Abstract
The Mediterranean coast holds a wide and rich variety of wetlands, some of which are protected by international laws, but at the same time have been historically subjected to threatening activities such as agriculture, pollution and aquifer overexploitation. As part of a conservation and restoration project at the Marjal dels Moros coastal wetland (Eastern Iberian Peninsula) we initiated investigations to begin to characterise aquatic environments, and evaluate changes in the system experienced in recent times. To this aim, we collected four seasonal samples at seven points of the Marjal dels Moros wetland, varying in salinity, permanence and other environmental conditions, and studied the recent past (early modern to contemporary remains) and present (living) communities of Ostracoda. We found relatively poor species richness overall, possibly related to the brackish and seasonal character of large parts of the wetland. Of the seven species encountered the most common ostracods were tolerant to these stressing conditions: Heterocypris salina, Sarscypridopsis aculeata, Ilyocypris gibba and Cypridopsis vidua. A comparison of dead and living assemblages showed no significant differences, suggesting that the overall ostracod metacommunity was resilient to the perturbations experienced in the area, despite some particular locations recording notable shifts in their communities during the sampling period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spatial patterns in the distribution of aquatic invertebrates are strongly related to the environmental conditions of the habitat (e.g. Cottenie 2005; De Bie et al. 2012), and freshwater Ostracods are not an exception (Mesquita-Joanes et al. 2012). However, it is sometimes hard to ascertain these niche effects in small-scale areas with strong dynamics in environmental changes, such as temporary ponds in coastal wetlands, which are frequently affected by disturbances of anthropogenic and natural origin. In this sense, ostracods are useful to understand such dynamics, as we can compare their past assemblages with the living community and detect recent changes in habitat traits (Poquet et al. 2008; Valls et al. 2013).
Ostracods are microscopic crustaceans encased by two calcified valves. They can occur in most aquatic ecosystems, including lakes, ponds, streams, rivers, estuaries and oceans. The distribution of non-marine ostracods is mainly influenced by temperature, salinity, ionic composition, type of substrate, macrophyte coverage, flow velocity, nutrients, depth and dispersal vectors (Meisch 2000). Ostracod shells of past times remain in the taphocoenoses and therefore serve as proxies to understand the environmental evolution of impacted wetlands (Ruiz et al. 2003; Marco-Barba et al. 2013), and isotopical ratios from ostracod shells provide a consistent way to determine salinity, hydrological and carbon cycle changes (Durazzi 1977; Xia et al. 1997; Von Grafenstein et al. 1999). Moreover, ostracods can be used as sentinels of human impact in freshwater ecosystems (Allen and Dodson 2011; Ruiz et al. 2013). Hence, their calcareous shells are considered to have an excellent potential as (paleo-)environmental indicators. Past assemblages can therefore be compared with modern communities, allowing understanding the ecological setting of past environments (Griffiths and Holmes 2000).
Previous works at the Eastern Iberian Peninsula have described how anthropogenic impacts transformed natural wetlands by modifying environmental conditions (Poquet et al. 2008) or by introducing exotic species (Escrivà et al. 2012). Here, we focus our interest on a highly impacted wetland in the Western Mediterranean, the Marjal dels Moros, which has been historically affected by environmental degradation, agricultural management and seawater intrusion (Navarro 1989; Bordás-Valls et al. 1999, Bohigues 2001). Understanding the past and present environmental status in this coastal wetland is a key factor by which to initiate integrated and efficient management decisions. We describe the present ostracod biodiversity and ecology in Marjal dels Moros, a protected coastal wetland in Valencia (E. Spain) in relation to environmental conditions, and compare living with dead (recent) assemblages in order to test for metacommunity shifts during the past decades as a response to human impacts. Chemical and biological characteristics were explored to search for explanations of ostracod distributions. In addition, valves preserved in taphocoenoses were also examined to understand the environmental changes experienced in recent historical periods. Studies of this type may constitute a basic tool to understand metacommunity responses to human impacts in coastal wetlands during the last century.
