Abstract
Sphagnum wetlands in subtropical high-mountain regions have been severely destroyed by human activities, necessitating restoration efforts. We studied the effects of substrate and planting method on Sphagnum palustre L. growth and the underlying mechanisms to determine the optimal conditions for S. palustre restoration. S. palustre collected from natural wetlands was grown on nine substrates and with four planting methods in a greenhouse. The results show that S. palustre grew best in mountain yellow-brown soil without added peat or river sand and when planted as intact plants. Substrate pH and P content and capitula P content negatively correlated with S. palustre productivity, while initial biomass of S. palustre at planting positively correlated with productivity. S. palustre restoration on local mountain soil in subtropical high-mountain regions is practical, which may provide a new perspective for restoring peatlands. Traditional restoration method using the 10 cm upper parts of S. palustre as transplanted materials does not destroy the source S. palustre populations in habitats where plants are collected. However, we argue that a planting method using only capitula (top 1–2 cm) may be a better choice for S. palustre restoration, due to the similar productivity but less impact to source S. palustre populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sphagnum wetlands are the most important components of Northern Hemisphere peatlands (Limpens et al. 2017; Bengtsson et al. 2018). Because Sphagnum wetlands are located in areas with nutrient-poor (ombrotrophic), waterlogged (anoxic), cold and acidic conditions (Hajek et al. 2011; Rydin and Jeglum 2013; Manninen et al. 2016; Binet et al. 2017), their rate of decomposition is usually lower than their rate of production, which contributes to the significant accumulation of organic matter (OM) that serves as an important carbon pool (Gorham 1991; Clymo et al. 1998; Granath et al. 2014; Hommeltenberg et al. 2014). The genus Sphagnum comprises approximately 250–400 species (Shaw et al. 2016), among which Sphagnum palustre is a common species with a wide distribution (Daniels and Eddy 1990). In the subtropical regions of southern China, patches of S. palustre-dominated wetlands are found in the high-altitude areas of the Huangshan Mountains, Yunnan-Kweichow Plateau and western Hubei Mountains (Ma et al. 2008), which are the sources of the headstreams of a number of rivers and play an important role in water storage. However, large areas of subtropical high-mountain Sphagnum wetlands have been diminished because of habitat destruction and overcollection for horticultural purposes, leading to reduced performance of ecological functions (Wang et al. 2013), which emphasizes the urgent need for restoring these wetlands.
The growth of Sphagnum is determined by a wide variety of environmental factors (Price et al. 2003; Chapin et al. 2004; Thompson and Waddington 2008; Medvedeff et al. 2015). Nutrient availability (especially of nitrogen [N] and phosphorus [P]) is an important factor for Sphagnum growth when water availability is sufficient (Weltzin et al. 2001; Hoosbeek et al. 2002; Kim et al. 2014). In natural bogs, Sphagnum keeps growing upward from the apical part (capitulum), while the lower part gradually dies, isolating the Sphagnum from the underlying mineral soil or minerotrophic groundwater (Clymo 1973; van Breemen 1995). Therefore, it is generally thought that the nutrient supply for Sphagnum mainly depends on atmospheric deposition (Bridgham et al. 1996; Malmer et al. 2003; Bragazza and Freeman 2007; Limpens and Heijmans 2008; Granath et al. 2009; Nishimura and Tsuyuzaki 2014). However, during the restoration of destroyed peatlands with bare peat surfaces, transplanted Sphagnum plants are in full contact with the substrate, raising the question of whether the substrate will affect Sphagnum growth via its background nutrient contents. Until now, research and restoration work have been mostly performed on original peatlands or abandoned cutover peatlands (Rochefort and Price 2003; Vasander et al. 2003; Graf et al. 2008; Andersen et al. 2010; Karofeld et al. 2016) where local peat was the only substrate used. However, whether peat is the only substrate that can be used or if it is the optimal substrate for Sphagnum restoration remains uncertain. In addition, Sphagnum mires in subtropical high-mountain regions are intermittently distributed in small patches, and peat use is thus limited by the complicated topographic conditions and the distribution of mires. In contrast, mountain yellow-brown soil (“mountain soil” hereafter, which is classified in the Alfisol order, Moist Warm Alfisol suborder, in accordance with the classification system of Chinese soil; Zhu et al. 2010) is widely distributed, and high levels of precipitation commonly create rivulets in these subtropical high-mountain regions. This effect of water flow results in mixed substrates with a wide range of proportions of mountain soil and river sand. Considering these situations in subtropical high-mountain regions in China, we used local mountain soil as an alternative base substrate for S. palustre. Moreover, different proportions of peat (to improve the texture and nutritional environment of the substrate) or river sand (to simulate the mixture of mountain soil and river sand caused by rivulets) were mixed into the mountain soil to explore the suitability of different substrates for S. palustre as well as understand the possible mechanisms underlying the substrate effects.
In addition to the substrate effects, the effects of water and nutrient transport and supply inside Sphagnum mosses are also of key importance (Aldous 2002; Kim et al. 2014). Sphagnum mosses grows apically from the capitula (Clymo 1970) and has a well-developed system for water conduction in the capillary spaces among pendent branches around the stem, which provides an effective vertical transport path for water and soluble ions from the basal part to the capitulum (Rydin and Clymo 1989; van Breemen 1995; Thompson and Waddington 2008; Chiwa et al. 2016). Consequently, nutrient transport depends largely on water availability. In addition to the groundwater level, the distance water is transported from the basal part to the capitulum is determined by stem length and may affect water and nutrient supply efficiency, further affecting S. palustre growth (Aldous 2002; Kim et al. 2014). This implies that different collection depths and different planting methods for S. palustre (i.e., whether S. palustre is collected and transplanted with the stem and the length of the stem) could result in differences in final productivity. Additionally, the commonly used transplant materials in previous Sphagnum restoration practices have been the upper parts of plants of a certain length (usually within 10 cm) (Clymo and Duckett 1986; Campeau and Rochefort 1996; Bugnon et al. 1997; Rochefort et al. 2003; Waddington et al. 2003; Pouliot et al. 2015). Therefore, most of the attention was focused on the performance of the transplanted upper part of Sphagnum diaspores, while the growth status of the damaged Sphagnum plants in the source populations was overlooked. As the vitality of Sphagnum plant parts decreases sharply with increasing distance from the capitula (Rochefort et al. 2003), the regenerative potential of damaged plants after collection deserves more attention.
