Abstract
Bog vegetation, which is dominated by Sphagnum mosses, depends exclusively on aerial deposition of mineral nutrients. We studied how the main mineral nutrients are distributed between intracellular and extracellular exchangeable fractions and along the vertical physiological gradient of shoot age in seven Sphagnum species occupying contrasting bog microhabitats. While the Sphagnum exchangeable cation content decreased generally in the order Ca2+ ≥ K+, Na+, Mg2+ > Al3+ > NH4 +, intracellular element content decreased in the order N > K > Na, Mg, P, Ca, Al. Calcium occurred mainly in the exchangeable form while Mg, Na and particularly K, Al and N occurred inside cells. Hummock species with a higher cation exchange capacity (CEC) accumulated more exchangeable Ca2+, while the hollow species with a lower CEC accumulated more exchangeable Na+, particularly in dead shoot segments. Intracellular N and P, but not metallic elements, were consistently lower in dead shoot segments, indicating the possibility of N and P reutilization from senescing segments. The greatest variation in tissue nutrient content and distribution was between species from contrasting microhabitats. The greatest variation within microhabitats was between the dissimilar species S. angustifolium and S. magellanicum. The latter species had the intracellular N content about 40% lower than other species, including even this species when grown alone. This indicates unequal competition for N, which can lead to outcompeting of S. magellanicum from mixed patches. We assume that efficient cation exchange enables Sphagnum vegetation to retain immediately the cationic nutrients from rainwater. This may represent an important mechanism of temporal extension of mineral nutrient availability to subsequent slow intracellular nutrient uptake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ombrotrophic (rain-fed) bogs are peatlands in which the surface layers are hydrologically isolated from the surrounding landscape. Therefore, the bog plants receive mineral nutrients exclusively from both wet and dry atmospheric deposition. Sphagnum mosses play a major role in the fixation of carbon and mineral nutrients in bogs, the latter due to the ability of whole moss plants to take up nutrients. Sphagna are thus allowed to control nutrients entering the top soil. These traits are an important advantage in the competition with rooting vascular plants in such nutrient-poor habitats as ombrotrophic bogs (Malmer et al. 1994; Aldous 2002b).
The mechanisms of nutrient transport and uptake at the plant–water interface are generally the same in both vascular plants and mosses. Cell walls of the root tip and the moss leaf have an acidic character—they release protons from exchange sites, mostly carboxylic groups of uronic acid (Knight et al. 1961). Therefore, they become negatively charged and ready to form electrostatic interactions with cations. The cation exchange is an extracellular, passive process in which cations (including protons) compete for an exchange site. Although the physiological role of cation exchange in mosses (or plants generally) has never been exactly established (Dainty and Richter 1993), it can be regarded as a concentrating mechanism improving the availability of cations for their further intracellular uptake mediated by specific transport sites (Bates 1989; Büscher et al. 1990; Wells and Brown 1990).
Several authors have reported that shoots of Sphagnum species, living or dead, have an unusually high cation exchange capacity (CEC), i.e., capacity to bind a cation in a solution at a given pH and concentration of that cation, in comparison with other plants (Anschütz and Geßner 1955; Clymo 1963; Brehm 1968). Sphagnum species or ecotypes occupying elevated bog hummocks have a higher CEC than those growing in lower-situated and wet microhabitats such as carpets and particularly hollows (Clymo 1963; Spearing 1972). Regardless of the role of CEC in plants generally, the high CEC enables sphagna to maintain efficient cation exchange in bogs, notably in Sphagnum hummocks, although the CEC is reduced by strongly acidic conditions due to reduced dissociation of the ion-exchanger in the cell walls.
Although many studies have dealt with mineral nutrients, particularly tissue cation content, in several Sphagnum species (e.g., Pakarinen 1978, 1981; Aulio 1980, 1982; Lembrechts and Vanderborght 1985; Malmer 1988; Malmer et al. 1992; Wojtuń 1994; Kempter and Frenzel 2007) none of them has distinguished between the extracellular exchangeable and intracellular pools of cations. Yet, the two main compartments prefer different cations in Sphagnum (Brehm 1968) and in other mosses (Brown and Buck 1979; Bates 1982, 1987, 1992, 1997; Koedam and Büscher 1982; Wells and Brown 1996; Brown and Brūmelis 1996; Brūmelis and Brown 1997; Brūmelis et al. 2000). Thus, the aim of our study is to quantify the cation compartmentalization along the vertical physiological gradient of shoot age in six Sphagnum species from contrasting bog microhabitats. We test the hypothesis, proposed by Malmer (1993), that also in Sphagnum the polyvalent cations (Ca2+, Mg2+, and Al3+) accumulate predominantly on the extracellular ion exchangers and in older shoot segments with reduced protoplasts, while the monovalent K+ and NH4 + ions do not accumulate on exchangers but are mainly taken up into cells and also reutilized from old segments. Due to the small physiological importance of sodium in plants, we suppose that exchangeable Na+ prevails in sphagna. We determined the intracellular N and P content along the physiological gradient of shoot age to find the degree of potential N and P reutilization from older shoot segments as an important ecological trait of mineral nutrient economy.