Materials and Methods
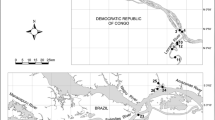
The Marjal dels Moros wetland covers 300 ha and is located in the Eastern Iberian Peninsula. Water inputs are from rain, waste-water, wells (extracted with pumps) and seawater intrusions (Fig. 1). The basal Plio-Quaternary sediment deposits transported by seasonal streams originating in the Calderona Mountains from the West, together with sedimentation from Palància River from the North, mark the initial evolution of this wetland in the Holocene (Sanchis and Ferri 1997; Cuesta 2001). Historical studies confirm the presence of Roman and Muslim populations and activities in the area between the 1st and 13th centuries, which involved building an irrigation network in the Marjal dels Moros (Box 1991, Sanchis and Ferri 1997), later modified but still apparent in the wetland (Fig. 1), the first evidence of anthropogenic impacts in the zone. Human settlement and agriculture (mainly ricefields) increased during the High Middle Ages, but fell of between the 14th and 17th centuries because of health-related governmental rules against rice culturing, and then increased again until the late 20th century (Cavanilles 1795; Gómez et al. 1988; Sanchis and Ferri 1997; Box 1991; Bohigues 2001). Moreover, salt extractions and hunting activities were common in the pre-industrial period (Bohigues 2001). In recent decades, pollution by (steel) industry, waste-water, aquifer overexploitation and introduction of exotic species were the main impacts that affected this wetland (Box 1991; Sanchis and Ferri, 1997; Cuesta 2001; Sancho 2001). Presently, it is part of the Natura 2000 Network, having been declared a Special Protection Area (SPA) in 1996, mainly due to the presence of endemic endangered fishes such as Aphanius iberus (Cuvier and Valenciennes 1846) and Valencia hispanica (Cuvier and Valenciennes 1846), crustaceans such as Palaemonetes zariquieyi Sollaud, 1939 and a high diversity of birds (Roure 2001).
Map of the Marjal dels Moros wetland. Asterisks: sample points; Circles: Water wells; Arrows: Water inputs from a near wastewater treatment plant; Dashed lines: irrigation network; Double lines: road tracks; Single lines: remains of limits of old agricultural fields; Pale gray colour: (temporary) inundated zones
Benthic samples were collected seasonally from autumn 1997 through to summer 1998 using hand nets in seven points of the wetland (unless they were dry). Sites were selected on the basis of distance to the sea, hydroperiod and other environmental conditions (Fig. 1, Table 1). Invertebrate samples were stored in 10 % formaldehyde. At the same time, physical and chemical data were also collected (temperature, conductivity, salinity, dissolved oxygen concentration and pH) with portable probes (VWR® EC300, WTW® Oxi 330i and VWR™ SympHony).
Although no dating was obtained from the sediment substrate sampled with the hand net, we estimate that it could encompass ostracod remains averaged over a maximum period of some decades to a few hundreds of years. Sedimentation rates in coastal Mediterranean wetlands in the area (e.g. Lake Albufera, Pego-Oliva wetland) have been established to vary from about 0.1 to 1.0 mm y−1 (Sanjaume et al. 1992; Torres et al. 2013; Carmona and Ruiz, 2014). As we sampled the first few centimeters of pond bottom surface with a hand net, assuming a maximum sediment depth sampled of around 2–3 cm, the oldest age of the ostracod remains analyzed can be estimated to be of about 20 to 300 years. As many of the sites sampled are temporary, when they dry out the sediment is exposed to the climatological conditions. In these circumstances, wind erosion during summer might reduce sedimentation rates and consequently increase the age window of the obtained remains up to the highest estimated values, but these can be highly variable among sites. Also, wind could remove and mix assemblages from different time intervals.
In the laboratory, aquatic macroinvertebrates were identified following Meisch (2000) for ostracods and Tachet et al. (1981) for other invertebrates. Living and dead ostracods were handpicked, identified and counted under a stereomicroscope, until at least 200 individuals were collected or alternatively all of them when samples contained a lower number. Selected valves of each species were attached to a double-sticky tape on an aluminum stub and coated with Au-Pd. A Philips XL-30 scanning electron microscopy (SEM) at the University of Valencia was used to take photographs of the ostracod valves.
Invertebrate Shannon diversity (H’) was calculated for each sample. Binary multivariate logistic regression was performed to check for effects of environmental conditions on the presence of living ostracods using the SPSS program v19.0 (IBM 2010). Analysis of Similarities (ANOSIM) was carried out to check for differences in ostracod communities between seasons and between bio- and taphocoenoses, as a means to test for significant differences between (recent) past and present ostracod communities. Bray-Curtis distances were used in ANOSIM, which was carried out with the software PAST (Hammer et al. 2001). Ostracod biocoenoses data were initially ordinated by detrended correspondence analysis (DCA) to estimate the gradient length, and redundancy analysis (RDA) was performed afterwards to investigate the relationship between ostracod species and environmental variables. Principal component analysis (PCA) was carried out to compare ostracod taphocoenoses (disarticulated valves and empty shells) and biocoenoses. DCA, RDA and PCA were generated with the CANOCO 5.0 software (ter Braak and Šmilauer 2012). In RDA, Monte Carlo permutation tests (n = 999) were used to analyze the statistical significance of the relationship between species and environment variables with a Forward Selection of Variables (FSV) procedure.
Results
The studied Marjal dels Moros wetland showed a wide range of variation in the physicochemical environmental data (Table 1). Two notable sites, numbers five and seven, had high mean electrical conductivity (>15,000 μS·cm−1). The highest values in these two sites were measured in autumn and spring, with the maximum peak reached (26,400 μS·cm−1) at site five. Sites five and seven dried in summer months. Low conductivities at site one (on average 3942 μS·cm−1) were caused by addition of water pumped from a nearby canal. Water depth at stations five and seven was of the order of 15 cm or less; the remaining sites were >24 cm deep, with the highest depth recorded in site number one (62 cm). Seasonal variations in water temperature were considerable. Differences between maximum and minimum temperature ranged as much as about 20° at sites four and seven. Summer temperatures in all stations were above 25 °C and winter values were always lower than 15 °C. Macroinvertebrate diversity was highest at site two, which also presented the highest mean values of pH and dissolved oxygen concentration.