We studied the substrate composition and length of shoot fragment as factors influencing the growth of S. palustre in subtropical high-mountain regions and the possible mechanisms underlying these effects. Specifically, we tested the following hypotheses: (1) Background nutrient contents in different substrate compositions will affect the N and P contents in the capitula, further affecting S. palustre growth; therefore, the growth of S. palustre in substrates mixed with peat will be different from that in substrates mixed with river sand. (2) Water and nutrient supply, which will depend on the length of the shoot fragment, will affect the water and NP contents in the capitula, further affecting S. palustre growth; therefore, S. palustre growth will be different when transplanted with different lengths of shoot fragments. (3) The regenerative ability of S. palustre is functional below 10 cm of the capitula, therefore, collecting approximately 10 cm of the upper part of S. palustre plants will not have destructive effects on the regenerative potential of the damaged plants.
Materials and Methods
Study Site and Species
The Qizimei Mountain National Nature Reserve (29° 39′ 30″~30° 05′ 15″ N, 109° 38′ 30″~109° 47′ 00″ E) is located in southwestern Hubei, China, and encompasses an area of 34,550 ha (Liu et al. 2006). The region lies in the subtropical zone and is characterized by a subtropical humid monsoon climate with definitive vertical differentiation (i.e., the temperature lapse rate is −0.6 °C 100 m−1). The study site was set in the reserve of a high-mountain area: altitude of 1800 m, annual average temperature of 8.9 °C, annual precipitation of 1876 mm, and annual sunshine duration of 1520 h.
A total of approximately 940 ha of Sphagnum wetlands are distributed from an altitude of 1650 to 1950 m in patches of various sizes. The soil profiles in these Sphagnum wetlands have thicknesses ranging from 48 to 100 cm and are divided into three layers: a sod layer (pH 6.7 ± 1.2), deposited peat layer (pH 5.7 ± 1.2) and gleying layer (pH 5.3 ± 1.1) (Mao et al. 2009). The results of the most recent survey indicate that a total of 197 species of embryophytes, including 3 bryophytes, 8 ferns, 4 gymnosperms and 182 angiosperms, exist in the area (Wang et al. 2013; Zhao et al. 2013). Sphagnum palustre L. is the only Sphagnum species at the study location and is the dominant species in wetland areas with developed hummocks. The other common species have been described in detail in Li et al. (2018).
Experimental Design
A 20-m × 15-m experimental greenhouse was constructed on a wasteland in the core zone of the reserve. The wasteland had been over-grown with weeds dominated by Erigeron annuus (L.) Pers. and Inula japonica Thunb. before our greenhouse was constructed. To keep the greenhouse ventilated, transparent plastic film and shade nets were placed overhead, while only shade nets were placed around the sides. All experiments were conducted in the greenhouse (data of temperature and humidity are given in Online Resource 1). A two-factor design consisting of nine substrates and four planting methods (see below) was applied to transplanted S. palustre plants. Each combination of treatments was replicated five times, and there were 180 samples in total.
In February 2016, large quantities of substrate material [mountain soil, peat, and river sand] were excavated from natural Sphagnum wetlands, a nearby wasteland and a rivulet, after which the materials were air-dried for 72 h in the sun and then sifted through a 3-mm mesh screen. Afterward, peat or river sand was mixed evenly into the mountain soil in proportions (on the basis of mass) of 20%, 40%, 60% and 80% (20%M + 80%P, 40%M + 60%P, 60%M + 40%P, 80%M + 20%P, 20%M + 80%R, 40%M+ 60%R, 60%M + 40%R and 80%M + 20%R; M, mountain soil; P, peat; and R, river sand), and equal amounts of the mixtures were placed in plastic pots. A substrate that included only mountain soil, without peat or river sand (M, 0%), was used as a control group. Each substrate was used in 20 pots as replicates. In March 2016, S. palustre plants were collected from a local natural Sphagnum wetland and assigned to four treatments randomly: P1, intact plant (capitulum with long stem, 20 cm in total); P2, the upper 8 cm of the intact plant (capitulum with short stem); P3, 1 cm of the apical part of the intact plant (capitulum); and P4, the remaining part of the intact plant after the upper 8 cm was removed (long stem). Shoot bundles within the same treatment composed of 5 shoots (or capitula) of similar sizes without side shoots or multiple capitula were weighed to ensure that they were as close as possible to being equal in mass and then were transplanted into plastic pots. The lower part of every shoot bundle in P1, P2 and P4 was inserted into the substrate, leaving an initial aboveground length of 5 cm, while the capitula in P3 were pressed gently to place them in full contact with the substrate surface. Local spring water (pH was approximately 6.0, conductivity was approximately 500 μS cm−1) was sprayed evenly above the S. palustre plants 3–5 times per week to ensure sufficient soil and air humidity.
Measurements of Growth Indicators and Elements
Each substrate used in the experiment was subsampled and subjected to physical and chemical analyses in accordance with the methods of Lao (1988). For the physical analyses, we used oven drying (105 °C) to determine the water content (WC), a cutting ring was used to determine the volume-weight (VW), a pycnometer was used to determine the specific gravity (SG), and the formula Porosity (%) = (1-\( \frac{VW}{SG} \)) × 100 was used to calculate the porosity. The solid phase ratio (SR), liquid phase ratio (LR) and gas phase ratio (GR) were calculated by the formulae SR(%) = (1-Porosity) × 100, LR(%)=\( \frac{WW- DW}{\rho V} \)×100 (WW, sample wet weight; DW, sample dry weight; ρ, the density of water; V, cutting ring volume), and GR(%) = (Porosity-LR) × 100, respectively. For the chemical analyses, a potentiometer was used to determine the pH of each substrate; H2SO4–HClO4 was used to digest substrates, and the indophenol blue method and molybdenum-antimony anti-spectrophotometric method were used to analyze the total N (TN) and total P (TP) contents, respectively. Additionally, alkaline hydrolysis diffusion was used to determine soil hydrolysable N (HN) contents, the Olsen method was used to determine available P (AP) content, and the oil-melt method was used to determine the OM content (data are given in Online Resource 2).
From March until November 2016, the number of capitula and average shoot length of S. palustre plants in each pot were measured monthly on fixed dates. In addition, a digital photograph with a reference frame included in it was taken at the same time, and coverage was analyzed using ArcMap (version 10.2, ESRI, USA) (data are given in Online Resources 3, 4).
In November 2016, all tissues of the S. palustre plants were harvested, and the fresh weight (biomass) per pot was measured immediately. After which, all tissues were first dried at ambient temperature and then at 70 °C for 48 h. Afterward, all dried Sphagnum capitula were removed and ground to a fine powder to measure the TN and TP contents (on a dry-mass basis). The methodology was identical to that used for the substrate (data are given in Online Resource 5).