The second aim is to study cation compartmentalization in three pairs of Sphagnum species which coexist closely and for long periods in hummocks, lawns, and hollows. We test two contrasting hypotheses, namely that the mineral nutrient content of Sphagnum mosses is controlled either by moss species (Aulio 1982), or by growth pattern and habitat conditions (e.g., Pakarinen 1978; Malmer 1988; Malmer et al. 1992).
Materials and methods
Plant material collection
We collected the Sphagnum mosses from an ombrotrophic raised bog Rokytecká slať (Bohemian Forest—Šumava National Park and Biosphere Reserve, Czech Republic, 49°01.4′N, 13°25.1′E, 1,115 m a.s.l.) in October, at the end of the 2005 and 2006 growing seasons. The bog consists of a strip of Norway spruce (Picea abies) and bog-pine (Pinus × pseudopumilio) lagg forest (transition between mineral soil and bog peat) surrounding a large treeless mire expanse differentiated into the vertical hummock–hollow pattern.
We used polyethylene gloves to separate bunches of entire shoots from moss cushions or mats. In 2005, we chose six Sphagnum species dominating contrasting microhabitats: S. cuspidatum floating in bog pools and inundated elongated depressions oriented perpendicularly to the slope (flarks); S. majus from wet flarks and hollows; S. magellanicum from lawns and low flat hummocks; S. rubellum and S. fuscum from elevated hummocks; and S. capillifolium from the lagg forest. In 2006, we collected three pairs of Sphagnum species co-occurring in mixed cushions: S. cuspidatum and S. majus in wet flarks, S. angustifolium and S. magellanicum in low hummocks and lawns, and S. rubellum and S. fuscum from elevated hummocks. We collected five replicates (Sphagnum cushions) per species or species pair. The minimum distance between replicate samples was 2 m. Moist samples were stored in polyethylene bags.
Sample preparation
We took the samples to the laboratory, stored them at 5°C, and processed them within 2 days. We divided each shoot into four segments representing their physiological state: (1) capitulum (apical segment, 3–8 mm long), (2) subapical segment (10 mm), (3) last living segment (10 mm) beginning 13–35 mm below the apex (90 mm in S. cuspidatum), and (4) dead segment (10–20 mm). We left an at least 5-mm gap between the last two segments. We distinguished between the third and fourth segment according to the green color. Where this feature failed (dark samples of S. fuscum and S. rubellum), we used imaging fluorometer (FluorCam, Photon System Instruments Ltd., Czech Republic) to visualize the chlorophyll content by the method of chlorophyll a fluorescence. We verified the reliability of this method earlier on green shoots of S. capillifolium—the variable chlorophyll fluorescence of dark-adapted shoots decreased abruptly where the green shoot color turned pale-yellow, which indicates chlorophyll breakdown and cell death. In the case of S. fuscum, we obtained only three segments (the second and third ones were identical). In 2006, we studied only capitula and dead (first and fourth) segments. Based on shoot apical growth measured in the 2000 and 2001 seasons (unpublished data), the apical segments were 2–4 months old (3 weeks in S. cuspidatum) followed by segments not older than 1 year. The age of dead segments was very uniform across the species (1.4–1.9 years).
Analyses of mineral nutrient content
We washed all the samples in distilled water for 5 min in order to remove mineral nutrients occurring in the external water located between leaves and within hyaline cells which are opened by pores and serve as water reservoir. Then we squeezed the excess water and placed the samples (1–2 g of fresh weight, FW) into closeable bottles, added 40 ml of 20-mM HCl, and shook thoroughly for 90 s (after Clymo 1963; Brehm 1968; Büscher et al. 1990). The eluates were analyzed for the contents of K+, Na+, Ca2+ and Mg2+; in the case of the 2005 samples, also NH4 +, and in 2006 also Al3+ were analyzed. These eluted cations were supposed to be the extracellular, exchangeable fraction bound by anionic exchange site located on the cell-wall surface. We expressed the total exchangeable metal content in milliequivalents per gram dry weight. After the elution, we washed the samples in distilled water, oven-dried them at 80°C, weighed, and analyzed the subsamples (about 1–3 mg each) for the intracellular content of the cations listed above (after mineralization with nitric acid), of phosphorus (perchloric acid), and of organic nitrogen (sulphuric acid). The elements in the tissue after the acidic elution are referred to as the intracellular, unexchangeable fraction located within chlorophyllous cells. We assume the organic N represents the total N content. Contents of organic nitrogen (mineralized to NH4 +) as NH4 +, and phosphorus were analyzed colorimetrically by flow injection analysis (Foss Tecator AB, Sweden) and the metal cations by atomic absorption spectrometry (Varian Inc., Australia).
Determination of CEC
We determined the CEC as the amount of exchangeable NH4 + at pH of 7.2 in samples saturated with NH4 + (Spearing 1972). First, we sealed subsamples (70 mg) of the oven-dried material of the apical and fourth segments of Sphagnum mosses into polyester mesh bags (mesh size 150 μm) and hydrated them in distilled water under intermittent vacuum (to remove air bubbles) for 3 h. Then, we immersed all 60 bags into 2 l of 0.5 M ammonium acetate for 5 min. The pH of the shaken solution containing the bags was set to 7.2 by adding ammonia. After the saturation, all the bags were washed several times in a large volume of distilled water for 1 h. The exchangeable ammonium ions were then eluted with 1 M KCl for 15 min. The eluate was diluted eight times and analyzed for NH4 + as above.
