widely acknowledged few studies have addressed these two factors in concert, and none of the studies has focused on the Kalahari sand system. We experimentally studied how annual late dry season fires and grazing affect herbaceous plant species composition, above- and belowground biomass, and soil and grass nutrient concentrations in the nutrient-poor semi-arid Kalahari system in northern Botswana. Four treatments (fire, grazing, fire + grazing, and no-fire–no-grazing) were applied for two consecutive years in the late dry season. Plant species composition was affected by treatment and year. The no-fire–no-grazing treatment was distinctly different from all the other treatments in terms of species composition. Beta diversity was lower on the fire treatment and grazing treatment, but not where fire and grazing were combined. Fire and grazing alone or in combination did not have a substantial effect on biomass, soil and plant nutrients or plant species alpha diversity. Plant nitrogen was the only element that differed between treatments, with high concentrations on all the grazed treatments in the first year and low levels on the fire-alone treatment during the second year. The results show that fire and grazing mainly affect species composition and large-scale biodiversity patterns as indicated by the no-fire–no-grazing treatment being distinctly different from other treatments, suggesting the evolutionary adaptation of this dystrophic Kalahari sand system to herbivory and fire.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Frequent fires and herbivory by terrestrial vertebrates are widely recognized as major natural agents of spatial heterogeneity and modifiers of savanna ecosystems (Trollope 1984; Skarpe 1992; Bond and van Wilgen 1996; Van Langevelde and others 2003; Archibald and others 2005). Hence, fire and grazing have been studied extensively in the savannas, but often independently in spite of the fact that they regularly occur together with potential synergistic effects (McNaughton 1985; Hobbs and others 1991; Johnson and Matchett 2001; Fuhlendorf and Engle 2004; Archibald and others 2005; Midgley and others 2010).
Fire removes moribund vegetation and may create a window of opportunity for the establishment of species-rich communities that also host rare species that may otherwise have been suppressed by dominant species (Pickett and White 1985; Enright and others 1994). However, because of high sensitivity or low tolerance of rare species, frequent fires may lead to their local extinction (Brockway and others 2002). Fire may increase soil available forms of nitrogen (N), calcium (Ca), magnesium (Mg), and other mineral elements (Wan and others 2001; Lavoie and others 2010) as well as N, sodium (Na), potassium (K), and phosphorus (P) in plants (Moe and Wegge 1997). Fire has also been associated with increases in plant tissue nutrient quality (Van de Vijver and others 1999; Brockway and others 2002), plant species composition (Enright and others 1994), primary production (Hulbert 1988), plant growth rates (Gignoux and others 1997), and cover (Brockway and others 2002). However, some studies have found little or no significant effect of fire on soil, plant chemical properties or on plant species richness (Dougill and others 1999; Van de Vijver and others 1999; Jensen and others 2001; Brockway and others 2002; Dougill and Thomas 2004; Holdo and others 2011). Although repeated burning may cause increases or no changes in the concentrations of some elements (Wan and others 2001), significant losses may occur in aboveground biomass, foliar carbon (C) and N (Trollope 1984; McNaughton and others 1988; Hobbs and others 1991; Wan and others 2001), as well as in total soil N in mostly temperate soils (Kaye and Hart 1998; Deluca and Zouhar 2000).
Similarly, grazing removes aboveground grass biomass both in its green or dry state, and may in the process create gaps for recolonization of open spaces by new species or local extinction of rare and sensitive species (McNaughton 1985; Olff and Ritchie 1998). Many studies have observed grazing to stimulate growth of some grazing-tolerant plant species, and also to contribute to increased plant tissue nutrient quality, species diversity, and productivity (Frank and McNaughton 1993; Augustine and McNaughton 1998; Ritchie and others 1998; Augustine and Frank 2001). Belowground biomass can also be influenced by grazing or fire, despite the fact that vegetative organs are often protected by a soil layer from the disturbances occurring aboveground (Frost 1984; Sullivan and Rohde 2002). However, studies on belowground root biomass are generally few (Böhm 1979; De Castro and Kauffman 1998; Frank and others 2002), and those investigating effects of grazing or fire have found inconsistent results. Root biomass can be reduced by grazing as a result of more resources being allocated to the remaining aboveground tissues to compensate for the grazed tissues (Holland and Detling 1990; Ritchie and others 1998; Gao and others 2008) or it can increase or not be affected by fire or grazing (McNaughton and others 1998; Van de Vijver and others 1999; Frank and others 2002).
Interactive effects of fire and grazing have often been overlooked despite knowledge that grazers are frequently attracted to burned patches by the green and nutritive plants found in those patches (Daubenmire 1968; Moe and Wegge 1997; Archibald and others 2005; Mills and Fey 2005). In the process, grazers consume grass biomass, and with increasing grazing intensity the amount of grass that would otherwise be spared to serve as fuel load for the dry season fire is eventually reduced. Grazing, therefore, has the ability to moderate fire effects on vegetation and soils (Hobbs and others 1991). During grazing, herbivores may also deposit dung and urine that subsequently enrich the grazed patches with readily available plant nutrients (Ruess and McNaughton 1987; Hobbs 1996). Burning also removes biomass, but deposits ash and increases soil temperature, respiration, and nutrient turn-over rates (Hobbs and others 1991; Van de Vijver and others 1999; Johnson and Matchett 2001). The combined effects of fire and grazing may be additive resulting in enhanced nutrient cycling processes (McNaughton and others 1988; Johnson and Matchett 2001) or compensatory, with the strength of one factor becoming negligible or weakened by the presence of the other factor (Hobbs and others 1991). Grazing and burning thus offer alternate soil and plant nutrient enrichment pathways and have the potential to transform the plant structure, composition, and productivity in a complex manner when acting together (McNaughton 1985; Hobbs and others 1991; Mills and Fey 2005).
The objectives of this study were to experimentally test how annual fires and continuous grazing, alone or in combination affect (i) plant species composition and diversity, (ii) above- and belowground herbaceous plant biomass, and (iii) soil and grass nutrient concentrations in the Kalahari sand system in northern Botswana.
In light of the above, we predicted that (i) fire and grazing would change plant species composition (Enright and others 1994), and that (ii) plant richness and diversity would be lower in areas protected from fire and grazing by large herbivores as well as under the combined disturbance of fire and grazing, but relatively higher in burned or grazed areas. We further predicted that (iii) fire and grazing would decrease herbaceous biomass (Hobbs and others 1991; Van Langevelde and others 2003) and (iv) that fire would increase soil N, Ca, Mg, and other mineral elements (Wan and others 2001; Lavoie and others 2010), and this would be reflected in increased concentrations in emerging plant tissue materials following intake from nutrient-enriched soils (Trollope 1984; McNaughton and others 1988; Wan and others 2001). (v) Because of dung and urine deposition, and accelerated nutrient turn-over rates facilitated by grazing, vegetation in grazed plots would be higher in plant nutrient concentrations compared to vegetation in burned and unburned plots (Hobbs and Huenneke 1992; Moe and Wegge 2008), except P which is normally reduced on grazed areas (Moe and Wegge 2008; Wang and others 2011).