Statistical Analysis
We divided the eight mixed substrates into two groups: one group consisted of the four mixtures with peat, and the other group consisted of the four mixtures with river sand (mixture proportions of 20%, 40%, 60% and 80%). One-way ANOVA was used to test the differences in the physical and chemical properties between the two groups (mixture types) and the differences among the five substrate mixture proportions (including 0% mountain soil) within each group. Two-way ANOVA was used to analyze the effects of the substrate mixture type and planting method, as well as their interactive effects, on S. palustre growth (the response variables included length growth, number of capitulum increments, coverage change and biomass accumulation). Moreover, a MANOVA approach was used to evaluate the effects of substrate mixture type and planting method, as well as their interactive effects, on overall S. palustre growth (four indicators combined). Within each type of substrate mixture, one-way ANOVA was used to analyze the effects of the substrate mixture proportions on S. palustre growth under different planting methods. Analyses were also performed to determine the mechanisms underlying the effects of the substrate composition and planting method on S. palustre growth. We used multiple linear regression to test the relationship between S. palustre biomass accumulation and all thirteen measured physical and chemical properties of the substrate. One-way ANOVAs were used to test the differences in initial S. palustre biomass under different substrate mixture types, different substrate mixture proportions and different planting methods. Two-way ANOVA was performed to analyze the effects of substrate mixture type and planting method, as well as their interactive effects, on N and P contents and the N:P ratio in capitula. Within each type of substrate mixture, one-way ANOVA was used to analyze the effects of the substrate mixture proportion on N and P contents and the N:P ratio in capitula. Finally, multiple linear regression was used to analyze the relationships between S. palustre biomass accumulation and initial biomass, capitula N and P contents and the N:P ratio. Pearson’s correlation coefficients (r values) were calculated to test the relationships between the N and P contents in substrates and those in capitula.
The analyses described above included only data from P1, P2 and P3. For P4, we focused on the regenerative potential of damaged S. palustre plants. Considering the particular factors in P4 (the capitula were initially removed, and minimal longitudinal growth occurred), we used number of capitulum increments as the growth indicator representing regenerative potential. The growth of P4 plants was compared with that of the plants subjected to the other three planting methods by one-way ANOVAs.
Prior to analysis, the data were checked for normality and homogeneity of variance. Any variables generating unequal variance were log or square root transformed. All statistical analyses were performed using IBM SPSS Statistics (version 19.0).
Results
Effects of Substrate Mixture Type on S. palustre Growth
Among the physical indicators of the substrates, the WC, porosity, LR and GR were higher in substrates mixed with peat than in substrates mixed with river sand, while the VW, SG and SR were lower in substrates mixed with peat than in substrates mixed with river sand. Among the chemical indicators of the substrate, TN, HN, AP and OM contents were higher in substrates mixed with peat than in substrates mixed with river sand, while the pH and TP content were not significantly different between the two types of substrate mixtures (Table 1).
There were no interactive effects between substrate mixture type and planting method on S. palustre growth (Table 2). For each of the four S. palustre growth indicators, no significant differences were found between the effects of substrates mixed with peat and the substrates mixed with river sand [length growth: 4.1 ± 1.6 cm (peat), 4.3 ± 1.8 cm (river sand); number of capitulum increments: 4.4 ± 4.3 (peat), 5.3 ± 5.4 (river sand); coverage change: 28.91 ± 16.02 cm2 (peat), 27.61 ± 16.24 cm2 (river sand); biomass accumulation: 5.56 ± 3.62 g (peat), 5.33 ± 3.29 g (river sand)]. Therefore, the type of substrate mixture did not significantly affect S. palustre growth.
Effects of Substrate Mixture Proportion on S. palustre Growth
Among the physical indicators, with increasing proportion of peat, the WC, porosity and GR increased, the VW, SG, SR decreased, and the LR did not show significant differences. All chemical indicators (pH, TN, TP, HN, AP and OM) increased with the increasing proportion of peat. Among the physical indicators, with increasing proportion of river sand, the VW, SG, SR and GR increased, and the WC, porosity and LR decreased. Among the chemical indicators, with increasing proportion of river sand, the pH and TP content increased, and the TN, HN, AP and OM contents decreased (Table 3).
S. palustre biomass accumulation tended to decrease as the proportion of peat increased, irrespective of the planting method (P1: F(4, 20) = 31.02, P < 0.001; P2: F(4, 20) = 11.08, P < 0.001; P3: F(4, 20) = 7.00, P = 0.001) (Fig. 1a). The same trend was also observed in plants growing in substrates mixed with river sand (P1: F(4, 20) = 4.18, P = 0.01; P2: F(4, 20) = 3.00, P = 0.04; P3: F(4, 19) = 7.41, P = 0.001) (Fig. 1b).
Effects of the proportion (0%, 20%, 40%, 60%, 80%) of (a) peat (P) or (b) river sand (R) in the mixture on the biomass accumulations (means ± SDs, during eight months) of Sphagnum palustre under three planting methods (P1, P2 and P3). [n = 25 for each planting method in the two substrate mixtures, except for P3 in mixtures with river sand (n = 24). Different letters above the bars indicate significant differences (P < 0.05) by one-way ANOVA; Tukey tests were used for multiple comparisons. Each part (a, b) of the figure was created by Microsoft Excel 2013, and the whole figure was created by Photoshop]
Effects of the Planting Method on S. palustre Growth
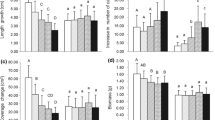
Each of the four growth indicators showed significant differences among the three planting methods (Table 2, Fig. 2). Length growth was highest in P1, intermediate in P2, and lowest in P3, while the other three growth indicators (number of capitulum increments, coverage change and biomass accumulation) responded similarly to the planting method, with the highest values in P1 and no remarkable differences in values between P2 and P3.
Effects of planting method (P1, P2 and P3) on a length growth, b number of capitulum increments, c coverage change and d biomass accumulation (means ± SDs, during 8 months) (n = 119 for each growth indicator) in Sphagnum palustre. [Different letters above the bars indicate significant differences (P < 0.05) by two-way ANOVA; Tukey tests were used for multiple comparisons. Each part (a, b, c and d) of the figure was created by Microsoft Excel 2013, and the whole figure was created by Photoshop]
S. palustre Growth in Planting Method 4
Ninety-eight percent of the transplanted S. palustre plants in P4 were able to grow new capitula from their stems though the upper 8 cm, and all initial capitula were removed; moreover, new capitula were able to grow from any part of the plant stem. The number of capitulum increments in P4 (5.2 ± 3.6) was significantly lower than that in P1 (10.9 ± 5.0) (F(1,88) = 38.51, P < 0.001) but significantly higher than that in P2 (2.6 ± 3.1, F(1,88) = 19.15, P < 0.001) and P3 (3.1 ± 2.7, F(1,88) = 12.47, P < 0.001).
Similar to S. palustre growth under the other three planting methods, the number of capitulum increments in P4 was not affected by the type of substrate mixture (peat: 4.4 ± 2.4, river sand: 4.8 ± 3.8, F(1,37)=0.11, P = 0.74), but tended to decrease as the proportions of peat or river sand increased (peat: F(4, 20) = 9.69, P < 0.001; river sand: F(4, 20) = 5.09, P = 0.005) (Fig. 3).