Atmospheric nutrient deposition
Data on the aerial atmospheric deposition of the studied nutrients were provided by the Czech Hydrometeorological Institute. We averaged data from two monitoring sites (Lake Plešné and Lake Čertovo, 41 km SE and 41 km NW apart, respectively), at an altitude similar to that of our study site. We compared the deposition data with the mean nutrient contents in S. fuscum and S. rubellum, the representatives of hummocks as purely ombrotrophic microhabitat, using the “moss enrichment factor”, i.e., the ratio between the element concentration in the moss and that in precipitation (Malmer et al. 1992).
Statistical analyses
We performed all statistical tests using STATISTICA software, version 7.1 (StatSoft Inc., USA). We analyzed the data on element content using ANOVA: one-way repeated-measures ANOVA (or t test for dependent samples in the case of comparing two segments) to test the differences between segments of different age within each species, between species within each segment of a certain age, and between species within each habitat in the case of data of 2006. Hierarchical ANOVA was applied to test the data of 2005 for differences between the species in the element content among the segments (interaction species × segment): element_content = species + segment + replicate (habitat) + species × segment. To test the differences between habitats (2006 data), we applied hierarchical ANOVA to each segment: element_content = species (habitat) + habitat + replicate (habitat). We used the factorial hierarchical ANOVA to test whether the habitats differed in their element content among segments (interaction habitat × segment): element_content = species (habitat) + habitat + segment + replicate (habitat) + habitat × segment. The nested parameter “replicate” was always the random factor.
To visualize the correlations between different cation contents, compartments, and shoot segments and their relationships to moss species, we used the principal component analysis (PCA) using Canoco for Windows 4.5 (Lepš and Šmilauer 2003). The data were centered and standardized in order to make the variables comparable.
Results
Cation compartmentalization
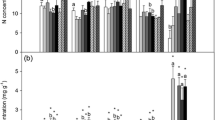
Compartmentalization of the metallic cations showed a similar pattern across the species, habitats and shoot segments. In general, the extracellular exchange sites of Sphagnum species were occupied by cations in the following approximate order (moles per unit moss DW are always shown): Ca2+ ≥ K+ > Na+ = Mg2+ > NH4 + in 2005 (Table 1) and Ca2+ > Mg2+ = K+ = Na+ > Al3+ in 2006 (Table 2). The exchangeable NH4 + content was below the analytical detection limit; it is therefore assumed to be zero and is not shown in Table 1. The exchangeable contents of all four elements were, however, generally very variable both between the species within each shoot segment and, except for Na+, between the segments within each species. Thus, any generalization is ambiguous. The intracellular, unexchangeable metal content decreased in the general order: K » Na ≥ Mg ≥ Ca = Al in both years (Tables 1, 2). The proportion of exchangeable to total cation content (Fig. 1) was always highest for Ca2+ (75% on average) followed by Mg2+ (35%), Na+ (18%), K+ (11%), and Al3+ (7%).
Exchangeable cation fraction (in %) from the total cation pool in apical (first) and dead (fourth) shoot segments. Symbols below the columns of the segments denote P values of the t test for dependent samples testing the differences between shoot segments: # P < 0.001; + 0.01 < P < 0.001; * 0.05 < P < 0.01; n P > 0.05. P values given in separate line denote statistical difference within groups (one-way and nested ANOVA): *** P < 0.001; ** 0.01 < P < 0.001; * 0.05 < P < 0.01; n.s. P > 0.05
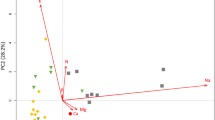
PCA (Fig. 2) revealed relationships between element contents in both compartments. In the samples of 2005, the first principal component axis explained 30% of the total variation and was related to the intracellular element contents. The second axis (27%) was dominated by exchangeable element contents and the third axis (22%; not shown in the PCA diagram) by intracellular contents of Ca and exchangeable Na+, and CEC. There was no general relationship between the exchangeable and intracellular contents of K and Na within all Sphagnum species, while this correlation was positive for Ca in both segments (0.88 for the apical and 0.66 for the dead one, P < 0.0001, n = 30) and negative for Mg in the dead segments (R = −0.57, P = 0.001).
PCA ordination diagrams (based on data of 2005 and 2006) displaying correlations between element contents located within cells (-cell) and in exchangeable form (marked by + as cations), total exchangeable cation equivalents (total eq+), and potential cation exchange capacity (CEC). Centroids of shoot segments and moss species are projected onto these correlations. Pairs of the connected species belong to the same microhabitat
In the samples of 2006, the intracellular N and P contents formed the main gradient (32% of the explained total variation; Fig. 2; see also Table 2), while the intracellular cation contents dominated the second (23%) and third (12%, not shown) axis. Exchangeable cation contents correlated with both, the first and second axis. The exchangeable and intracellular contents in the apical segments correlated only for K and Ca (R = 0.62 and 0.76, P < 0.0001, n = 30) and in the dead segments for K, Na and Ca (R = 0.57, 0.73, and 0.48, P = 0.001, P < 0.0001, and P = 0.008, respectively).