Seven ostracod species occurred in the biocoenoses, plus four other species that appeared only in the taphocoenoses (see Table 2, Fig. 2). The most frequently observed species were Heterocypris salina (Brady 1868), Ilyocypris gibba (Ramdohr 1808), Sarscypridopsis aculeata (Costa 1847) and Plesiocypridopsis newtoni (Brady and Robertson 1870). Another common species was Cypridopsis vidua (OF Müller 1776). Heterocypris incongruens (Ramdohr 1808) and a juvenile of Potamocypris sp. were found in a single sample. Cyprideis torosa (Jones 1850), Eucypris virens (Jurine 1820), Pseudocandona marchica (Hartwig 1889) and Heterocypris sp. were recorded only from the taphocoenoses. All of these species are widely distributed in other circum-Mediterranean coastal wetlands (Meisch 2000). Living specimens of most Ostracoda species were more abundant in spring at all stations. No living ostracods were found at station number three. Heterocypris salina was the most frequently recorded ostracod, found at almost all sample sites, and comprising more than 50 % of ostracod individuals recorded from sites numbers two, five and six. In contrast, I. gibba and S. aculeata were less frequent but dominant in stations one and seven, respectively. These two were the only species for which adult individuals were found in autumn. The sample with the highest species richness (five species) was collected in spring at station number four.
SEM photographs of ostracod species found in Marjal dels Moros. a Cyprideis torosa (Jones 1850), noded shell, dorsal view; b Sarscypridopsis aculeata (Costa 1847) external view of left valve (LV); c Eucypris virens (Jurine 1820) inner view of LV; d-f: Ilyocypris gibba (Ramdohr 1808) d male dorsal view; e female dorsal view; f female inner view of LV; g-h: Cypridopsis vidua (O. F. Müller 1776); g dorsal view; h inner view of LV; i Plesiocypridopsis newtoni (Brady and Robertson 1870) external view of right valve (RV); j Pseudocandona marchica (Hartwig 1899) inner view of LV; k Heterocypris salina (Brady 1868) inner view of RV; l Heterocypris incongruens (Ramdohr 1808) inner view of RV
It is important to highlight that we recorded a bisexual population of I. gibba (Fig. 2), a species that is usually represented by only parthenogenetic females. In addition, it needs to be pointed out that the two C. torosa shells found in the taphocoenoses were noded, an indication of low salinity due to pathological effects linked to osmoregulation (Keyser and Aladin 2004; Frenzel et al. 2012; Marco-Barba et al. 2013). According to Ruiz et al. (2013), these taphocoenose valves are environmental indicators of salinities lower (i.e. < 6 g·L−1) than at present in some areas of Marjal dels Moros.
Binary multivariate logistic regression showed no environmental variables significantly related (p > 0.18) with ostracod presence. Ordination analyses provided a more detailed view of species-environment relationships, though. DCA resulted in a total inertia of 2.05 SD, a relatively short gradient that pointed to applying an RDA linear ordination method. Two significant variables were detected with FSV: water temperature and macroinvertebrate Shannon diversity (p = 0.002 and p = 0.036, respectively). Axis 1 accounted for 21.9 % of the explained variance, and together with axis 2 for 35.3 %. RDA (Fig. 3) ordination shows a high number of species at the negative part of the plane defined by both axes, corresponding to sites with high macroinvertebrate diversity, while H. incongruens is located on the opposite part of the graph. These differences between ostracod communities were not only spatially-related. Significant differences (p < 0.02) were noted between seasons in the ostracod assemblages according to ANOSIM. These changes are key to understand the factors that influenced the building of these ostracod communities. In RDA axis 1, high water temperature and conductivity values were ordered on the opposite trend along this axis compared to depth, suggesting a desiccation effect, and in relation to high temperatures in summer. Although two of these variables were not significant when tested with FSV (partly because of correlation with selected variables), responses of the species to the environment reflect the ecological conditions. In this way, positions of H. salina and I. gibba in the RDA point to the gradual changes in depth and conductivity due to desiccation, together with increasing temperatures from autumn to summer (Fig. 3).