Effects of the proportion (0%, 20%, 40%, 60%, 80%) of peat or river sand in the mixture on the number of capitulum increments (means ± SDs, during 8 months) of Sphagnum palustre under planting method 4. [n = 25 for each substrate mixture. Different letters above the bars indicate significant differences (P < 0.05) by one-way ANOVA; Tukey tests were used for multiple comparisons. The figure was created by Microsoft Excel 2013]
Effects of Physical and Chemical Properties on S. palustre Growth
According to the multiple linear regression analysis, among the thirteen indicators of the physical and chemical properties of the substrate, only TP and pH showed significant correlations with biomass accumulation (biomass accumulation = −7.53 TP-1.81 pH + 24.53; F(2,78) = 4.70, P = 0.01), and both indicators were negatively correlated with biomass accumulation.
Effects of Initial Biomass and Capitula N and P Contents on S. palustre Growth
No significant differences in the initial biomass of S. palustre were found between the two types of substrate mixture (F(1,117) = 0.06, P = 0.80) or among the different substrate mixture proportions (peat: F(4,70) = 0.11, P = 0.98; river sand: F(4,69) = 0.08, P = 0.99). However, pronounced differences in the initial biomass of S. palustre existed among the three planting methods (F(2,116) = 899.44, P < 0.001), with the highest values in P1 (7.24 ± 1.00 g), intermediate values in P2 (3.28 ± 0.34 g) and lowest values in P3 (1.53 ± 0.03 g).
There were no interactive effects between the substrate mixture type and the planting method on N and P contents and N:P ratios in the capitula of S. palustre (ANOVA results are given in Online Resource 6); however, each affected the N and P contents and N:P ratios in the capitula of S. palustre (Fig. 4). The N contents and N:P ratios were significantly higher in substrates mixed with peat than in substrates mixed with river sand (Fig. 4a and c), but the P contents were not obviously different (Fig. 4b). The N content remained unchanged under all three planting methods (Fig. 4d). P content was highest in P2, while no obvious difference existed between P contents in P1 and P3 (Fig. 4e). N:P ratios were the lowest in P2, while no obvious difference existed between ratios in P1 and P3 (Fig. 4f).
Effects of the a, b, c substrate mixture type (peat and river sand) (n = 72 for each indicator) and d, e, f planting method (P1, P2, P3) (n = 72 for each indicator) on N and P contents and the N:P ratios (means ± SDs) in capitula of Sphagnum palustre. [Different letters above the bars indicate significant differences P < 0.05 by two-way ANOVA; Tukey tests were used for multiple comparisons. Each part (a, b, c, d, e and f) of the figure was created by Microsoft Excel 2013, and the whole figure was created by Photoshop]
The substrate mixture proportion also affected the N and P contents and N:P ratios in the capitula of S. palustre (Fig. 5). For plants grown in substrates mixed with peat, as the proportions of peat increased, the N contents increased, N:P ratios remained unchanged, and P contents showed a slight but not significant increase. For plants grown in substrates mixed with river sand, as the proportions of sand increased, the N contents remained unchanged, P contents increased, and N:P ratios decreased.
Effects of the proportion (0%, 20%, 40%, 60%, 80%) of peat (n = 45 for each indicator) or river sand (n = 45 for each indicator) in the mixture on a N and b P contents and c the N:P ratios (means ± SDs) in capitula of Sphagnum palustre. [Different letters above the bars indicate significant differences P < 0.05 by one-way ANOVA; Tukey tests were used for multiple comparisons. Each part (a, b and c) of the figure was created by Microsoft Excel 2013, and the whole figure was created by Photoshop]
According to the multiple linear regression analysis, among the four factors, only the initial biomass and P content in capitula showed significant correlations with biomass accumulation (biomass accumulation = 1.16 initial biomass-7.14 P + 6.19; F(2,78) = 135.84, P < 0.001). Initial biomass was positively correlated with biomass accumulation, while the P content in capitula was negatively correlated with biomass accumulation.
Correlations Between the N and P Contents in Capitula and those in the Substrate
Both the N and P contents in the capitula of S. palustre were positively correlated with those in the substrate in which S. palustre was cultivated [the r values for the correlations between N contents in the substrate and that in the capitula of S. palustre in P1, P2 and P3 were 0.54 (P = 0.003), 0.58 (P = 0.002) and 0.52 (P = 0.005), respectively; the r values for the correlations between the P contents in the substrate and that in the capitula of S. palustre in P2 and P3 were 0.62 (P = 0.001) and 0.68 (P < 0.001), respectively]. However, there was no significant correlation between the P content in capitula and that in the substrate for P1 (r = 0.09, P = 0.66).
Discussion
Composition and Proportion of the Mixed Substrate
The effects of substrate mixture type on S. palustre growth differed from what we predicted. We initially expected significant differences in S. palustre growth between plants grown in substrate mixed with peat and those grown in substrate mixed with river sand as a result of differences in the physical and chemical properties of the substrates. However, the results show that S. palustre growth in the two substrate mixture groups was not obviously different even though the physical and chemical properties showed obvious and expected differences (Table 1). On the other hand, within each substrate mixture group, as the proportion of peat or river sand increased and the physical and chemical properties changed (Table 3), S. palustre growth decreased (Figs. 1 and 3).
This seems to be a contradictory result. We speculated that, although the growth of S. palustre can be affected by various biotic and abiotic factors (Gunnarsson 2005; Pouliot et al. 2015), in this particular situation, growth would be primarily limited by only one or few factors. In our results, the negative correlation between S. palustre productivity (biomass accumulation) and P contents in capitula suggested that the growth of S. palustre depends on this P content. Furthermore, the negative correlation between S. palustre productivity and P content in the substrate and the highly positive correlation between the N and P contents in the substrate and those in the capitula of S. palustre confirmed our speculation: the nutrient contents in the substrate affected the amount of nutrients taken up and assimilated in capitula and further affected S. palustre growth. This may be the main mechanism underlying the effects of substrate on S. palustre growth. Therefore, we speculated that the lack of an obvious difference in the background P contents between the two substrate mixture groups (Table 1) led to no obvious difference in the P contents of capitula (Fig. 4b). This resulted in no detectable differences in S. palustre growth when transplanted into two different substrates mixtures (Table 2). Similarly, the continuous increase in the substrate P content with increasing proportion of peat or river sand (Table 3) caused a corresponding increase (or a slight increase) in the P content in capitula (peat: from 0.63 ± 0.13 mg g−1 to 0.79 ± 0.11 mg g−1; river sand: from 0.63 ± 0.13 mg g−1 to 0.84 ± 0.12 mg g−1) (Fig. 5b), which further resulted in a continuous decrease in S. palustre growth (Figs. 1 and 3). On the other hand, the multiple regression analysis suggested that in addition to the P content, the increased pH of the substrate also negatively affected S. palustre growth. Therefore, another possibility was that no significant difference in the pH between the two substrate mixture groups (Table 1) resulted in no differences in S. palustre growth. In contrast, the continuous increase in pH in substrates with increasing proportions of peat or river sand (Table 3) resulted in a continuous decrease in S. palustre growth (Figs. 1 and 3).