Variation between species and microhabitats
The six species studied in 2005, which represented six bog microhabitats, were allocated in the PCA diagram (Fig. 2) along the second principal component axis representing the exchangeable cation content. The highest content of exchangeable and intracellular cations was in the apical segments of S. capillifolium, the only species collected from the lagg forest understorey. S. capillifolium contrasted with the only hollow species, S. majus, which was poor in exchangeable cations and also had a low CEC. The other species of elevated microhabitats (S. fuscum, S. rubellum, and S. magellanicum) were generally similar in nutrient contents and were located between the two extremes close to the center of the PCA diagram. S. cuspidatum, collected from bog pools, differed from all other species on the third principal axis (not shown) in its high content of exchangeable monovalent cations in the dead segments (Fig. 1, Tables 1, 2).
The allocation of species and microhabitats, studied in 2006, clearly followed the second principal axis in the PCA diagram. The hollow species contrasted with the hummock ones by a low CEC and exchangeable and intracellular Ca content, but a high exchangeable Al3+ content. Although the lawn species tended to have an intermediate character, they were closer to the hummock ones in most of the characteristics. The greatest interspecific variation within microhabitats was in the lawns (Table 2). Here, the N-poor S. magellanicum with a high CEC bound more exchangeable K+ and retained higher metallic element and P contents in the dead segments.
Element content along the physiological gradient
In 2005, we determined the intracellular content of six elements in four shoot segments—according to their physiological status (Table 1). The N, P, K, and Mg contents highly significantly correlated with one another across all species and segments (R = 0.79–0.92, P < 0.0001, n = 23 mean values of five independent replicates per species and shoot segment), while they showed no relation to the Ca and Na contents. Ca and Na correlated negatively with each other (R = −0.42, P = 0.048). Only Na, but not Ca, accumulated in dead cells. The N, P, and K contents were the highest in the capitula and/or in the subapical segments and were always the lowest in the dead segments; the last living segments approached the nutrient contents of the apical segments. The contents of N, P, and K are thus well explained by the physiological status of the plant parts. S. capillifolium, the only species collected from the lagg forest understorey, accumulated more Ca and Mg within the whole shoot than the other species (P < 0.001), while the two species of wet habitats, S. majus and S. cuspidatum, accumulated less Ca than the other species (P = 0.017). The floating S. cuspidatum accumulated more N, P, K, and Mg in its apical and subapical segments than the other species, but it lost most of K and Mg from the ageing segments. In 2006, the vertical physiological polarity between the apical and dead segments was much less distinct. Only the N and P, but not the metallic element contents, were lower in the dead segments and only Na accumulated there.
We compared the exchangeable cation contents in the two contrasting shoot segments—an apical and a dead one. The dead segments contained more exchangeable Ca2+ and Mg2+ than the apical ones in both 2005 and 2006 seasons and also Al3+ accumulated more in the dead segments (Tables 1, 2). The monovalent cations, however, behaved inconsistently. They prevailed at the exchange sites of the dead segments in 2006, but they concentrated in the capitula in many cases in 2005. The ratio of exchangeable to total cation contents was significantly higher in the dead segments for a half of the species and cations (Fig. 1).
Relationship between element accumulation and availability
Annual deposition (in mmol m−2 year−1) of the studied cations decreased in the order of NH4 + » Na+ > Ca2+ > K+ > Mg2+ » Al3+ in both seasons (Table 3). As opposed to the other elements, the high ammonium deposition, zero exchangeable N, and high intracellular N content demonstrate efficient N uptake by the mosses. Similarly, when comparing the moss enrichment factors (Table 3), the capitula showed an efficient intracellular accumulation of deposited K, Al and, to a certain extent, also Mg. In contrast, Sphagnum capitula retained a small amount of deposited Na (intracellular) and Ca2+ (exchangeable).
Discussion
Cation compartmentalization
Mosses are characterized by the lack of a thick cuticle as opposed to vascular plants. Therefore, they can utilize their large shoot surface area and its fixed negative charge in cell walls for effective exchange and uptake of cations into cells. This study is the first attempt to separate the exchangeable extracellular and intracellular cation fractions in Sphagnum mosses occurring in contrasting microhabitats of a bog (cf., e.g., Malmer 1993). The exchangeable cation content in the moss and cation concentration in external (pore) water are directed towards electrochemical equilibrium; they are regulated by several processes:
-
1.
Cation supply by aerial deposition;
-
2.
Cation transport from mineral substrate and translocation from senescent tissues;
-
3.
Cation uptake into cytoplasm;
-
4.
Cation leakage from cytoplasm;
-
5.
Cation affinity to exchange sites.