Redundancy analysis (RDA). Triplot graph of RDA showing ordination of species, samples and environmental variables from Marjal dels Moros defined by the first and second axes. Solid line arrow: significant variables (p < 0.05); dashed line arrows: not-significant variables (p > 0.05). See text for further explanation. HSA: Heterocypris salina (Brady 1868); HIN: Heterocypris incongruens (Ramdohr 1808); IGI: Ilyocypris gibba (Ramdohr 1808); CVI: Cypridopsis vidua (O. F. Müller 1776); SAC: Sarscypridopsis aculeata (Costa 1847); PNE: Plesiocypridopsis newtoni (Brady and Robertson 1870); POT: Potamocypris sp.; Cond: Electrical conductivity; Wtemp: Water temperature; Hmacroin: Macroinvertebrate Shannon-Wiener index. Sampling site codes as in Fig. 1. Season codes: A = autumn, B = winter, C = spring, D = summer
The ecological history of the wetland has been analyzed through ostracod community changes comparing dead and living assemblages, which may reflect variation in environmental conditions. In spite of the presence of some species in the dead assemblages that were not found alive, no significant overall differences were found between bio- and taphocoenoses (ANOSIM; p = 0.15, 999 permutations). The variance explained by a PCA (Fig.4) of biocoenoses and taphocoenoses accounted for 41.55 % of the variability in the case of the first component and up to 65.67 % including the second axis. Differences between present and past communities did not show a clear general pattern but a heterogeneous distribution instead, particular for each site. The most important changes observed along the first component corresponded to station four. In this site the most abundant valves corresponded to H. salina in the dead assemblages (taphocoenoses), but living communities were dominated by I. gibba, an oligohaline species. Along axis 2, the largest changes corresponded to station number five, moving from a past composition dominated by oligohaline species to others that prefer saline conditions. Taphocoenoses samples manifested such kind of inter-annual variability in all stations, highlighting the continuous dynamics of the structure of these communities over time.
Ostracod biocoenoses (grey squares, codes with the letter “L”; living community) and taphocoenoses (black circles, codes with “D”; dead assemblage) in Marjal dels Moros defined by first and second axes of principal component analysis (PCA). Site numeric codes (1–7) as in Fig. 1. CTO: Cyprideis torosa (Jones 1850); PNE: Plesiocypridopsis newtoni (Brady and Robertson 1870); PSE: Pseudocandona marchica (Hartwig 1899). HSA: Heterocypris salina (Brady 1868); HIN: Heterocypris incongruens (Ramdohr 1808); IGI: Ilyocypris gibba (Ramdohr 1808); CVI: Cypridopsis vidua (O. F. Müller 1776); SAC: Sarscypridopsis aculeata (Costa 1847); EVI: Eucypris virens (Jurine 1820); HSP: Heterocypris sp.; POT: Potamocypris sp.
Discussion
Coastal wetlands present high diversity of invertebrates and of Ostracoda in particular, factors that are not always taken into account in restoration and management programs, even knowing that human impacts and land use can alter ostracod communities. Our results are consistent with earlier limnological studies showing wide variability in environmental conditions; salinity and pH reflect similar values to previous studies in this wetland but oxygen content, on average, is slightly lower (Rodrigo and Colom 1999). In our analysis on the environmental factors that could affect the presence of ostracods as a group, we found no significant variables, this being most probably a result of both low statistical power and rapidly changing conditions. This type of changing environments, particularly in relation to hydroperiod and salinity, represent stressful habitats driving low species richness. Other Mediterranean coastal wetlands recorded higher number of ostracod species (>15) than this study (e.g. Altinsaçli 2004; Rossetti et al. 2004; Martinoy et al. 2006; Rueda Sevilla et al. 2006), yet this might be also caused by a higher sampling effort or larger surface area studied. As other surveys demonstrate, high salinities reduce ostracod diversity and, in general, faunal richness in continental waters (Geddes et al. 1981; Williams 1998). Physiologic and osmoregulation problems can be the key factor to this phenomenon. This is reflected in this study and in other Mediterranean wetlands with high electrical conductivity values that relate to lower ostracod richness (<10 species) (Samraoui et al. 1998; Valls et al. 2013). Certain ostracod species are however able to live in these stressful conditions. De Deckker and Forester (1988) suggest that ostracod abundance could increase with salinity, as it happens in hypersaline environments, but then the community would be made up of monospecific assemblages. In our study, H. salina is dominant in these saline conditions (Fig. 3), as it is also observed in other surveys nearby (Valls et al. 2013).
Other species do not seem to tolerate so high salinities, as it seems the case for S. aculeata, which was recorded in all stations except the sites one and five with the lowest and highest salinity values, respectively. Experiments under laboratory conditions and field studies suggest a salinity range for S. aculeata of 0.5–15 g·L−1, being optimal at 5 g·L−1 (Meisch 2000). Stations one (most landward) and five (most seaward) have salinity values outside the range for this species. The observed high salinities may be one reason why Marjal dels Moros, unlike other nearby areas, seems not to have been yet colonized by exotic ostracods. Similar circumstances were reflected in other coastal wetlands nearby as the Racó de l’Olla (Valls et al. 2013).