The effects of P on S. palustre growth are usually influenced by other nutrients, especially N (Gusewell and Koerselman 2002; Bubier et al. 2007; Wendel et al. 2011). Koerselman and Meuleman (1996) posited that the N:P ratio reflects relative nutrient availability and can be used as an indicator to evaluate the limiting nutrient. For species in the genus Sphagnum, some studies (Bragazza et al. 2004; Hajek and Adamec 2009; Chiwa et al. 2016) proposed that growth would be limited by N availability at N:P ratios less than 30, in which case P would have a negative effect on Sphagnum growth. In our experiment, the N:P ratio (9.22 ± 2.51) in S. palustre capitula was much lower than 30. In addition, Bragazza et al. (2005) suggested that N would play a limiting role when its concentration in Sphagnum capitula was approximately 6 mg g−1; this value is similar to ours (6.46 ± 1.19 mg g−1). Also, other studies reported higher concentrations of N with lower (or similar) concentrations of P in Sphagnum capitula from unpolluted areas (Brock and Bregman 1989; Heijmans et al. 2002; Limpens et al. 2003; Fritz et al. 2012). Therefore, one possibility for the negative effects of P on Sphagnum are related to imbalances in nutrient stoichiometry triggered by the low N concentration. However, this speculation requires additional experimental verification.
Species of the genus Sphagnum are generally acidophilic calcifuges and are highly sensitive to acidity and alkalinity (Quinty and Rochefort 2003; Vicherova et al. 2017). Previous studies showed that the optimal pH range for S. palustre is 4.5–6.0 (Andrus 1986; Chen et al. 2009; Ye et al. 2012; Tahvanainen and Haraguchi 2013; Harpenslager et al. 2015; Riegel and Wilde 2016) and that above pH 6.0, the growth of S. palustre decreases as pH increases (Wang 2010), which was in accordance with our results. There are two possible reasons for the negative effects of increased pH on S. palustre productivity in our study. On the one hand, pH was strongly and positively related to the P content in the substrate (Pearson coefficient r = 0.79, P < 0.001), and increased P content with increased substrate pH likely caused the decrease in S. palustre productivity. On the other hand, the increased substrate pH is usually associated with increased concentrations of Ca2+, Mg2+ and HCO3− (Clymo 1973; Gorham and Janssens 1992; Lamers et al. 1999), which may cause toxicity symptoms in S. palustre by interfering with cellular uptake of monovalent cations (such as K+ and NH4+) (Hajek and Adamec 2009), or by interfering with cellular metabolism (Vicherova et al. 2015, 2017), negatively affecting S. palustre growth (Hajek et al. 2006; Bengtsson et al. 2018).
Our results demonstrate that the yellow-brown soil, which is distributed widely in subtropical regions, is an optimal substrate for S. palustre growth, probably due to its favorable pH and P content. This finding has important implications as it indicates that S. palustre restoration does not dependent on the use of peat, allowing for S. palustre restoration on large, less restrictive scales.
Planting Method
As expected, different planting methods resulted in differences in S. palustre growth, with the best performance in P1 and no pronounced differences in growth between P2 and P3 (Fig. 2). The multiple regression analysis suggested that S. palustre productivity was positively correlated with initial biomass and negatively correlated with P content in capitula. Therefore, compared to values for the other two planting methods, the higher initial biomass and the lower P contents in capitula (Fig. 4e) in P1 contributed to the greatest amount of growth. For the remaining two planting methods, both the initial biomass and capitula P contents were higher in P2 than in P3 (Fig. 4e). Therefore, it is likely that the positive effects related to initial biomass together with the negative effects related to P content cancelled each other out, resulting in nonsignificant growth differences between P2 and P3.
The negative correlation between S. palustre productivity and the P contents in capitula has been discussed previously. The positive effects of initial biomass (or initial fragment size) on Sphagnum growth have been reported by earlier studies. Gunnarsson and Soderstron (2007) compared Sphagnum angermanicum growth under four planting methods and found that a larger diaspore size resulted in better growth; moreover, S. angermanicum showed the greatest growth when transplanting whole shoots. Robroek et al. (2007, 2009) reported similar results. These authors explained that the ability of Sphagnum species to supply its capitula with water increased with increasing fragment size, suggesting that Sphagnum diaspores of a larger size are better at creating and maintaining microhydrological conditions that are favorable for growth. In contrast, Sphagnum diaspores of a smaller size are more sensitive to the environment and to seasonal variations in temperature and moisture conditions, leading to less growth. In addition, Robroek et al. (2009) found that at the early stage of Sphagnum plant transplantation, the differences between the microenvironment in the new locality and that in the original habitat led to decreased competitive ability and further reduced coverage. In our experiment, compared to the growth environment in P2 and P3, that of the upper viable parts of Sphagnum plants in P1 was closer to the original environment in natural Sphagnum wetlands, as they grow on the same basal material, i.e., senescent but undecomposed parts of Sphagnum plants (also called ‘white peat’). This may also contribute to the better performance of S. palustre in P1.
In terms of planting method 4, 98 % of the damaged S. palustre plants were able to grow new capitula from any part of the plant stem, suggesting that damaged plants can still regenerate and were no longer affected by apical dominance after their capitula were removed (Rydin and Clymo 1989; Daniels and Eddy 1990). The number of capitulum increments in P4 was lower than that in P1, but higher than that in P2 and P3, suggesting that the regenerative potential of the damaged S. palustre plants was decreased when the upper parts were removed, but was stronger than that of S. palustre plants with only several centimeters of the upper part of the plant. Therefore, our study demonstrated that the common peatland restoration method using the upper parts of S. palustre as transplanted materials is practical and has no destructive effects on the source S. palustre populations in the areas where they are collected. However, because Sphagnum vitality decreases continuously with increasing distance from the capitula, and the depth at which fragments of Sphagnum plants can regenerate new capitula varies greatly between species (Rochefort et al. 2003), the collection depth needs to be determined carefully. From this perspective, we can expect that planting intact plants would cause greatest effects on the source Sphagnum populations, while planting only the capitula would cause minimal effects. Therefore, compared with the traditional transplanting method (P2), the method involving transplanting only capitula has the similar productivity but does less damage to the source S. palustre populations, making it more suitable for S. palustre restoration.