Methods and Materials
Study Area
The study was done in Chobe National Park (17°49′–17°55′S, 24°50′–24°59′E), a wildlife protected area in semi-arid northern Botswana, in the open savanna woodland characterized by scattered large trees of Zambezi Teak (Baikiaea plurijuga Harms) and a long history of annual fires (Child 1968; Taolo 2003). The current open tree vegetation structure is attributed to frequent fires in this part of the park which reduced the density of the once dominating fire-sensitive woodlands of Baikiaea plurijuga (Taolo 2003). The soil is nutrient-poor Kalahari sand—a deep and poorly structured soil, with low water-holding capacity and high infiltration rates (Ben-Shahar and MacDonald 2002; Skarpe and others 2004).
Rainfall occurs only in hot summer months (October–April) with a peak in January and February (Figure 1). The maximum monthly mean temperature in summer months is 35°C. Annual average rainfall is about 650 mm. The period between May and July is cold and dry, with a mean temperature of 6°C. August to September is dry and hot (Bhalotra 1987; Ben-Shahar and MacDonald 2002). Most herbaceous plants reach their maximum growth in February and senescence in March as the rainfall intensity declines.
Annual fires are common in the dry season in the study site, occurring mainly in the dry and hot months (August–October), but fires are commonly put out in line with the no-fire policy of the park (Taolo 2003). However, some fires often manage to spread over a larger part of the park before they are put out. Records of fire incidents in the study area are poorly documented. Reports, however, indicate that the last fire recorded in this part of the area occurred in 1997 (DWNP, unpublished reports). Charred wood and charcoal remains found within 30 cm depth of the soil surface showed that fires were common in the study area.
The study site was located 300 m south of the Ngoma-Kasane tarmac road, about 10 km from the Chobe River. The dominant tree species in the study site is Baikiaea plurijuga. Common grass species are Aristida congesta R. et S, Schmidtia pappophoroides Stued, and Digitaria species (Child 1968; Taolo 2003).
The site is a wet season range for a large number of wildlife species that include Cape buffalo (Syncerus caffer Sparrman) and African elephant (Loxodonta africana Blumenbach) (Taolo 2003; Skarpe and others 2004). There are about 7,000 buffalo and 80,000 elephant in the Chobe area, which is a substantial proportion of the 150,000 elephant estimated for the whole northern Botswana (Taolo 2003; DWNP 2006).
Experimental Design
A randomized block design was used for the application of four treatments: (i) fire alone (fenced plots with burning), (ii) grazing alone (unfenced plots with no burning), (iii) fire + grazing (unfenced plots with burning), and (iv) no-fire–no-grazing (fenced and no burning) (Figure 2). Treatment plots, each measuring 12 m × 12 m, were placed 20 m from each other and were replicated in eight blocks. Each block covered an area of 44 m × 44 m. The blocks were set up in homogenous open woodland within the flat to gentle depressions of undulating fossil sand dunes (Child 1968). There were two blocks per depression and blocks within one depression were 100 m distant from each other. None of the blocks had trees or large shrubs in them. The depressions were about 2–3 km apart, and are used by different wildlife species for grazing (Taolo 2003) and as a passage route to the Chobe River, the only permanent surface water source in the park (Child 1968). The selection of the sites was done in the dry season when the herb layer was dominated by dry perennial grasses. The perennial grass species were, therefore, used to confirm similarity among the block locations.
The fence used was 1.8 m high, and had a mesh size of 10 cm × 10 cm to exclude both medium and large herbivores. Large plots permitted us to avoid sampling within 1 m distance from the edge of the plots, and within one spot more than once.
Plots were marked in mid-September 2002, and the exclosures erected and completed on 12 October 2002. Prior to burning, litter was collected from four randomly placed 1 m × 1 m subplots within each treatment plot to measure litter quantity. The litter was made up of all standing dead and loose litter on the ground floor. The collected litter was taken to camp for oven drying at 60°C for 72 h prior to weighing for litter quantity. The litter was then returned and redistributed on the 1 m × 1 m subplots where it was collected before fire was applied. Litter was returned to the treatment plots to minimize disturbances to the plots and reduce loss of fuel load. Fire was applied in the late hot dry season on October 14–15, 2002 and October 15–16, 2003, in the evening between 1700 and 1900 h when air temperature and wind speed were low. When it was not possible to burn all plots in one evening, fire was applied to the remaining plots the following morning between 0600 and 0800 h.
Aboveground vegetation parameters were recorded in the wet season at the peak of the following growing seasons (February 1–15) in 2003, and in 2004. Root biomass was sampled between February 25 and March 03, 2003, and February 20–26, 2004.
Species Composition and Vegetation Cover
Species composition was recorded from four 1 m × 1 m permanent subplots which were randomly located within each treatment plot. The random location of the permanent subplots was restricted by subdividing each treatment plot with a thin thread into four quadrants of 6 m × 6 m each (Figure 2). Within each quadrant one permanent subplot was located from which a total percent areal vegetation cover was visually estimated, and plant species present as well as their individual numbers were recorded, during the 2 years of study.
Shoot and Root Biomass
Within each treatment plot, another four 1 m × 1 m temporary subplots were randomly selected for sampling of shoot and root biomasses. All live and standing dead shoot biomass was clipped at the ground level in all plots, and separated into graminoids and forbs (De Castro and Kauffman 1998). However, there was very little standing dead shoot biomass remaining from the last dry season at the time of sampling. Litter found within the quadrants on the ground surface was collected separately and oven-dried at 60°C for 72 h prior to weighing. Roots were excavated within the temporary subplots used for sampling shoot biomass. Root biomass was sampled by removing a monolith (50 cm × 50 cm, 30 cm deep) from within each subplot (Böhm 1979; De Castro and Kauffman 1998). Monolith sites within each treatment plot were at least 4 m apart. A pilot study had showed that the accumulative root biomass of the herbaceous plants in the study sites reaches 90% at 30 cm depth within 0–50-cm soil depth (data not shown). The 5–10 cm depth contributed the largest root biomass of 29%, with the 0–15 cm depth contributing 67% of the root biomass. Bulbs and large taproots were excluded.