Previous paleopalynological (Navarro 1989) and hydrological (Pernía et al. 1996) studies showed changes with seawater intrusions and dominance of halophile plants through the history of Marjal dels Moros. Such kinds of changes were reflected by ostracod remains in sites two, three, four and six, with the presence of C. torosa and H. salina in the taphocoenoses. The presence of noded individuals of Cyprideis torosa is of particular interest, because these indicate the presence of permanent water bodies with salinities <5 g·L−1 (Frenzel et al. 2012), usually corresponding to limnocrenic springs in the region (Mezquita et al. 2005; Rueda et al. 2013). Such an environment might have been therefore naturally present in the Park in previous decades (Box 1991; Sanchis and Ferri 1997). Although C. torosa was represented in taphocoenoses, we did not find any living individual in the biocoenoses, even if conductivity conditions would appear to be optimal in some of the studied sites. However, C. torosa needs permanent water bodies, unlike all of the other species found in this survey (Meisch, 2000).
Human activities and large hydrological changes (Box 1991; Sanchis and Ferri 1997; Bohigues 2001) could be the key factors for the absence of ostracods in some places and community shifts in others. For example, agriculture at the second half of the 20th century increased the use of fertilizers and pesticides that affect directly ostracod survival rate (Takamura and Yasuno 1986; Aguilar-Alberola and Mesquita-Joanes 2012). Species replacements occurred in some sites, probably produced for the same reasons. Agriculture may contribute in the spatial differences in the Marjal dels Moros and could be a possible factor in the differences between the taphocoenoses and the biocoenoses found in the area (Table 2).
The environmental history of another Mediterranean wetland, the Lake Albufera de Valencia Natural Park, is paradigmatic of a landscape strongly influenced by human activities (eutrophication, pollution by pesticides and fertilizers...) where changes in ostracod palaeoassemblages through time notably differ from the actual biocoenoses of the area (Marco-Barba et al. 2013; Valls et al. 2014). We expected that a similar drastic replacement could have happened in the nearby wetland of Marjal dels Moros. Increasing agriculture activities and seawater intrusions might have influenced ostracod communities, as observed in Albufera and other coastal wetlands (Poquet et al. 2008; Marco-Barba et al. 2013). However, we found no major changes in the whole wetland system, as no significant differences were observed between past and present ostracod communities overall, indicating notable resilience of this metacommunity to environmental changes. Indeed, the invertebrate communities of brackish and coastal wetlands are usually encompassing a particular set of species adapted to stressing changes in salinity and other abiotic factors (Margalef 1983; Barnes 1994). Further palaeoecological studies in Marjal dels Moros taking into account ostracod tolerances and other proxies would help deciphering longer-term paleohydrological and paleosalinity changes in this system (e.g. Curry 1999; Ruiz et al. 2003; Reed et al. 2012).
We observed intra-annual changes in community structure occur, as indicated by the ANOSIM results. The detected average spring increase in ostracod abundance was expected from general patterns of ostracod population dynamics (Horne 1983). A previous study in Marjal dels Moros also found a spring increase in benthic invertebrate populations and a decrease in summer (Oltra and Armengol-Díaz 1999), probably related to increased temperatures and salinities in the hottest period. Similar conditions that reflected the replacement in the taphocoenoses, are affecting the actual communities. A wide range of conductivities was observed in Marjal del Moros, but also differences in water depth. This scenario changed between spring and summer with the reduction in depth and an increase in salinity. In these periods, H. salina was dominant and increased its populations in sites five and six (with low depth and high salt content). Conversely, I. gibba dominated in summer communities at sites one and six (with higher depth and lower salt content). As reflected in the RDA analysis H. salina was abundant in sites with higher conductivities, while I. gibba was collected in high proportions in sites with higher depth in summer. The tolerance ranges of these species are in agreement with previous studies in the Iberian Peninsula (Mezquita et al. 2005). Ilyocypris gibba populations were also found in a wide range of salinities in near localities in coastal wetlands (e.g. Rueda Sevilla et al. 2006; Poquet et al. 2008; Rueda et al. 2013; Valls et al. 2013), but at mean salinity values lower than in the present survey. However, sexual populations of this species are rare and seem to be mostly occupying circum-Mediterranean habitats (Meisch 2000). Further studies on the biogeographical pattern of distribution of sexual and parthenogenetic I. gibba populations are needed to discuss processes involved, but previous studies with other species with similar patterns such as E. virens suggest that both biogeographic history linked to glacial-interglacial cycles, together with climate effects on hydroperiod and habitat predictability are the key factors to understand geographical parthenogenesis in ostracods (Schmit et al. 2013).
Our results on the ecology of ostracod communities in Marjal dels Moros provide support for the influence of environmental factors on species distribution, even at small spatial scales and habitats with wide dynamics in abiotic variables, but also stress the resilience of invertebrate communities adapted to live in coastal wetlands experiencing wide changes in abiotic factors. Long-term ecological studies are usually needed to determine the human factors that influence community changes in freshwaters and devising measures to control them, but biotic remains, such as ostracod shells, might help in quickly evaluating major differences between present and past environmental conditions related to human impacts. Preservation and restoration efforts should therefore take into account ostracod living and dead assemblages as an important tool in the management planning of protected wetlands.