Conclusion and Implications
The results of this study show that the substrate composition and length of shoot fragment significantly affect the growth of S. palustre, which is the dominant peat-forming species in the subtropical high-mountain peatland regions of China. The substrate can affect S. palustre growth via its background nutrient contents. Although close correlations between the nutrient contents in soils and those in vascular plants have been reported by other studies, our results still have important implications for the transplantation and restoration of Sphagnum mosses considering their absence of roots and vascular tissues. In addition to the nutrient contents in the substrate, the pH of the substrate also plays an important role in S. palustre growth. Compared with peat, which is used as the only substrate in traditional peatlands restoration technique, the local yellow-brown soil, which is widely distributed in subtropical mountain regions, seemed to be a better substrate for S. palustre growth. The results show that S. palustre restoration practices can be independent of the distribution and range of peatlands, which may provide a new approach for restoring peatland ecosystems in these regions. Furthermore, our study demonstrated that the common restoration technique of collecting the upper parts of S. palustre plants for transplantation does not destroy the source populations in natural Sphagnum wetlands. However, considering the similar productivity and less impact to the source S. palustre populations, a planting method using only capitula may be a better choice for S. palustre restoration.
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Aldous AR (2002) Nitrogen translocation in Sphagnum mosses: effects of atmospheric nitrogen deposition. New Phytologist 156:241–253. https://doi.org/10.1046/j.1469-8137.2002.00518.x
Andersen R, Rochefort L, Poulin M (2010) Peat, water and plant tissue chemistry monitoring: a seven-year case-study in a restored peatland. Wetlands 30:159–170. https://doi.org/10.1007/s13157-009-0015-0
Andrus RE (1986) Some aspects of Sphagnum ecology. Canadian Journal of Botany 64:416–426. https://doi.org/10.1139/b86-057
Bengtsson F, Rydin H, Hajek T (2018) Biochemical determinants of litter quality in 15 species of Sphagnum. Plant and Soil 425:161–176. https://doi.org/10.1007/s11104-018-3579-8
Binet P, Rouifed S, Jassey VEJ, Toussaint ML, Chiapusio G (2017) Experimental climate warming alters the relationship between fungal root symbiosis and Sphagnum litter phenolics in two peatlands. Soil Biology and Biochemistry 105:153–161. https://doi.org/10.1016/j.soilbio.2016.11.020
Bragazza L, Freeman C (2007) High nitrogen availability reduces polyphenol content in Sphagnum peat. Science of the Total Environment 377:439–443. https://doi.org/10.1016/j.scitotenv.2007.02.016
Bragazza L, Tahvanainen T, Kutnar L, Rydin H, Limpens J, Hajek M, Grosvernier P, Hajek T, Hajkova P, Hansen I, Iacumin P, Gerdol R (2004) Nutritional constraints in ombrotrophic Sphagnum plants under increasing atmospheric nitrogen deposition in Europe. The New Phytologist 163:609–616. https://doi.org/10.1111/j.1469-8137.2004.01154.x
Bragazza L, Limpens J, Gerdol R, Grosvernier P, Hajek M, Hajek T, Hajkova P, Hansen I, Iacumin P, Kutnar L, Rydin H, Tahvanainen T (2005) Nitrogen concentration and δ15N signature of ombrotrophic Sphagnum mosses at different N deposition levels in Europe. Global Change Biology 11:106–114. https://doi.org/10.1111/j.1365-2486.2004.00886.x
Bridgham SD, Pastor J, Janssens JA, Chapin C, Malterer TJ (1996) Multiple limiting gradients in peatlands: a call for a new paradigm. Wetlands 16:45–65. https://doi.org/10.1007/BF03160645
Brock TCM, Bregman R (1989) Periodicity in growth, productivity, nutrient content and decomposition of Sphagnum recurvum var. mucronatum in a fen woodland. Oecologia 80:44–52. https://doi.org/10.1007/BF00789930
Bubier J, Moore T, Bledzki LA (2007) Effects of nutrient addition on vegetation and carbon cycling in an ombrotrophic bog. Global Change Biology 13:1–19. https://doi.org/10.1111/j.1365-2486.2007.01346.x
Bugnon JL, Rochefort L, Price J (1997) Field experiment of Sphagnum reintroduction on a dry abandoned peatlands in eastern Canada. The Society of Wetland Scientists 17:513–517. https://doi.org/10.1007/BF03161517
Campeau S, Rochefort L (1996) Sphagnum regeneration on bare peat surfaces: field and greenhouse experiments. Journal of Applied Ecology 33:599–608. https://doi.org/10.2307/2404988
Chapin CT, Bridgham SD, Pastor J (2004) pH and nutrient effects on above-ground net primary production in a Minnesota, USA bog and fen. Wetlands 24:186–201. https://doi.org/10.1672/0277-5212(2004)024[0186:PANEOA]2.0.CO;2
Chen X, Bu ZJ, Wang SZ et al (2009) Niches of seven bryophyte species in Hani peat land of Changbai Mountains. Chinese Journal of Applied Ecology 20:574–578. https://doi.org/10.13287/j.1001-9332.2009.0076
Chiwa M, Sheppard LJ, Leith ID et al (2016) Sphagnum can ‘filter’ N deposition, but effects on the plant and pore water depend on the N form. Science of the Total Environment 559:113–120. https://doi.org/10.1016/j.scitotenv.2016.03.130
Clymo RS (1970) The growth of Sphagnum: method of measurement. Journal of Ecology 58:13–49. https://doi.org/10.2307/2258168
Clymo RS (1973) The growth of Sphagnum – some effects of environment. Journal of Ecology 61:849–869. https://doi.org/10.2307/2258654
Clymo RS, Duckett JG (1986) Regeneration of Sphagnum. New Phytologist 102:589–614. https://doi.org/10.1111/j.1469-8137.1986.tb00834.x
Clymo RS, Turunen J, Tolonen K (1998) Carbon accumulation in peatland. Oikos 81:368–388. https://doi.org/10.2307/3547057
Daniels RE, Eddy A (1990) Handbook of European Sphagna, 2nd edn. Institute of Terrestrial Ecology, Huntingdon
Fritz C, van Dijk G, Smolders AJP et al (2012) Nutrient additions in pristine Patagonian Sphagnum bog vegetation: can phosphorus addition alleviate (the effects of) increased nitrogen loads. Plant Biology 14:491–499. https://doi.org/10.1111/j.1438-8677.2011.00527.x
Gorham E (1991) Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecological Applications 1:182–195. https://doi.org/10.2307/1941811