Four blocks were randomly chosen for root sampling, and were used in both years. Monoliths were transported to the camp in firm containers where the soil was wet sieved through 1-mm wire mesh to obtain live roots (McNaughton and others 1998; Ritchie and others 1998). The roots were washed clean and separated into graminoids and forbs according to their color and morphology (McNaughton and others 1998). Roots that broke easily when bent using forceps were considered dead and discarded. All biomass samples were air-dried and then oven-dried at 60°C for 72 h before being weighed.
Soil Nutrient Concentration
Soil samples were collected at the same time as the aboveground biomass samples in both years. Four soil samples (0–10 cm depth, core diameter 5 cm) were collected randomly per treatment plot for all the eight blocks (n = 128). The soil was air-dried and later oven-dried at 40°C for 48 h. Total C, total N, P, K, Na, Ca, and Mg were determined for each sample. Chemical analysis was done at the Norwegian Institute for Agricultural and Environmental Research at the Norwegian University of Life Sciences. The soil was passed through a 2-mm sieve and nutrient elements extracted using ammonium lactate. Total C and N were determined by dry combustion (using 2400 CHN Elemental Analyser, Perkin-Elmer) (Nelson and Sommers 1996). Emissions of P, K, Na, Ca, and Mg were read on an optical emission spectrometer (OES Optima-5300 DV, Perkin-Elmer precisely) (Soltanpour and others 1996).
Grass Nutrient Concentration
Nutrient concentration of the live grass shoot biomass collected in 2003 and 2004 was determined for each of the subplots. The dried biomass was ground in a Wiley Mill (Arthur H. Thomas Comp, Philadelphia, USA) using a 2-mm sieve before being analyzed for N, P, K, Na, Ca, and Mg (AOAC 1995).
Aristida congesta and Schmidtia pappophoroides were the two dominant grass species selected to monitor monthly trends in nutrient levels following treatment application. Both S. pappophoroides and A. congesta are perennial species; though A. congesta can have a shorter lifespan (Gibbs-Russel and others 1990; Weber and others 2000). The two species were sampled within one continuous wet season of 2003/2004 on November 23–24, December 26, 2003, and January 25, 2004 from each treatment in the eight blocks by selecting only green foliage. The first rains of this wet season occurred on October 17, 2003 making it possible to sample green foliage a month later. Care was taken to avoid interfering with the whole plot where other mid-wet season samples were to be collected.
The grass tissue was air-dried and subsequently oven-dried at 60°C for 72 h. The tissues from each sampling period were analyzed for N, P, K, Na, Ca, and Mg at the Agricultural Research Soil and Plant Analyses Research Support laboratories (SPARS) in Sebele, Botswana. A sulfuric–selenium–salicylic acid mixture was used as the digestion reagent. The sample–reagent mixture was then heated at 330°C for 2 h before being cooled. Nitrogen was determined following the Kjeldahl process (AOAC 1995). Phosphorus was read on an UV photo-spectrometer (Shimadzu Corp. Japan). Potassium emission was measured on a flame photometer (Corning 410). Calcium, Mg, and Na were measured using atomic absorption spectrophotometer (Varian Techron, Australia) (AOAC 1995).
Data Analysis
We determined relationships between species composition, treatment, and year using canonical correspondence analysis (CCA) (ter Braak 1986) within the Vegan package in the R-software version 2.11.1 (Oksanen and others 2012). We removed infrequent species with less than three occurrences, as commonly done in vegetation gradient analyses (McCune and others 2000). We used treatment, year, and their interactions as explanatory variables for species composition. The CCA runs were constrained by blocks.
We also used the generalized Morisita’s similarity index (Chao and others 2008; Jost 2008), associated with 95% confidence intervals based on 200 bootstrap replications, to compare the plant compositional similarities between treatments. This index uses abundance data and it can compare multiple assemblages. The free software SPADE was used for this analysis (http://chao.stat.nthu.edu.tw/SPADE_UserGuide.pdf).
Species alpha diversity (between treatment heterogeneity) and evenness were calculated using Shannon–Wiener’s indices (Magurran 2004). Species richness (number of species per plot) was also calculated. Data were either log- or arcsine square root transformed for normality and homogeneity of variance, prior to analysis of variance (ANOVA). A two-factor repeated-measure ANOVA using the General Linear Model was used to determine differences between treatments, with biomass, alpha diversity indices, and nutrient levels as response factors measured on two occasions. A two-way ANOVA was also used to determine differences within years. The Kruskall–Wallis H test was used when assumptions of normality and homogeneity of variance were not met when determining differences in nutrient concentrations of individual grass species between treatments and months. Means that were significantly different at p < 0.05 were separated by a post hoc Student–Newman Keuls method.
Beta diversity which is within treatment heterogeneity (Anderson 2006; Anderson and others 2006) was determined using the function betadisper in Vegan in R (Oksanen and others 2012). Tukey’s HSD was used as a post hoc test to evaluate pair-wise contrasts between treatments.
Results
Species Composition, Diversity, and Cover
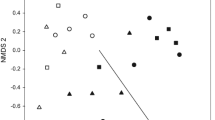
Species composition within the plots was significantly affected by treatments (F 3,52 = 1.65, p = 0.002) and year (F 1,52 = 3.25, p = 0.002) (Figure 3). The CCA 1 axis mainly captured the differences between the 2 years, whereas CCA 2 axis separated the treatments. The grass species Panicum maximum, Eragrostis superba and Schmidtia pappophoroides, and forb species Solanum incanum, Clerodendrum species, Sida cordifolia, and Striga asiatica were associated with the no-fire–no-grazing treatment. The forb species Oxygonum sinuatum and Ipomea species were associated with fire-alone, whereas the grass species Urochloa trichopus and Pogonathria squarrosa and forb species Eurphobia crotonoides and Corchorus tridens were mostly associated with the grazing treatment. Grass species Hyparrhenia hirta and Dactylotenium gigateum and forb species Cyperus esculatum, Cyperus margaritaceous, and Fimbristylis hispidula were mostly associated with the fire–grazing treatment.
Canonical Correspondence Analysis (CCA) biplot diagram of herbaceous plant species composition and fire, grazing, fire + grazing, control, and years (2003 and 2004) as environmental variables. Solid arrows represent a gradient influenced by treatments (fire, grazing, fire +grazing, and control) and broken arrows represent a gradient influenced by years. For plant species that could not be identified to species level, only the genus names are written and then followed by abbreviation “spp” (for example, Clerodendrum spp.). Forb species that could not be identified altogether were coded as Forb1, 2, 3… and unidentified fern as Fern.
The species similarities among plots subjected to different combinations of grazing and fire were high (>0.9), and confidence intervals overlapped (Table 1). However, when the different combinations of fire and grazing were compared to the no-fire–no-grazing treatment similarities were less and statistically different from the comparisons between the treatments involving fire and grazing.