References
Aguilar-Alberola JA, Mesquita-Joanes F (2012) Acute toxicity tests with cadmium, lead, sodium dodecyl sulfate, and Bacillus thuringiensis on a temporary pond ostracod. Int Rev Hydrobiol 97(4):375–388
Allen PE, Dodson SI (2011) Land use and ostracod community structure. Hydrobiologia 668(1):203–219. doi:10.1007/s10750-011-0711-7
Altinsaçli S (2004) Investigation on ostracoda (crustacea) fauna of some important wetlands of Turkey. Pak J Biol Sci 7(12):2130–2134. doi:10.3923/pjbs.2004.2130.2134
Barnes RSK (1994) The brackish-water fauna of northwestern Europe. Cambridge University Press, Cambridge, p. 287
Bohigues C (2001) L’antropització de la marjal dels Moros. Braçal 24:205–212(in Catalan)
Bordás-Valls V, Hurtado-Soler A, Batlle-Sales J (1999) Estudio de los índices climáticos de degradación en los principales ecosistemas lagunares de la provincia de Valencia. Cuadernos de Geografía 65-66:313–324 (in Spanish)
Box M (1991) Humedales y áreas lacustres. In: Morales A (ed) Atlas temático de la Comunidad Valenciana I. Levante-EMV, Valencia, pp. 121–140(in Spanish)
Carmona P, Ruiz JM (2014) Procesos geomorfológicos en llanos de inundación y Lagos costeros mediterráneos. El cambio ambiental histórico en la Albufera de Valencia (España). Cuaternario y Geomorfología 28(3–4):95–106 (in Spanish)
Cavanilles AJ (1795) Observaciones sobre la historia natural, geografia, agricultura, población y frutos del reyno de Valencia Vol. 2. Imprenta Real, Madrid. 236 pp. (in Spanish).
Cottenie K (2005) Integrating environmental and spatial processes in ecological community dynamics. Ecol Lett 8(11):1175–1182. doi:10.1111/j.1461-0248.2005.00820.x
Cuesta F (2001) Gestió i manteniment dels recursos hídrics de La Marjal dels Moros. Braçal 24:49–54(in Catalan)
Curry BB (1999) An environmental tolerance index for ostracodes as indicators of physical and chemical factors in aquatic habitats. Palaeogeogr Palaeoclimatol Palaeoecol 148:51–63
De Bie T, De Meester L, Brendonck L, Martens K, Goddeeris B, Ercken D, Hampel H, Denys L, Vanhecke L, Van der Gucht K, Van Wichelen J, Vyverman W, Declerck SA (2012) Body size and dispersal mode as key traits determining metacommunity structure of aquatic organisms. Ecol Lett 15(7):740–747. doi:10.1111/j.1461-0248.2012.01794.x.
De Deckker P, Forester RM (1988) The use of ostracods to reconstruct continental palaeoenvironmental records. In: De Deckker P, Colin JP, Peypouquet JP (eds) Ostracoda in the Earth Sciences, 1st edn. Elsevier Science Publishers, Amsterdam, pp. 175–200
Durazzi JT (1977) Stable isotopes in the ostracod shell: a preliminary study. Geochim Cosmochim Acta 41:1168–1170
Escrivà A, Smith RJ, Aguilar-Alberola JA, Kamiya T, Karanovic I, Rueda J, Schornikov EI, Mesquita-Joanes F (2012) Global distribution of Fabaeformiscandona subacuta: an exotic invasive Ostracoda on the Iberian Peninsula? J Crustac Biol 32:949–961. doi:10.2307/41,691,450
Frenzel P, Schulze I, Pint A (2012) Noding of Cyprideis torosa valves (Ostracoda)–a proxy for salinity? New data from field observations and a long-term microcosm experiment. Int Rev Hydrobiol 97(4):314–329
Geddes MC, De Deckker P, Williams WD, Morton DW, Topping M (1981) On the chemistry and biota of some saline lakes in Western Australia. Hydrobiologia 82:201–222. doi:10.1007/BF00048717
Gómez JA, Pardo R, Urios V (1988) Humedales. In: Sanchis EJ (ed) Guía de la naturaleza de la Comunidad Valenciana II. Levante-EMV, Valencia, pp. 653–670 (in Spanish)
Griffiths HI, Holmes JA (2000) Non-Marine ostracods and Quaternary palaeoenvironments. Quaternary Research Association, London
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Palaeontological Statistics Software package for education and data analysis. Palaeontol Electron 4(1):9
Horne DJ (1983) Life-cycles of podocopid Ostracoda - a review (with particular reference to marine and brackish-water species). In: Maddocks RF (ed) Applications of Ostracoda. Proceedings of the Eighth International Symposium on Ostracoda. University of Houston, Texas, pp. 581–590
IBM (2010) IBM SPSS Statistics for Windows, Version 19.0. IBM Corporation, Armonk.