Gorham E, Janssens JA (1992) Concepts of fen and bog re-examined in relation to bryophyte cover and the acidity of surface waters. Acta Societatis Botanicorum Poloniae 61:7–20. https://doi.org/10.5586/asbp.1992.001
Graf MD, Rochefort L, Poulin M (2008) Spontaneous revegetation of cutaway peatlands of North America. Wetlands 28:28–39. https://doi.org/10.1672/06-136.1
Granath G, Strengbom J, Breeuwer A, Heijmans MMPD, Berendse F, Rydin H (2009) Photosynthetic performance in Sphagnum transplanted along a latitudinal nitrogen deposition gradient. Oecologia 159:705–715. https://doi.org/10.1007/s00442-008-1261-1
Granath G, Limpens J, Posch M (2014) Spatio-temporal trends of nitrogen deposition and climate effects on Sphagnum productivity in European peatlands. Environmental Pollution 187:73–80. https://doi.org/10.1016/j.envpol.2013.12.023
Gunnarsson U (2005) Global patterns of Sphagnum productivity. Journal of Bryology 27:269–279. https://doi.org/10.1179/174328205X70029
Gunnarsson U, Soderstron L (2007) Can artificial introductions of diaspore fragments work as a conservation tool for maintaining populations of the rare peatmoss Sphagnum angermanicum? Biological Conservation 35:450–458. https://doi.org/10.1016/j.biocon.2006.10.014
Gusewell S, Koerselman W (2002) Variation in nitrogen and phosphorus concentrations of wetland plants. Urban & Fischer Verlag 5:37-61. https://doi.org/10.1078/1433-8319-0000022
Hajek T, Adamec L (2009) Mineral nutrient economy in competing species of Sphagnum moss. Ecological Research 24:291–302. https://doi.org/10.1007/s11284-008-0506-0
Hajek M, Horsak M, Hajkova P et al (2006) Habitat diversity of central European fens in relation to environmental gradients and an effort to standardise fen terminology in ecological studies. Perspective in Plant Ecology Evolution and Systematics 8:97–114. https://doi.org/10.1016/j.ppees.2006.08.002
Hajek T, Balance S, Limpens J et al (2011) Cell-wall polysaccharides play an important role in decay resistance of Sphagnum and actively depressesed decomposition in vitro. Biogeochemistry 103:45–57. https://doi.org/10.1007/s10533-010-9444-3
Harpenslager SF, van Dijk G, Kosten S, Roelofs JGM, Smolders AJP, Lamers LPM (2015) Simultaneous high C fixation and high C emissions in Sphagnum mires. Biogeosciences 12:4465–4494. https://doi.org/10.5194/bgd-12-4465-2015
Heijmans MMPD, Klees H, Berendse F (2002) Competition between Sphagnum magellanicum and Eriophorum angustifolium as affected by raised CO2 and increased N deposition. Oikos 97:415–425. https://doi.org/10.1034/j.1600-0706.2002.970311.x
Hommeltenberg J, Schmid HP, Drosler M (2014) Can a bog drained for forestry be a stronger carbon sink than a natural bog forest? Biogeosciences 11:3477–3493. https://doi.org/10.5194/bg-11-3477-2014
Hoosbeek MR, van Breemen N, Vasander H (2002) Potassium limits potential growth of bog vegetation under elevated atmospheric CO2 and N deposition. Global Change Biology 8:1130–1138. https://doi.org/10.1046/j.1365-2486.2002.00535.x
Karofeld E, Muur M, Vellak K (2016) Factors affecting re-vegetation dynamics of experimentally restored extracted peatland in Estonia. Environmental Science and Pollution Research 23:13706–13717. https://doi.org/10.1007/s11356-015-5396-4
Kim S, Kim Y, Kim Y et al (2014) Effects of planting method and nitrogen addition on Sphagnum growth in microcosm wetlands. Paddy and Water Environment 12(Supp.):185-192. https://doi.org/10.1007/s10333-014-0427-1
Koerselman W, Meuleman AFM (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. Journal of Applied Ecology 33:1441–1450. https://doi.org/10.2307/2404783
Lamers LPM, Farhoush C, van Groenendael JM, Roelofs JGM (1999) Calcareous groundwater raises bogs; the concept of ombrotrophy revisited. Journal of Ecology 87:639–648. https://doi.org/10.1046/j.1365-2745.1999.00380.x
Lao JC (1988) Handbook of soil agro-chemistrical analysis. Agricultural Publishing House, Beijing
Li TT, Wang ZX, Lei Y (2018) Vegetation ecology of Sphagnum wetlands in subtropical subalpine regions: a case study in Qi Zimei Mountains. In: Greller A, Fujiwara K, Pedrotti F (eds) Geographical changes in vegetation and plant functional types. Geobotany Studies (Basics, Methods and Case Studies). Springer, Cham, pp 281–288
Limpens J, Heijmans MMPD (2008) Swift recovery of Sphagnum nutrient concentrations after excess supply. Oecologia 157:153–161. https://doi.org/10.1007/s00442-008-1046-6
Limpens J, Berendse F, Klees H (2003) N deposition affects N availability in interstitial water, growth of Sphagnum and invasion of vascular plants in bog vegetation. The New Phytologist 157:339–347. https://doi.org/10.1046/j.1469-8137.2003.0067.x
Limpens J, Bohlin E, Nilsson MB (2017) Phylogenetic or environmental control on the elemental and organo-chemical composition of Sphagnum mosses? Plant and Soil 417:69–85. https://doi.org/10.1007/s11104-017-3239-4
Liu SX, Qu JP, Jiang YF et al (2006) Hubei Qizimeishan nature reserve scientific survey and research report. Hubei publishers of science and technology, Wuhan
Ma GL, Lei Y, Wang ZX et al (2008) Plant diversity of Sphagnum mire at Qizimei Mountations in Western Hubei Province. Journal of Wuhan Botanical Research 26(5):482–488
Malmer N, Albinsson C, Svensson BM, Wallen B (2003) Interferences between Sphagnum and vascular plants: effects on plant community structure and peat formation. Oikos 100:469–482. https://doi.org/10.1034/j.1600-0706.2003.12170.x
Manninen S, Kivimaki S, Leith ID et al (2016) Nitrogen deposition does not enhance Sphagnum deposition. Science of the Total Environment 571:314–322. https://doi.org/10.1016/j.scitotenv.2016.07.152
Mao R, Wang ZX, Lei Y et al (2009) Profile characteristics and element vertical distribution in Sphagnum wetlands in Qizimei Mountains nature reserve, Hubei. Acta Pedologica Sinica 46(1):159–163
Medvedeff C, Bridgham SD, Meister LP et al (2015) Can Shagnum leachate chemistry explain differences in anaerobic decomposition in peatlands? Soil Biology & Biochemistry 86:34–41. https://doi.org/10.1016/j.soilbio.2015.03.016
Nishimura A, Tsuyuzaki S (2014) Effects of water level via controlling water chemistry on revegetation patterns after peat mining. Wetlands 33:117–127. https://doi.org/10.1007/s13157-013-0490-1