Species alpha diversity (F 3,21 = 0.61, p = 0.62), richness (F 3,21 = 1.00, p = 0.41), and evenness (F 3,21 = 0.85, p = 0.48) did not differ between treatments, but differed between years (Tables 2, 3). Species alpha diversity and evenness were significantly higher in 2004 than in 2003. Species richness, however, was higher in 2003 than in 2004. Perennial grass species were more dominant in terms of numbers than annual grass species in both years (Perennials = 10; Annuals = 4) (Supplementary Table). Overall, forb species accounted for a larger number of the herbaceous plant species in the system in both 2003 and 2004 (forb = 38, grass = 14). The total number of individual plants of each plant species was about two-fold in 2004 compared to 2003 (data not shown). Aristida congesta (1600), Digitaria eriantha (1300), Dactyloteniun giganteum (900), Pogonarthria squarrosa (800), and Schmidtia pappophoroides (500) had many individuals in 2004 compared to 2003 in which the number of individuals was lower and species abundance rankings shifted (Dactyloteniun giganteum—559, Aristida congesta—492, Pogonathria squarrosa—433, Schmidtia pappophoroides—271, Gisekia africana—216, Digitaria eriantha—167).
Species beta diversity (heterogeneity within the treatments) was significantly affected by treatment (Figure 4). Fire alone (Tukey’s HSD, p = 0.03) and grazing alone (Tukey’s HSD, p = 0.02) had lower beta diversity compared to the control treatment. There were no differences in beta diversity between the control and the combined fire and grazing treatment.
There was no significant difference between treatments in the vegetation cover when both years were used (Repeated measures F 3,21 = 0.40, p = 0.75), but there was a significant treatment–year interaction effect (F 3,21 = 4.50, p = 0.01) and year-effect (F 1,21 = 34.1, p < 0.0001). The grazing-alone and the no-fire–no-grazing treatments had a significantly higher cover in 2004 compared to the two fire treatments (Two-way ANOVA F 3,21 = 6.51, p = 0.003) (Figure 5). There was no significant effect of treatments on the herbaceous cover in 2003 (F 3,21 = 0.72, p = 0.5).
The litter quantity measured in the growing season was significantly different between treatments (F 3,21 = 12.80, p < 0.0001). Treatments that were affected by fire (fire alone and fire + grazing) had less litter compared to treatments that were not affected by fire (no-fire–no-grazing, and grazing) in both 2003 (F 3,21 = 0.77, p < 0.0001) and 2004 (F 3,21 = 16.1, p < 0.0001). In general, there was more litter in 2003 (75 g m−2) compared to 2004 (57 g m−2) (F 1,21 = 6.56, p = 0.02) when all treatment plots were considered.
Shoot and Root Biomass
There was no significant difference in shoot biomass or in root biomass between treatments or years (Tables 2, 3). Also, when separated into the grass and forb groups, no significant difference in the shoot biomass was found between treatments. Neither the grass root nor the forb root biomass differed between treatments (grass: F 3,6 = 0.65, p = 0.61, forb: F 3,6 = 0.16, p = 0.92) or years (grass: F 1,6 = 2.75, p = 0.15, forb: F 1,6 = 1.85, p = 0.22). However, there was a significant difference between years in the shoot biomass of the grasses (F 1,21 = 11, p = 0.003), and of the forbs (F 1,21 = 34.7, p < 0.0001). The shoot biomass of the grasses was higher in 2004 than in 2003, whereas that of the forbs was higher in 2003 than in 2004.
Soil and Grass Shoot Nutrients
None of the soil nutrients studied differed significantly between treatments (Tables 4, 5). There was, however, a significant difference between years in soil C, N, P, Na, K, and pH. There was a higher concentration of soil C, P, and K in 2003 than in 2004. Soil N and Na were higher in 2004 than in 2003. Soil Ca and Mg concentrations were not different between years. Soil pH was higher in 2003 than in 2004.
There was a significant difference in grass shoot N concentration between treatments (F 3,21 = 5.38, p = 0.01) (Figure 6). In 2003, grasses that were subject to grazing alone, and to both fire and grazing had a higher concentration of N than grasses that were only burned, and those excluded from both burning and grazing (F 3,21 = 4.20, p = 0.02). In 2004, grasses in the plots that were only grazed, burned and grazed, and not burned and grazed had a higher N concentration compared to grasses in plots that were only burned (F 3,21 = 6.55, p = 0.003). Essentially, grazing increased plant N, whereas burning lowered plant N. Furthermore, the grass N level was significantly higher in 2003 than in 2004 (F 1,21 = 184, p < 0.0001). There was no significant treatment–year interaction effect on the N concentration. No significant difference between treatments was observed in the concentration of P (F 3,18 = 0.87, p = 0.47), K (F 3,12 = 0.13, p = 0.94), Ca (F 3,21 = 0.41, p = 0.74), Mg (F 3,21 = 0.94, p = 0.44), and Na (F 3,18 = 3.09, p = 0.05). Between years, the concentrations of P (F 1,18 = 41.9, p < 0.0001), K (F 1,12 = 63.4, p < 0.0001), Ca (F 1,21 = 12.2, p = 0.003), and Mg (F 1,21 = 53.9, p < 0.0001) were significantly lower in 2004 than in 2003.
In general, there was a significant decrease in the concentration of all nutrients (p < 0.0001) of A. congesta and S. pappophoroides within 3 months (November 2003, December 2003, and January 2004), except for K and Na in A. congesta (Figure 7a, b). The concentration of K was higher in December and January than in November (Kruskall–Wallis H 2 = 13.7, p = 0.01), whereas the concentration of Na did not differ between months (Kruskall–Wallis H 2 = 5.53, p = 0.06). There was no significant difference between treatments in the concentration of each of the nutrient elements of A. congesta and S. pappophoroides in November, December and January (p > 0.05), except for the Mg concentration in S. pappophoroides, which was slightly lower in January in the fire + grazing treatment than other treatments (Kruskall–Wallis H 3 = 10.4, p = 0.02) (Figure 7b).
Mean (±SE) monthly percent concentration of A nitrogen, phosphorus, potassium, and B calcium, magnesium, and sodium in green shoot tissues of Aristida congesta and Schmidtia pappophoroides grasses in relation to fire-only, grazing-only, fire + grazing, and no-fire–no grazing (none) treatments. Fire was applied in October.