Keyser D, Aladin N (2004) Noding in Cyprideis torosa and its causes. Studia Quaternaria 21:19–24
Marco-Barba J, Mesquita-Joanes F, Miracle MR (2013) Ostracod palaeolimnological analysis reveals drastic historical changes in salinity, eutrophication and biodiversity loss in a coastal Mediterranean lake. The Holocene 23(4):556–567. doi:10.1177/0,959,683,612,466,752
Margalef R (1983) Limnología. Ed. Omega S.A, Barcelona (in Spanish)
Martinoy M, Boix D, Sala J, Gascón S, Gifre J, Argerich A, Barrera R, Brucet S, Badosa A, López-Flores R, Méndez M, Utgé JM, Quintana XD (2006) Crustacean and aquatic insect assemblages in the Mediterranean coastal ecosystems of Empordà Wetlands (NE Iberian Peninsula). Limnetica 25(3):665–682
Meisch C (2000) Freshwater Ostracoda of Western and Central Europe. Spektrum Akademischer Verlag, Heidelberg
Mesquita-Joanes F, Smith AJ, Viehberg F (2012) The ecology of Ostracoda across levels of biological organisation from individual to ecosystem: a review of recent developments and future potential. In: Horne DJ, Holmes JA, Rodríguez-Lázaro J, Viehberg F (eds) Ostracoda as proxies for Quaternary climate change. Elsevier, Amsterdam, pp. 15–35
Mezquita F, Roca JR, Reed JM, Wansard G (2005) Quantifying species-environment relationships in non-marine Ostracoda: Examples using Iberian data. Palaeogeogr Palaeoclimatol Palaeoecol 225:93–117. doi:10.1016/j.palaeo.2004.02.052
Navarro LF (1989) El hombre y la alteración del medio. Aportes de la palinología al estudio de dos yacimientos arqueológicos del periodo subatlántico en la franja costera del País Valenciano Cuadernos de Geografía 46:127–148 (in Spanish).
Oltra R, Armengol-Díaz X (1999) Limnología de los humedales mediterráneos susceptibles de albergar Samaruc y Fartet: (II) Zooplancton. In: Planelles-Gomis M (ed) Peces Ciprinodóntidos Ibéricos Fartet y Samaruc. Generalitat Valenciana, Valencia, pp. 79–97 (in Spanish)
Pernía JM, Cuesta F, Ballesteros B, Barba-Romero J, García E (1996) Los recursos hídricos en la Comunidad Valenciana. Instituto Tecnológico GeoMinero de España, Valencia (in Spanish)
Poquet JM, Mezquita F, Rueda J, Miracle MR (2008) Loss of Ostracoda biodiversity in Western Mediterranean wetlands. Aquatic Conservation 18:280–296. doi:10.1002/aqc.831
Reed J, Mesquita-Joanes F, Griffiths H (2012) Multi-indicator conductivity transfer functions for Quaternary palaeoclimate reconstruction. J Paleolimnol 47:251–275
Rodrigo MA, Colom W (1999) Limnología de los humedales valencianos susceptibles de albergar Samaruc y Fartet: (I) Físico-química. In: Planelles-Gomis M (ed) Peces Ciprinodóntidos Ibéricos Fartet y Samaruc. Generalitat Valenciana, Valencia, pp. 59–79 (in Spanish)
Rossetti G, Bartoli M, Martens K (2004) Limnological characteristics and recent ostracods (Crustacea, Ostracoda) of freshwater wetlands in the Parco Oglio Sud (Northern Italy). Ann Limnol-Int J Limnol 40(4):329–341. doi:10.1051/limn/2,004,030
Roure I (2001) Fauna de la Marjal dels Moros. Braçal 24:55–61 (in Catalan)
Rueda Sevilla J, Aguilar-Alberola JA, Mezquita i Juanes F (2006) Contribución al conocimiento de los crustáceos (Arthropoda, Crustacea) de las Malladas de la Devesa del Parque Natural de la Albufera (Valencia). Boletín de la Asociación Española de Entomología 30(1–2):9–29 (in Spanish)
Rueda J, Mesquita-Joanes F, Valentín A, Dies B (2013) Inventario de los macroinvertebrados acuáticos del “Ullal de Baldoví” (Sueca, Valencia, España) tras un programa de restauración. Bol Real Soc Esp Hist Nat 107:1–9 (in Spanish)
Ruiz F, Abad M, Bodergat AM, Carbonel P, Rodríguez-Lázaro J, González-Regalado ML, Toscano A, García EX, Prenda J (2013) Freshwater ostracods as environmental tracers. Int J Environ Sci Technol 10(5):1115–1128. doi:10.1007/s13762-013-0249-5
Ruiz F, González-Regalado ML, Muñoz JM, Pendón JG, Rodrıguez-Ramirez A, Cáceres L, Vidal JR (2003) Population age structure techniques and ostracods: applications in coastal hydrodynamics and paleoenvironmental analysis. Palaeogeogr Palaeoclimatol Palaeoecol 199(1):51–69. doi:10.1016/S0031-0182(03)00,485-1.