In Sphagnum mosses from an ombrotrophic bog, the aerial deposition can be assumed to be the same in species growing in the open parts; only S. capillifolium received also nutrients intercepted by the lagg forest canopy as indicated by high exchangeable and intracellular contents of K and Ca in the apical segment. Mosses are capable of an effective mineral nutrient transport from the substrate (Bates and Farmer 1990) but no nutrient transport from mineral substrate is assumed in ombrotrophic bogs. Only the nutrients released from decaying biomass in the upper, aerated peat layer (acrotelm) can move upwards. However, the hollow species receive also nutrients washed out from hummocks, and species growing in pools (S. cuspidatum in 2005) also receive nutrients from deep peat layers. Desiccation of the living moss usually causes cation leakage from cell cytoplasm during which most of the effused Mg2+ (Brown and Buck 1979) or K+ (Brown and Brūmelis 1996) is retained on the exchange sites and reutilized during the recovery after rewetting (Bates 1997). Cation transport to the cytoplasm is the only process actively regulated by moss cells. Although ammonium ion was the dominant cation in rainwater, it was absent in the exchangeable form in our study. It indicates that the cation exchange is not a process competing with the intracellular uptake of NH4 +. This seems to be true also for K+, which has a very similar affinity to the Sphagnum cation exchanger as NH4 + (Breuer and Melzer 1990), but has a deposition ten times lower. A very low concentration of NH4 + tracer in mire ground water was found by Williams et al. (1999) 2 weeks after the tracer application to a Sphagnum vegetation; this points out that Sphagnum has efficient N retention (up to 100%; Williams et al. 1999; Li and Vitt 1997), exclusively by intracellular uptake.
Assuming the identical character of the cell-wall cation exchanger among our sphagna, the cation affinity to the exchanger generally correlates with its valency, hydrated atomic radius, and concentration in the ambient water solution (Bates 2000), but it also strongly depends on relative cation concentrations in the solution and the degree of exchanger dissociation (pH). These relationships were described in details by Dainty and Richter (1993).
Generally, our results of exchangeable and intracellular K, Ca and Mg contents in Sphagnum apical segments are very similar to the only one published result for Sphagnum (S. magellanicum, Brehm 1968). Our sphagna also showed a similar pattern of cation compartmentalization to that in other mosses from minerotrophic habitats in which the relative exchangeable cation contents also decrease in the order Ca2+ > Mg2+ > K+ (Brown and Buck 1979; Bates 1987; Wells and Brown 1990, 1996; Brown and Brūmelis 1996; Brūmelis and Brown 1997; Brūmelis et al. 2000). The upper values of the intracellular Ca and Mg contents in our sphagna (Tables 1, 2) were similar to the mean values found in six of 20 studied non-Sphagnum moss species from minerotrophic habitats (17–24 and 13–29 μmol g−1 d.w. for Ca and Mg) and the mean intracellular K content was even the same (Brown and Buck 1979; Bates 1987; Brown and Brūmelis 1996; Wells and Brown 1996). Our sphagna differed strikingly from the 20 moss species by low contents of exchangeable Ca2+ and partly also Mg2+ but not K+. The affinity of K+ to the cell-wall exchanger could be enhanced in Sphagnum by a low content of competing polyvalents and low pH (cf. Dainty and Richter 1993). Assuming that sphagna have a lower cytoplasm volume in the entire tissue volume than other mosses (having large empty hyaline cells), the intracellular content of Ca and Mg was roughly the same in ombrotrophic Sphagnum and minerotrophic mosses and the intracellular content of K was even higher. By contrast, S. magellanicum collected from a bog situated 10 km away from sea (Brehm 1968) contained two to five times as much intracellular Na and about ten times as much exchangeable Na+ as our S. magellanicum. Despite its low physiological demand and functions, Na is taken up into the cells where it accumulates and obviously has an osmotic function. On the other hand, increased deposition of Mg in this maritime bog led to the accumulation of exchangeable Mg2+ while the intracellular content remained similar to our values, although Mg is an important macronutrient. Despite no need of Al in plant cells, at least 94% of the total Al represented the intracellular fraction while only up to 6% of Al3+ was bound in exchangeable form.
Although significant differences existed between our sphagna, the intracellular cation content was of the same order of magnitude in species from environments of contrasting cation availability. Thus, the differences of the total metallic cation content are determined rather by the pool of exchangeable cations. Although Ca2+ was not the dominant metallic cation in the total cation income (Table 3) as compared to various mineral soils (Büscher et al. 1990), it was the dominant exchangeable cation in most cases, like in the other mosses mentioned above. Only the hollow species S. majus (both years) and S. cuspidatum (2006) contained little exchangeable Ca2+ (and also Mg2+), probably due to a lower cell wall charge density (represented by low CEC), which reduces the binding of polyvalent cations in favor of monovalents (Dainty and Richter 1993). Exchangeable monovalents, particularly Na+, accumulated in dead segments of the preferentially hollow and pool species S. cuspidatum and S. majus, probably also because they had not been retained as efficiently as bivalents in hummocks.
Element contents along the physiological gradient and nutrient reutilization
The results showed a considerable year-to-year variability in the exchangeable cation content in the apical segment and a smaller variability in the dead one. The spatial variability between replicates was relatively small, which indicates that the exchangeable cation pool of the upper shoot is more susceptible to environmental factors. The higher total exchangeable cation content in the apical segment in 2005 could result from a two to three times higher precipitation (and thus nutrient deposition; data not shown) during each of the 3 months before sampling in 2005 than in 2006. On the other hand, Malmer (1988) showed that there was no correlation between K, Ca and Mg annual wet deposition and the total element content in Scandinavian sphagna.