Discussion
Species Composition, Diversity, and Cover
Although fire and grazing are important determinants of African savannas they are rarely studied in concert. Several studies have documented the effects of fire and grazing separately and the few that studied the interaction of these two factors have found strong interactive effects (Johnson and Matchett 2001; Mills and Fey 2005). Supporting our first prediction, there were differences in plant species composition between the treatments. The most pronounced difference we have documented in this study is between the no-fire–no-grazing treatment and the other treatments. Both fire and grazing have traditionally been regarded as strong agents of savanna heterogeneity (McNaughton 1985; Skarpe 1992; Van Langevelde and others 2003). In this study, we have shown that the beta diversity (that is, within treatment heterogeneity) is higher when herbivores are excluded. The strong effects of removing fire and grazing can also be seen from the similarity indices where the no-fire–no-grazing treatment is highly different from the other treatments. Recently, in a more eutrophic savanna in Uganda, Okullo and Moe (2012) also found that beta diversity was higher when large herbivores were excluded from savanna areas. Contrary to common perceptions, Milchunas and others (1988) have argued that in areas with a long evolutionary history of grazing, excluding herbivores may be considered as a strong disturbance. This view is supported by our study as indicated by the strong effect of grazing and fire removal on species composition, and the small differences between the other three treatments. Savanna systems, in general, and our study system, in particular, are adapted to fire and grazing. The Kalahari sand system in northern Botswana is dominated by large bodied bulk feeders like elephant and buffalo. Large herds of buffalo numbering over 7,000, and a substantial part of the current population of about 150,000 elephant in northern Botswana congregate along the Chobe river system in the dry season (Taolo 2003; Skarpe and others 2004; DWNP 2006). Although elephant (4 elephants per km2) and buffalo occur in higher densities in the study area during the dry season when grass forage is less important, these animals are also present during the wet season when grass is more important (Skarpe and others 2004). Elephant and buffalo are less selective than smaller grazers and consequently, this feeding behavior may contribute to a homogeneous herbaceous species pattern at the landscape scale.
The grass species Panicum maximum, Eragrostis superba, and Schmidtia pappophoroides and forb species Solanum incanum, Sida cordifolia, and Clerodendrum species were associated with the no-fire–no-grazing treatment. These grasses are good forage species and can tolerate light disturbances in the form of light intensity fires and grazing, but can disappear under heavy grazing and frequent fires (Sweet 1982; O’Connor 1991; Van Oudtshoorn 1992; Skarpe 1986). The three forb species are also indicators of disturbed ecosystems, though Solanum incanum can occur in both disturbed and undisturbed sites in climax grassland communities (Belsky 1987; Kutt and Woinarski 2007). There were many forbs and few grass species associated with the fire and grazing treatments, with the grazing-alone treatment showing less strength in species association compared to the other three treatments. Usually, forb species occur in higher frequencies in burned than in unburned and grazed areas, especially in semi-arid systems (Ruthven III and others 2000). Moisture conditions can be a critical determinant of forbs response to disturbances such as grazing. In mesic environments, grazing has been found to stimulate forb abundance (Winter and others 2012), whereas in semi-arid systems an opposite response has been observed (Ruthven III and others 2000).
Contrary to our second prediction, we did not find significant changes in alpha diversity or species richness between any of the treatments.
Plant Biomass and Cover
Neither fire nor grazing changed the plant shoot and root biomass, or concentrations of most nutrients in the open Baikiaea woodland of the Kalahari sands in semi-arid northern Botswana. This result was not in line with our prediction that fire and grazing would decrease plant biomass. Only the concentration levels of grass shoot N were affected by the treatments. Thus, the herbaceous component of the Baikiaea woodland seems to a large extent adapted to herbivory and fire, as has also been argued in other studies done in sandy semi-arid ecosystems (Dougill and others 1998, 1999; Sullivan and Rohde 2002). Because of the high frequency of fires in semi-arid systems, fine fuels do not accumulate in large quantities and fire intensities are, therefore, relatively low and consequently their impact, particularly on the herbaceous vegetation and soils is also low. It may be argued that the lack of vegetation and soil response to fire and herbivory could be a result of the short duration of the study. We do indeed acknowledge that more long-term experiments could modify our findings. However, the many inter-annual differences we did find in this study show that herbaceous vegetation does respond rapidly to changes in rainfall.
It is widely acknowledged that rainfall and soils are the primary determinants of savanna vegetation (Sankaran and others 2005), with rainfall particularly important in semi arid and arid regions (Oba and others Oba and others 2000; Fynn and O’Connor 2000; Sullivan and Rohde 2002; Sankaran and others 2005). Fires and herbivory are mainly modifiers or secondary determinants (Skarpe 1992; Fynn and O’Connor 2000; Sankaran and others 2005). Our study shows that, whereas fire and herbivory did not cause considerable change to the savanna vegetation over 2 years, variations in rainfall had a significant influence. The high amount of rainfall in 2004 as compared to 2003 probably contributed to the higher plant biomass, species diversity (see also Oba and others 2000), and evenness observed in 2004. Species richness was, however, lower in 2004 in spite of the higher rainfall received in that year. Rainfall also affected the treatments in that vegetation cover was different between treatments in the high rainfall year. The vegetation cover was reduced by burning but increased under grazing and in the no-fire–no-grazing treatment. Grass biomass was higher and forb biomass lower when rainfall was high, suggesting that grass suppressed forbs in years of high rainfall.
This study is not the only study that has found limited effects of fire on nutrient levels in southern African savanna systems. Frequent fires caused no significant changes to soil N mineralization, total N, and organic carbon at depths ranging from 0 to 60 cm over a 50-year period in Kruger National Park (Coetsee and others 2010; Holdo and others 2011). The lack of difference in soil organic carbon following fire was attributed to the recalcitrance of the carbon pools, which was suggested to be reinforced by fire (Holdo and others 2011).
The fuel load, a reflection of fire intensity, was within the normal ranges experienced in this particular area and in similar semi-arid systems in the region. The natural grass fuel load in Chobe National Parks commonly ranges between 2000 and 9930 kg ha−1 (Smith and others 2005). In our study, the total herbaceous fuel load prior to burning in 2002 was 2420 ± 280 kg ha−1 (mean ± SE). In 2003, 1 year after the application of the first year treatment the fuel load was 1710 ± 100 and 1940 ± 100 kg ha−1 (mean ± SE) for fire-alone and fire + grazing treatments, respectively. Hence, the fuel load in this study was comparable, though on the lower side, to the fuel load previously recorded in Chobe National Park (Smith and others 2005). A fuel load of about 2500 kg ha−1 commonly yields a fire intensity of 2000 kW m−1 (Trollope and others 2002). However, the overall fire intensity in this study might have been lower than expected particularly when determined from the grass fuel load.