Samraoui B, Segers H, Maas S, Baribwegure D, Dumont HJ (1998) Rotifera, Cladocera, Copepoda, and Ostracoda from coastal wetlands in northeast Algeria. Hydrobiologia 386(1–3):183–193. doi:10.1023/A:1,003,538,730,152.
Sanchis C, Ferri M (1997) La marjal dels Moros, sistema natural y producto antrópico Dinámica Litoral-interior: actas del XV Congreso de Geógrafos Españoles, (Santiago, 15–19 setembro 1997), Vol. 1, pp. 257–266 (in Spanish).
Sancho V (2001) La restauració del cordó Litoral, reserva marina i aqüicultura. Braçal 24:199–204(in Catalan)
Sanjaume E, Segura F, López García MJ, Pardo JE (1992) Recent sedimentation in the Valencia Lagoon: Preliminary results. J Coast Res 8(3):668–698
Schmit O, Bode SN, Camacho A, Horne DJ, Lamatsch DK, Martens K, Martins MJF, Namiotko T, Rosseti G, Rueda-Sevilla J, Schôn I, Vandekerkhove J, Mesquita-Joanes F (2013) Linking present environment and the segregation of reproductive modes (geographical parthenogenesis) in Eucypris virens (Crustacea: Ostracoda). J Biogeogr 40(12):2396–2408. doi:10.1111/jbi.12174
Tachet H, Bournard M, Richoux PH (1981) Introduction à l’étude des macroinvertébrés des eaux douces. Université Lyon-Association Française de Limnologie, Lyon
Takamura K, Yasuno M (1986) Effects of pesticide application on chironomid larvae and ostracods in rice fields. Appl Entomol Zool 21(3):370–376
ter Braak CJF , Šmilauer P (2012) Canoco reference manual and user’s guide: software for ordination, version 5.0. Ithaca USA: Microcomputer Power, New York.
Torres T, Ortiz JE, Martín-Sánchez D, Arribas I, Moreno L, Ballesteros B, Blázquez A, Domínguez JA, Rodríguez Estrella T (2013). The long Pleistocene record from the Pego-Oliva marshland (Alicante-Valencia, Spain). In: Martini IP, and Wanless HR (eds.) Sedimentary coastal zones from high to low latitudes: similarities and differences. Geological Society, Special Publication (London), 388:1–24. doi:10.1144/SP388.2.
Valls L, Rueda J, Mesquita-Joanes F (2013) Dynamics of Ostracoda (Crustacea) assemblages in a Mediterranean pond system (Racó de l’Olla, Albufera Nat. Park) with focus on the exotic species Candonocypris novaezelandiae (Baird, 1843). Ann Limnol-Int J Limnol 49(3):237–247. doi:10.1051/limn/2,013,053
Valls L, Rueda J, Mesquita-Joanes F (2014) Rice fields as facilitators of freshwater invasions in protected wetlands: the case of Ostracoda (Crustacea) in the Albufera Natural Park (E Spain). Zool Stud 53:68. doi:10.1186/s40555-014-0068-5
Von Grafenstein U, Erlenkeuser H, Trimborn P (1999) Oxygen and carbon isotopes in modern fresh-water ostracod valves: assessing vital offsets and autecological effects of interest for palaeoclimate studies. Palaeogeogr Palaeoclimatol Palaeoecol 148:133–152
Williams WD (1998) Salinity as a determinant of the structure of biological communities in salt lakes. Hydrobiologia 381:191–201. doi:10.1023/A:1,003,287,826,503
Xia J, Engstrom DR, Ito E (1997) Geochemistry of ostracod calcite: 2. the effects of water chemistry and seasonal temperature variation on Candona rawsoni. Geochim Cosmochim Acta 61(2):383–391
Acknowledgments
The study was mainly funded by the Conselleria de Medi Ambient de la Generalitat Valenciana and the “Life” European environmental program. Partial funding has been received from the Spanish Ministries of Science and Innovation, and of Economy and Competitiveness through projects ECOINVADER (CGL2008-01296/BOS) and ECOLAKE (CGL2012-38909). We are grateful to the Microscopy Unit of the SCSIE-UVEG for their help with the SEM. We would like to thank Ignacio Lacomba and Yuri Rueda for providing facilities and for their help during fieldwork, and two anonymous reviewers and D. J. Horne for their suggestions to earlier versions of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valls, L., Zamora, L., Rueda, J. et al. Living and Dead Ostracod Assemblages in a Coastal Mediterranean Wetland. Wetlands 36, 1–9 (2016). https://doi.org/10.1007/s13157-015-0709-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-015-0709-4