The intracellular N, P and, in 2005, also K, accumulated significantly in upper segments. Because the exchangeable content of these elements was low or negligible (Fig. 1) the results are comparable and consistent with the total contents reported by other authors (Pakarinen 1978; Malmer 1988). The lower content of these macronutrients in the dead segments indicates their possible translocation to the upper segments. Rydin and Clymo (1989) observed an internal upward translocation of C and P through the Sphagnum “stem”. Aldous (2002a) found that the upward N translocation was an important basis for N retention in the field, particularly in such a relatively N-unpolluted area as was our collection site, where the annual N deposition of 0.5 g m−2 year−1 was limiting for Sphagnum growth (Table 3; cf. Bragazza et al. 2004). The intracellular element contents along the vertical physiological gradient could be affected by a temporal variation in the nutrient deposition and the subsequent uptake. This can be a reason for an absence of a pattern in the vertical distribution of metallic elements; only Na accumulated in the dead segments. Although our results do not enable us to estimate accurately the proportion of N and P reutilized from senescing shoot segments upwards to the apical segments (Tables 1, 2), this proportion was only 0–50% (36% in average) for N and 32–72% (54 in average) for P in our sphagna. Vascular plants inhabiting nutrient-poor bogs and fens reutilize N more efficiently (40–50%) but their P reutilization (50–60%) is roughly the same as in our sphagna (Aerts et al. 1999).
Species and habitat controls on shoot nutrient content
The results separated our species into four groups according to their microhabitat: forest, hummock + lawn, hollow, and aquatic species (Fig. 2). The most conspicuous differences in shoot nutrient characteristics were found in S. cuspidatum submerged in pools (2005) and occupying wet hollows (2006). Submerged form of this species hosted a rich algal microflora (Lederer and Soukupová 2002), which can significantly increase N input by cyanobacterial N fixation, particularly in the given microhabitat (Granhall and Selander 1973). The smallest interspecific differences in nutrient characteristics of the shoots were found in hummocks and also in hollows which were dominated by species pairs of closely related species belonging to the sections Acutifolia (hummocks) and Cuspidata (hollows). In these habitats, the environmental conditions determined the Sphagnum nutrient content, consistently with the results of Malmer (1988) and Malmer et al. (1992).
The environmental conditions of contrasting mire microhabitats are, however, highly specific and enable the selection of species according to their adaptations. Thus, the morphologically and ecophysiologically similar species occupying the same microhabitat use to be also taxonomically related. Lawns were, however, dominated by the unrelated species S. magellanicum (sec. Sphagnum) and S. angustifolium (sec. Cuspidata). S. angustifolium, which prefers wetter microhabitats in boreal mires, had low CEC, like the other species of this section. Thus, CEC in Sphagnum do not reflect only the position above the water level. However, the low CEC seemed to affect only the exchangeable K+, but not the polyvalents. The much lower N content in S. magellanicum than in S. angustifolium (by about 40%) can result from a lower intracellular uptake rate of NH4 + and NO3 − in S. magellanicum (also by about 40%) than in species of the section Cuspidata (Jauhiainen et al. 1998). We found that N content of S. magellanicum in 2005 was similar to that in the hummock and hollow species in both 2005 and 2006. Thus, S. angustifolium seems to overplay S. magellanicum in the competition for N. This leads to the decrease of the N:P ratio to 19 (based on weight content) in S. magellanicum and stronger N limitation, which occurs at the N:P ratio < 30 in sphagna (Bragazza et al. 2004). In the other species, P limitation could take place (N:P = 31–39) accompanied by possible K co-limitation in S. angustifolium (cf. Bragazza et al. 2004).
From the long-term view, the enhanced N acquisition by S. angustifolium may either represent a mechanism enabling its coexistence with S. magellanicum, or may lead up to outcompeting of S. magellanicum. S. magellanicum has a higher water-holding capacity, which is necessary for surviving of scattered individuals of S. angustifolium with a lower water-holding capacity (cf. Rydin 1986). The slower growth of N-limited S. magellanicum will result in slower peat accumulation. The water table will rise closer to the moss surface and favor S. angustifolium, which will not be further limited by water availability. Probably this outcompetition of S. magellanicum by a species of wetter habitats will further be promoted if the atmospheric N deposition increases, as pointed out by Twenhöven (1992).
The role of cation exchange in Sphagnum
Many authors have proposed several roles of the unusually high CEC of Sphagnum in its biology. Dainty and Richter (1993) concluded that the main role of the cation exchanger could be proton production to acidify bog water and, thus, suppress vascular plant competitors. The acidity also suppresses microbial decomposers and, moreover, the high CEC can limit the availability of essential cations for the decomposers in peat (Thomas and Pearce 2004); both properties thus participate in bog formation.
Cation-exchange processes of plant tissue are often assumed to affect intracellular ion uptake. Mosses growing on acidic substrates have lower CEC than neutrocline taxa, thus avoiding binding of aluminium, which is highly mobile under acidic conditions (Büscher et al. 1990). Al3+ toxicity is unlikely in ombrotrophic Sphagnum mosses but due to high CEC, they are limited to acidic conditions to avoid excessive binding (condensation) of Ca2+ which arises already from pH > 5 (Clymo 1973; Dainty and Richter 1993). The excessive binding of polyvalents may act as a barrier against exchange and intracellular uptake of other cations (cf., Wells and Brown 1990; Mautsoe and Beckett 1996). Nevertheless, these processes need more experimental evidence.