Similar to fire, grazing in semi-arid grassy regions often does not affect grasses adversely, particularly at the population and community level (Trollope and others 2002; Milchunas and others 1989). Although grazing intensity was not measured during this study, the number and spoors (footprints, dung and urine deposits) of elephant, buffalo, sable antelope, and kudu sighted in the area during the dry and wet seasons revealed that unfenced plots were grazed or trampled. About 3000 buffalo are estimated to use the study area during the wet season, as well as over 80,000 elephant that graze the area during the wet and the dry seasons (Taolo 2003; Skarpe and others 2004). Herds of over 15 sable antelope are common in the area. However, grazing is considered to be light during the wet season as wildlife grazers are widely distributed at this time of the year. Trampling is often heavy in the experimental area during the dry season as large herds of ungulates traverse the area to reach and congest along the Chobe River which provides a permanent supply of surface water. The dominance of perennial grass species in the experimental area further suggests that grazing was generally low. Normally, light grazing promotes growth and abundance of perennial grasses in areas receiving high rainfall (Fynn and O’Connor 2000), such as in the Chobe ecosystem. Had intense grazing occurred, it would have removed much of the perennial grasses and promoted an increase in growth of annual grasses and forbs.
Soil and Plant Chemistry
Soil chemistry showed no response to fire and grazing. Similar results have been found in previous studies of effects of grazing (Dougill and others 1998; Dougill and Thomas 2004), and of 2-year (Brockway and others 2002) and 7-year annual fires (Tongway and Hodgkinson 1992). Although there was no change in soil total N, available soil N may have changed in the upper layer of the soil. Usually when there is a change in soil N due to either fire or grazing, it occurs immediately after fire, within 0–5-cm soil depth and in the available forms of N (NH4 + and NO3 −)(Ruess and McNaughton 1987; DeBano and others 1998; Dougill and others 1998; Wan and others 2001). Volatilization of N from plant matter normally occurs during combustion (Wan and others 2001), and this may have led to no transfer of N into the soil. Normally, soil total N does not change due to fire even in long-term studies (Coetsee and others 2010).
Grazing alone, and grazing and fire combined resulted in a significantly higher concentration of plant N, hence partially supporting theories that grazing stimulates an increase in plant nutrient concentrations (Singer and Schoenecker 2003) and burning decreases plant nitrogen (Hobbs and others 1991; Lavoie and others 2010). In our study, the fire-alone treatment had a lower concentration of N in grass shoots. Some studies have found that although fire can increase nutrient concentrations in plant tissues (Brockway and others 2002), the increase is not due to the soil nutrient status, but to the leaf–stem ratio and tissue rejuvenation (Daubenmire 1968; Van de Vijver and others 1999).
The nutrient concentrations of the individual grass species showed no response to fire or grazing. Van de Vijver and others (1999) have observed that although nutrient concentrations were higher in plant tissues from burned plots compared to those from unburned plots, the differences did not last for 3 months, and were not due to ash generated by fire as indicated by their ash-exclusion treatments.
In conclusion, areas subjected to fire and grazing have a distinctly different vegetation composition from that of no-fire–no-grazing, even over a 2-year period, indicating a rapid response to these treatments. Beta diversity (within treatment heterogeneity) was higher on the no-fire–no-grazing treatment compared to the fire-alone and grazing-alone treatments, indicating that herbivores and fire increases homogeneity at the landscape scale. Our results support Milchunas and others (1988) in that exclusion of herbivores, and in our case also fire, can be viewed as the disturbance factor in ecosystems that have co-evolved with large grazing and browsing herbivores as well as fire. Species diversity and concentrations of key soil and plant nutrients are maintained under fire and grazing. Thus, any changes in plant composition are unlikely to adversely affect the overall functioning of the dystrophic Kalahari sand system.
References
AOAC. 1995. Official methods of analysis. 16th edn. Arlington (VA): Association of Official Analytical Chemists.
Anderson MJ. 2006. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–53.
Anderson MJ, Ellingsen KE, McArdle BH. 2006. Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–93.
Archibald S, Bond WJ, Stock WD, Fairbanks DHK. 2005. Shaping the landscape: fire–grazer interactions in an African savanna. Ecol Appl 15:96–109.
Augustine DJ, McNaughton SJ. 1998. Ungulate effects on the functional species composition of plant communities: herbivore selectivity and plant tolerance. J Wildl Manag 62:1165–83.
Augustine DJ, Frank DA. 2001. Effects of migratory grazers on spatial heterogeneity of soil nitrogen properties in a grassland ecosystem. Ecology 82:3149–62.
Belsky AJ. 1987. Revegetation of natural and human-caused disturbances in the Serengeti National Park, Tanzania. Plant Ecol 70:51–60.
Ben-Shahar R, MacDonald DW. 2002. The role of soil factors and leaf protein in the utilization of mopane plants by elephants in northern Botswana. BMC Ecol 3:1–6.
Bhalotra YPR. 1987. Climate of Botswana. Part II: elements of climate. 1. Rainfall. Department of Meteorological Services. Ministry of Works, Transport and Communications, Gaborone.
Böhm W. 1979. Methods of studying root systems. New York: Springer.
Bond WJ, Van Wilgen BW. 1996. Fire and plants. Population and community biology Series 14. London: Chapman and Hall.
Brockway DG, Gatewood RG, Paris RB. 2002. Restoring fire as an ecological process in shortgrass prairie ecosystems: initial effects of prescribed burning during the dormant and growing seasons. J Environ Manage 65:135–52.
Chao A, Jost L, Chiang SC, Jian YH, Chazdon R. 2008. A two-stage probabilistic approach to multiple-community similarity indices. Biometrics 64:1178–86.
Child G. 1968. An ecological survey of northeastern Botswana. UNDP Report to the government of Botswana, F.A.O. Technical Assistance No. 2563, Rome.
Coetsee C, Bond WJ, February EC. 2010. Frequent fire affects soil nitrogen and carbon in an African savanna by changing woody cover. Oecologia 162:1027–34.
Daubenmire R. 1968. Ecology of fire in grasslands. In: Cragg JB, Ed. Advances in ecological research. New York: Academic Press. p 209–66.
DeBano LF, Neary DG, Ffolliott PF. 1998. Fire’s effects on ecosystems. New York: Wiley. pp 263–83.
De Castro EA, Kauffman JB. 1998. Ecosystem structure in the Brazilian Cerrado: a vegetation gradient of aboveground biomass, root mass and consumption by fire. J Trop Ecol 14:263–83.
DeLuca TH, Zouhar KL. 2000. Effects of selection harvest and prescribed fire on the soil nitrogen status of ponderosa pine forests. For Ecol Manage 138:263–71.
Dougill AJ, Heathwaite AL, Thomas DSG. 1998. Soil water movement and nutrient cycling in semi-arid rangeland: vegetation change and system resilience. Hydrol Process 12:443–59.
Dougill AJ, Thomas AD. 2004. Kalahari sand soils: spatial heterogeneity, biological soil crust and land degradation. Land Degrad Dev 15:233–42.