Pouliot R, Hugron S, Rochefort L (2015) Sphagnum farming: a long-term study on producing peat moss biomass sustainably. Ecological Engineering 74:135–147. https://doi.org/10.1016/j.ecoleng.2014.10.007
Price JS, Heathwaite AL, Baird AJ (2003) Hydrological processes in abandoned and restored peatlands: an overview of management approaches. Wetlands Ecology and Management 11:65–83. https://doi.org/10.1023/A:1022046409485
Quinty F, Rochefort L (2003) Peatland restoration guide, 2nd edn. Canadian Sphagnum Peat Moss Association and New Brunswick Department of Natural Resources and Energy. Québec, Québec
Riegel W, Wilde V (2016) An early Eocene Sphagnum bog at Schoningen, northern Germany. International Journal of Coal Geology 159:57–70. https://doi.org/10.1016/j.coal.2016.03.021
Robroek BJM, Limpens J, Breeuwer A et al (2007) Interspecific competition between Sphagnum mosses at different water tables. Functional Ecology 21:805–812. https://doi.org/10.1111/j.1365-2435.2007.01269.x
Robroek BJM, van Ruijven J, Schouten MGC, Breeuwer A, Crushell PH, Berendse F, Limpens J (2009) Sphagnum re-introduction in degraded peatlands: the effects of aggregation, species identity and water table. Basic and Applied Ecology 10:697–706. https://doi.org/10.1016/j.baae.2009.04.005
Rochefort L, Price J (2003) Restoration of Sphagnum dominated peatlands. Wetlands Ecology and Management 11:1–2. https://doi.org/10.1023/A:1022010802129
Rochefort L, Quinty F, Campeau S, Johnson K, Malterer T (2003) North American approach to the restoration of Sphagnum dominated peatlands. Wetlands Ecology and Management 11:3–20. https://doi.org/10.1023/A:1022011027946
Rydin H, Clymo RS (1989) Transport of carbon and phosphorus compounds about Sphagnum. Proceedings of the Royal Society B 237:63–84. https://doi.org/10.1098/rspb.1989.0037
Rydin H, Jeglum JK (2013) The biology of peatlands, 2nd edn. Oxford University Press, Oxford, New York. https://doi.org/10.1093/acprof:osobl/9780199602995.001.0001
Shaw AJ, Devos N, Liu Y et al (2016) Organellar phylogenomics of an emerging model system: Sphagnum (peatmoss). Annals of Botany 118:185–196. https://doi.org/10.1093/aob/mcw086
Tahvanainen T, Haraguchi A (2013) Effect of pH on phenol oxidase activity on decaying Sphagnum mosses. European Journal of Soil Biology 54:41–47. https://doi.org/10.1016/j.ejsobi.2012.10.005
Thompson DK, Waddington JM (2008) Sphagnum under pressure: towards an ecohydrological approach to examining Sphagnum productivity. Ecohydrology 1:299–308. https://doi.org/10.1002/eco.31
van Breemen N (1995) How Sphagnum bogs down other plants. Trends in Ecology & Evolution 10:270–275. https://doi.org/10.1016/0169-5347(95)90007-1
Vasander H, Tuittila ES, Lode E, Lundin L, Ilomets M, Sallantaus T, Heikkilä R, Pitkänen ML, Laine J (2003) Status and restoration of peatlands in northern Europe. Wetlands Ecology and Management 11:51–63. https://doi.org/10.1023/A:1022061622602
Vicherova E, Hajek M, Hajek T (2015) Calcium intolerance of fen mosses: physiological evidence, effects of nutrient availability and successional drivers. Perspectives in Plant Ecology Evolution and Systematics 17:347–359. https://doi.org/10.1016/j.ppees.2015.06.005
Vicherova E, Hajek M, Smilauer P et al (2017) Sphagnum establishment in alkaline fens: importance of weather and water chemistry. Science of the Total Environment 580:1429–1438. https://doi.org/10.1016/j.scitotenv.2016.12.109
Waddington JM, Rochefort L, Campeau S (2003) Sphagnum production and decomposition in a restored peatland. Wetlands Ecology and Management 11:85–95. https://doi.org/10.1023/A:1022009621693
Wang XY (2010) Effect of pH and nutrition elements on growth of Sphagnum plants. Guizhou Agricultural Science 38:80–83
Wang ZX, Lei Y, Xiong KC et al (2013) Comprehensive scientific investigation and research of Sphagnum wetlands for Qizimei mountains national nature reserve in Hubei. China Forestry Publishing House, Beijing
Weltzin JF, Harth C, Bridgham SD et al (2001) Production and microtopography of bog bryophytes: response to warming and water-table manipulations. Oecologia 28:557–565. https://doi.org/10.1007/s004420100691
Wendel S, Moore T, Bubier J (2011) Experimental nitrogen, phosphorus, and potassium deposition decreases summer soil temperatures, water contents, and soil CO2 concentrations in a northern bog. Biogeosciences 8:585–595. https://doi.org/10.5194/bg-8-585-2011
Ye R, Jin Q, Bohannan B, Keller JK, McAllister SA, Bridgham SD (2012) pH controls over anaerobic carbon mineraliza- tion, the efficiency of methane production, and methanogenic pathway in peatlands across an ombrotrophic-minerotrophic gradient. Soil Biology and Biochemistry 54:36–47. https://doi.org/10.1016/j.soilbio.2012.05.015
Zhao ST, Li EH, Cai XB et al (2013) Research on the higher plant diversity of subalpine Sphagnum mire in western Hubei province, China. Resources and Environment in the Yangtze Basin 22:468–475
Zhu HJ, Chen JF, Chen SL et al (2010) Soil geography, 2nd edn. Higher Education Press, Beijing
Acknowledgments
We thank Kai Tian, Binmei Xiong, Ze Li, Qianqian Peng, Yu Chen, and Shichao Li for their help with growth indicator measurements and Lan fang Yang, Qichi Yang, Yuyang Xu, and Bangjun Lin for their help with physical and chemical property measurements of plants and substrates. This experiment complies with all laws of the People’s Republic of China, where it was performed.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 41471041); the Technology Innovation Planning Project of Hubei Province, China (2017ABA161); and the Open Foundation of the Hubei Key Laboratory of Regional Development and Environmental Response, China (2017(A) 001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement of Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, TT., Liu, T., Lei, Y. et al. Effects of the Substrate and Planting Method on Sphagnum palustre Growth in Subtropical High-Mountain Regions and the Underlying Mechanisms. Wetlands 39, 879–893 (2019). https://doi.org/10.1007/s13157-019-01127-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-019-01127-0