The bog acidity diminishes the dissociation of the cation exchanger and, therefore, the apparent CEC. Thus, high CEC is also necessary for maintaining efficient retention of cations supplied by rainwater. Aldous (2002b) reported that vascular plants received <1% of N recently added by wet deposition. Such a partitioning of nutrient resources between mosses (utilizing nutrients from atmospheric deposition) and vascular plants (utilizing nutrients by peat mineralization) gives Sphagnum mosses competitive advantage (Malmer et al. 1994) and allows their coexistence.
We expect that the cation exchange enhances the intracellular cation uptake by the following mechanism. Rainwater has a higher pH (4.8–5.1; Table 3) than water in our Sphagnum habitats (<4.2 in pools, Lederer and Soukupová 2002, and most probably close to 3.5 in hummocks, Clymo 1963). During rain, the cations supplied will be immediately exchanged for H+. This will acidify the solution in moss habitat, but the rainwater itself will have a counteracting effect. After rain, evaporation will concentrate the solution among Sphagnum apices. This will again acidify the solution, and cations will be eluted from the cell-wall exchangers and become available for the intracellular uptake. Also, the active H+ efflux, counterbalancing ion transport into the cell (Raven et al. 1998), will acidify the solution. The active intracellular uptake is, in comparison with the cation exchange, a slow biochemical process (cf. Jauhiainen et al. 1998). Efficient cation retention by cation exchange may represent an important mechanism for temporal extension of mineral nutrient availability for their intracellular uptake in nutrient-limited Sphagnum habitats.
References
Aerts R, Verhoeven JTA, Whigham DF (1999) Plant-mediated controls on nutrient cycling in temperate fens and bogs. Ecology 80:2170–2181
Aldous AR (2002a) Nitrogen retention by Sphagnum mosses: responses to atmospheric nitrogen deposition and drought. Can J Bot 80:721–731
Aldous AR (2002b) Nitrogen translocation in Sphagnum mosses: effect of atmospheric nitrogen deposition. New Phytol 156:241–253
Anschütz I, Geßner F (1955) Der Ionenaustausch bei Torfmoosen (Sphagnum). Flora 144:179–236
Aulio K (1980) Nutrient accumulation in Sphagnum mosses. I. A multivariate summarization of the mineral element composition of 13 species from an ombrotrophic raised bog. Ann Bot Fenn 17:307–314
Aulio K (1982) Nutrient accumulation in Sphagnum mosses. II. Intra- and interspecific variation in four species from an ombrotrophic raised bog. Ann Bot Fenn 19:93–101
Bates JW (1982) The role of exchangeable calcium in saxicolous calcicole and calcifuge mosses. New Phytol 90:239–252
Bates JW (1987) Nutrient retention by Scleropodium purum and its relation to growth. J Bryol 14:565–580
Bates JW (1989) Retention of added K, Ca and P by Scleropodium purum growing under an oak canopy. J Bryol 15:589–605
Bates JW (1992) Mineral nutrient acquisition and retention by bryophytes. J Bryol 17:223–240
Bates JW (1997) Effects of intermittent desiccation on nutrient economy and growth of two ecologically contrasted mosses. Ann Bot 79:299–309
Bates JW (2000) Mineral nutrition, substratum ecology, and pollution. In: Shaw AJ, Goffinet B (eds) Bryophyte biology. Cambridge University Press, Cambridge, pp 248–311
Bates JW, Farmer AM (1990) An experimental study on calcium acquisition and its effect on the calcifuge moss Pleurozium schreberi. Ann Bot 65:87–96
Bragazza L, Tahvanainen T, Kutnar L, Rydin H, Limpens J, Hájek M, Grosvernier P, Hájek T, Hájková P, Hansen I, Iacumin P, Gerdol R (2004) Nutritional constraints in ombrotrophic Sphagnum plants under increasing atmospheric nitrogen depositions in Europe. New Phytol 163:609–616
Brehm K (1968) Die Bedeutung des Kationenaustausches fiir den Kationengehalt lebender Sphagnen. Planta 79:324–345
Breuer K, Melzer A (1990) Heavy metal accumulation (lead and cadmium) and ion exchange in three species of Sphagnaceae. Oecologia 82:461–467
Brown DH, Brūmelis G (1996) A biomonitoring method using the cellular distribution of metals in moss. Sci Total Environ 187:153–161
Brown DH, Buck GW (1979) Desiccation effects and cation distribution in bryophytes. New Phytol 82:115–125
Brūmelis G, Brown DH (1997) Movement of metals to new growing tissue in the moss Hylocomium splendens (Hedw.) BSG. Ann Bot 79:679–686
Brūmelis G, Lapina L, Tabors G (2000) Uptake of Ca, Mg and K during growth of annual segments of the moss Hylocomium splendens in the field. J Bryol 22:163–174
Büscher P, Koedam N, Van Speybroeck D (1990) Cation-exchange properties and adaptation to soil acidity in bryophytes. New Phytol 115:177–186
Clymo RS (1963) Ion exchange in Sphagnum and its relation to bog ecology. Ann Bot 27:309–324
Clymo RS (1973) The growth of Sphagnum: some effects of environment. J Ecol 61:849–869