Dougill AJ, Thomas DSG, Heathwaite AL. 1999. Environmental change in the Kalahari: integrated land degradation studies for nonequilibrium dryland environments. Ann Assoc Am Geogr 89:420–42.
DWNP. 2006. Aerial census of animals in Botswana. Internal report, Department of Wildlife and National Parks, Gaborone.
Enright NJ, Miller BP, Crawford A. 1994. Environmental correlates of vegetation patterns and species richness in the northern Grampians, Victoria. Aust J Ecol 19:159–68.
Frank DA, McNaughton SJ. 1993. Evidence for the promotion of aboveground grassland production by native large herbivores in Yellowstone National Park. Oecologia 96:157–61.
Frank DA, Kuns MM, Guido DR. 2002. Consumer control of grassland plant production. Ecology 83:602–6.
Frost PGH. 1984. The responses and survival of organisms in fire-prone environments. In: de Booysen VP, Tainton NM, Eds. Ecological studies 48: ecological effects of fire in South African ecosystems. Berlin: Springer. p 273–310.
Fuhlendorf SD, Engle DM. 2004. Application of the fire–grazing interaction to restore a shifting mosaic on tallgrass prairie. J Appl Ecol 41:604–14.
Fynn RWS, O’Connor TG. 2000. Effect of stocking rate and rainfall on rangeland dynamics and cattle performance in a semi-arid savanna, South Africa. J Appl Ecol 37:491–507.
Gao Y, Giese M, Lin S, Sattelmacher B, Zhao Y, Brueck H. 2008. Belowground net primary productivity and biomass allocation of a grassland in Inner Mongolia is affected by grazing intensity. Plant Soil 307:41–50.
Gibbs-Russel GE, Watson L, Koekemoer M, Smook L, Barker NP, Anderson HM, Dallwitz MJ. 1990. Grasses of southern Africa. Memoirs of the Botanical survey of South Africa No. 58. Leistner OA, Ed. Pretoria: National Botanic Gardens.
Gignoux J, Clobert J, Menant J. 1997. Alternative fire resistance strategies in savanna trees. Oecologia 110:575–83.
Hobbs NT, Schimel DS, Clenton EO, Ojima DS. 1991. Fire and grazing in the tallgrass prairie: contingent effects on nitrogen budgets. Ecology 72:1374–82.
Hobbs RJ, Huenneke LF. 1992. Disturbance, diversity, and invasion: implications for conservation. Coserv Biol 6:324–37.
Hobbs NT. 1996. Modification of ecosystems by ungulates. J Wildl Manag 60:695–713.
Holdo RM, Mack MC, Arnold SG. 2011. Tree canopies explain fire effects on soil nitrogen, phosphorus and carbon in a savanna ecosystem. Journal of Vegetation Science. doi:10.1111/j.1654-1103.2011.01357.x (early view on-line: 20 October 2011).
Holland EA, Detling JK. 1990. Plant response to herbivory and below ground nitrogen cycling. Ecology 71:1040–9.
Hulbert LC. 1988. Causes of fire effects in tallgrass prairie. Ecology 69:46–58.
Jensen M, Michelsen A, Gashaw M. 2001. Responses in plant, soil inorganic and microbial nutrient pools to experimental fire, ash and biomass addition in a woodland savanna. Oecologia 128:85–93.
Johnson LC, Matchett JR. 2001. Fire and grazing regulate belowground processes in tallgrass prairie. Ecology 82:3377–89.
Jost L. 2008. GST and its relatives do not measure differentiation. Mol Ecol 17(4):015–4026.
Kaye JP, Hart SC. 1998. Ecological restoration alters nitrogen transformations in a ponderosa pine bunchgrass ecosystem. Ecol Appl 8:1052–60.
Kutt AS, Woinarski JCZ. 2007. The effects of grazing and fire on vegetation and the vertebrate assemblage in a tropical savanna woodland in north-eastern Australia. J Trop Ecol 23:95–106.
Lavoie M, Starr G, Mack MC, Martin TA, Gholz HL. 2010. Effects of prescribed fire on understory vegetation, carbon pools, and soil nutrients in a longleaf pine-slash pine forest in Florida. Nat Areas J 30:82–94.
Magurran AE. 2004. Measuring biological diversity. Oxford: Blackwell.
McCune B, Rosentreter R, Ponzetti JM, Shaw DC. 2000. Epiphyte habitats in an old conifer forest in western Washington, USA. Bryologist 103:417–27.
McNaughton SJ. 1985. Ecology of a grazing ecosystem: the Serengeti. Ecol Monogr 55:259–94.
McNaughton SJ, Ruess RW, Seagle SW. 1988. Large mammals and process dynamics in African ecosystems. Bioscience 38:794–800.
McNaughton SJ, Banyikwa FF, McNaughton MM. 1998. Root biomass and productivity in a grazing ecosystem: the Serengeti. Ecology 79:587–92.
Midgley JJ, Lawes MJ, Chamaillé-Jammes S. 2010. Savanna woody plant dynamics: the role of fire and herbivory, separately and synergistically. Turner Review No. 19. Aust J Bot 58:1–11.
Milchunas DG, Sala OE, Lauenroth WK. 1988. A generalized model of the effects of grazing by large herbivores on grassland community structure. Am Nat 132:87–106.
Milchunas DG, Lauenroth WK, Chapman PL, Kazempour MK. 1989. Effects of grazing, topography, and precipitation on the structure of a semi-arid grassland. Plant Ecol 80:11–23.
Mills AJ, Fey MV. 2005. Interactive response of herbivores, soils and vegetation to annual burning in a South African savanna. Austral Ecol 30:435–44.
Moe SR, Wegge P. 1997. The effects of cutting and burning on grass quality and axis deer (Axis axis) use of grassland in lowland Nepal. J Trop Ecol 13:279–92.
Moe SR, Wegge P. 2008. Effects of deposition of deer dung on nutrient redistribution and on soil and plant nutrients on intensively grazed grasslands in lowland Nepal. Ecol Res 23:227–34.
Nelson DW, Sommers LE. 1996. Total carbon, organic carbon, and organic matter. In: Sparks DL, Ed. Methods of soil analysis. Part 3. Chemical methods. SSSA Book Series: 5. Madison. Wisconsin. pp 961–1010.
Oba G, Stenseth NC, Lusigi WJ. 2000. New perspectives on sustainable grazing management in arid zones of sub-saharan Africa. Bioscience 50:35–51.
O’Connor TG. 1991. Influence of rainfall and grazing on the compositional change of the herbaceous layer of a sandveld savanna. J Grassl Soc Southern Afr 8:103–9.
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H. 2012. Vegan: community ecology package. R package version 2.0-3. http://vegan.r-forge.r-project.org.
Okullo P, Moe SR. 2012. Large herbivores maintain termite-caused differences in herbaceous species diversity patterns. Ecology 93:2095–103.