Dainty J, Richter C (1993) Ion behavior in Sphagnum cell walls. Adv Bryol 5:107–128
Granhall U, Selander H (1973) Nitrogen fixation in a subarctic mire. Oikos 24:8–15
Jauhiainen J, Wallén B, Malmer N (1998) Potential 15NH4 15NO3 uptake in seven Sphagnum species. New Phytol 138:287–293
Kempter H, Frenzel B (2007) The geochemistry of ombrotrophic Sphagnum species growing in different microhabitats of eight German and Belgian peat bogs and the regional atmospheric deposition. Water Air Soil Pollut 184:29–48
Knight AH, Crooke WM, Inkson RHE (1961) Cation-exchange capacities of tissues of higher and lower plants and their related uronic acid contents. Nature 192:142–143
Koedam N, Büscher P (1982) Studies of possible role of cation exchange capacity in the soil preference of mosses. Plant Soil 70:77–93
Lederer F, Soukupová L (2002) Biodiversity and ecology of algae in mountain bogs (Bohemian Forest, Central Europe). Algol Stud 106:151–183
Lembrechts JFM, Vanderborght OLJ (1985) Mineral content of Sphagnum mosses in Belgian bog ecosystems. J Environ Qual 14:217–224
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using Canoco. Cambridge University Press, Cambridge
Li Y, Vitt DH (1997) Patterns of retention and utilization of aerially deposited nitrogen in boreal peatlands. Ecoscience 4:105–116
Malmer N (1988) Patterns in the growth and the accumulation of inorganic constituents in the Sphagnum cover on ombrotrophic bogs in Scandinavia. Oikos 53:105–120
Malmer N (1993) Mineral nutrients in vegetation and surface layers of Sphagnum-dominated peat-forming systems. Adv Bryol 5:223–248
Malmer N, Horton DG, Vitt DH (1992) Element concentrations in mosses and surface waters of western Canadian mires relative to precipitation chemistry and hydrology. Ecography 15:114–128
Malmer N, Svensson BM, Wallén B (1994) Interactions between Sphagnum mosses and field layer vascular plants in the development of peat-forming systems. Folia Geobot Phytotax 29:483–496
Mautsoe PJ, Beckett RP (1996) A preliminary study of the factors affecting the kinetics of cadmium uptake by the liverwort Dumortiera hirsuta. S Afr J Bot 62:332–336
Pakarinen P (1978) Production and nutrient ecology of three Sphagnum species in southern Finnish raised bogs. Ann Bot Fenn 15:15–26
Pakarinen P (1981) Metal content of ombrotrophic Sphagnum mosses in NW Europe. Ann Bot Fenn 18:281–292
Raven JA, Griffiths H, Smith EC, Vaughn KC (1998) New perspectives in the biophysics and physiology of bryophytes. In: Bates JW, Ashton NW, Duckett JG (eds) Bryology for the twenty-first century. Maney Publishing and the British Bryological Society, Leeds, pp 261–275
Rydin H (1986) Competition and niche separation in Sphagnum. Can J Bot 64:1817–1824
Rydin H, Clymo RS (1989) Transport of carbon and phosphorus compounds about Sphagnum. Proc R Soc Lond 237:63–84
Spearing AM (1972) Cation-exchange capacity and galacturonic acid content of several species of Sphagnum in Sandy Ridge bog, central New York State. Bryologist 75:154–158
Thomas PA, Pearce DME (2004) Role of cation exchange in preventing the decay of anoxic deep bog peat. Soil Biol Biochem 36:23–32
Twenhöven FL (1992) Competition between two Sphagnum species under different deposition levels. J Bryol 17:71–80
Wells JM, Brown DH (1990) Ionic control of intracellular and extracellular Cd uptake by the moss Rhytidiadelphus squarrosus (Hedw.) Warnst. New Phytol 116:541–553
Wells JM, Brown DH (1996) Mineral nutrient recycling within shoots of the moss Rhytidiadelphus squarrosus in relation to growth. J Bryol 19:1–17
Williams B, Silcock D, Young M (1999) Seasonal dynamics of N in two Sphagnum moss species and the underlying peat treated with 15NH4 15NO3. Biogeochem 45:285–302
Wojtuń B (1994) Element contents of Sphagnum mosses of peat bogs of lower Silesia (Poland). Bryologist 97:284–295
Acknowledgments
This paper is dedicated to our colleague and friend Dr. Jan Květ, South Bohemian University, České Budějovice, Czech Republic, on the occasion of his 75th birthday and for the long-term merits of his studies on wetland plants. Sincere thanks are due to Dr. Jan Květ for critically reading the manuscript and correction of the language. Special thanks are due to Ing. Jan Bastl, Mrs. Hana Strusková, and Mrs. Andrea Zajíčková for chemical analyses. This study was funded by the Research Project of the Academy of Sciences of the Czech Republic No. B600050503 “Mineral nutrient economy in Sphagnum mosses” and partly by the Research Programmes Nos. AV0Z60050516 and MSM6007665801.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hájek, T., Adamec, L. Mineral nutrient economy in competing species of Sphagnum mosses. Ecol Res 24, 291–302 (2009). https://doi.org/10.1007/s11284-008-0506-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-008-0506-0