Olff H, Ritchie ME. 1998. Effects of herbivores on grassland plant diversity. Trends Ecol Evol 13:261–5.
Pickett STA, White PS. 1985. Patch dynamics: a synthesis. In: Pickett STA, White PS, Eds. The ecology of natural disturbance and patch dynamics. San Diego: Academic Express. p 371–84.
Ritchie ME, David T, Knops JMH. 1998. Herbivore effects on plant and nitrogen dynamics in Oak savanna. Ecology 79:165–77.
Ruess RW, McNaughton SJ. 1987. Grazing and the dynamics of nutrient and energy regulated microbial processes in the Serengeti grasslands. Oikos 49:101–10.
Ruthven DCIII, Gallagher JF, Synatzske DR. 2000. Effect of fire and grazing on forbs in the Western South Texas plains. Southwestern Nat 45:89–94.
Sankaran M, Hanan NP, Scholes RJ, Ratnam J, Augustine DJ, Cade BS, Gignoux J, Higgins SI, Le Roux X, Ludwig F, Ardo J, Banyikwa F, Bronn A, Bucini G, Caylor KK, Coughenour MB, Diouf A, Ekaya W, Feral CJ, February EC, Frost PGH, Hiernaux P, Hrabar H, Metzger KL, Prins HHT, Ringrose S, Sea W, Tews J, Worden J, Zambatis N. 2005. Determinants of woody cover in African savannas. Nature 438:846–9.
Singer FJ, Schoenecker KA. 2003. Do ungulates accelerate or decelerate nitrogen cycling? For Ecol Manage 181:189–204.
Skarpe C. 1986. Plant community structure in relation to grazing and environmental changes along a north-south transect in the western Kalahari. Plant Ecol 68:3–18.
Skarpe C. 1992. Dynamics of savanna ecosystems. J Veg Sci 3:293–300.
Skarpe C, Aarrestad PA, Andreassen HP, Dhillion SS, Dimakatso T, Du Toit JT, Halley DJ, Hytteborn H, Makhabu S, Mari M, Marokane W, Masunga G, Modise D, Moe SR, Mojaphoko R, Mosugelo D, Motsumi S, Neo-Mahupeleng G, Ramotadima M, Rutina L, Sechele L, Sejoe TB, Stokke S, Swenson JE, Taolo C, Vandewalle M, Wegge P. 2004. The return of the giants: ecological effects of an increasing elephant population. Ambio 33:276–82.
Smith AMS, Wooster MJ, Drake NA, Dipotso FM, Falkowski MJ, Hudak AT. 2005. Testing the potential of multi-spectral remote sensing for retrospectively estimating fire severity in African Savannahs. Remote Sens Environ 97:92–115.
Soltanpour PN, Johnson GW, Workman SM, Jones JB, Miller RO. 1996. Inductively coupled plasma emission spectrometry and inductively coupled plasma-mass spectrometry. In: Sparks DL, Ed., Methods of soil analysis. Part 3. Chemical methods. SSSA Book Series: 5. Madison. Wisconsin. pp 91–139.
Sullivan S, Rohde R. 2002. On non-equilibrium in arid and semi-arid grazing systems. J Biogeogr 29:1595–618.
Sweet RJ. 1982. Bush control with fire in Acacia nigrescens/Combretum apiculatum savanna in Botswana. Proc Ann Congr Grassl Soc Southern Afr 17:25–8.
Taolo CL. 2003. Population ecology, seasonal movement and habitat use of the African buffalo (Syncerus caffer) in Chobe National Park, Botswana. PhD thesis. Norwegian University of Science and Technology, Trondheim.
ter Braak CJF. 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–79.
Tongway DJ, Hodgkinson KC. 1992. The effects of fire on the soil in a degraded semi arid woodland. III. Nutrient pool sizes, biological activity and herbage response. Soil fertility and plant nutrition. Aust J Soil Res 30:17–26.
Trollope WSW. 1984. Fire in savanna. In: Booysen de VP, Tainton NM, Ed., Ecological effects of fire in South African ecosystems. Ecological Studies 48. Berlin: Springer. pp 149–175.
Trollope WSW, Trollope LA, Harnett DC. 2002. Fire behaviour a key factor in the fire ecology of African grasslands and savannas. In: Viegas DX, Ed. Forest fire research and wildland fire safety. Rotterdam: Millpress. p 1–15.
Van de Vijver CADM, Poot P, Prins HHT. 1999. Causes of increased nutrient concentrations in post fire regrowth in an East African savanna. Plant Soil 214:173–85.
Van Langevelde F, Van de Vijver CADM, Kumar L, Van de Koppel J, De Ridder N, Van Andel J, Skidmore AK, Hearne JW, Stroosnijder L, Bond WJ, Prins HHT, Rietkerk M. 2003. Effects of fire and herbivory on the stability of savanna ecosystems. Ecology 84:337–50.
Van Oudtshoorn F. 1992. Guide to grasses of South Africa. Pretoria: Briza Publications.
Wan S, Hui D, Luo Y. 2001. Fire effects on nitrogen pools and dynamics in terrestrial ecosystems: a meta-analysis. Ecol Appl 11:1349–65.
Wang X, Yan Y, Cao Y. 2011. Impact of historic grazing on steppe soils on the northern Tibetan Plateau. Plant and Soil:1-11: online first Accepted 28 October 2011: doi:10.1007/s11104-011-1053-y.
Weber G, Moloney EK, Jeltsch F. 2000. Simulated long-term vegetation response to alternative stocking strategies in savanna rangelands. Plant Ecol 150:77–96.
Winter SL, Fuhlendorf SD, Goad CL, Davis CA, Hickman KR, Leslie DM Jr. 2012. Restoration of the fire–grazing interaction in Artemisia filifolia shrubland. J Appl Ecol 49:242–50.
Acknowledgments
The study was part of the Botswana-Norway Institutional Cooperation and Capacity building Project (BONIC) jointly funded by the Department of Wildlife and National Parks (DWNP) of Botswana and Norwegian Agency for Development (NORAD). We thank the Department of Agricultural Research in Sebele for providing a laboratory for chemical analysis and a number of DWNP officers for helping in the field. We also appreciate comments from RJ. Naiman and three anonymous reviewers which improved the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
GSM designed the study, performed research, analyzed data and wrote the manuscript; SRM contributed to data analysis, interpretation and write-up; BP designed and performed research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Masunga, G.S., Moe, S.R. & Pelekekae, B. Fire and Grazing Change Herbaceous Species Composition and Reduce Beta Diversity in the Kalahari Sand System. Ecosystems 16, 252–268 (2013). https://doi.org/10.1007/s10021-012-9611-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-012-9611-6