Abstract
The mineralogy of some carbonate rocks from the Abakaliki Basin has been investigated to determine the depositional environment classification and assess the suitability of the rocks for application in geotechnical construction and industrial process systems. The method of investigation included field mapping and petrographic and geochemical techniques to characterize their field characteristics, mineral types and texture and chemical contents. Mapping revealed two lithologic units of sandstone and shale that associate with the carbonates in a similar sequence. The carbonates contain fossils and are composed of calcite, quartz and rockclasts. The grains have angular to sub-rounded sphericity and are dispersed in hetereolithic groundmass. The major oxide composition shows average value of 32.30wt% SiO2, 26.37wt% CaO, 7.23wt% MgO, 6.56wt% Fe2O3, 4.38wt% Al2O3, and 3.23wt% K2O + Na2O in decreasing order of abundance. Concentrations of trace elements are significantly higher (> 100 ppm) for V, Cr, Sr, Zr, Cu, Ba, Pb and Zn and comparatively lower for Sb, Ge, Ce, Y, As, Hg, Tiand Au. The studied rocks qualify as biomicrite or wackestone and range from dolomitic, through carbonaceous to siliceous limestone deposited in a wide spectrum of environment from shallow marine to transitional setting. The rocks are marginally suitable for use as aggregates due to the presence of deleterious contents of quartz and organics. For cement production, their low content of CaO, relatively high SiO2, comparatively high LOI and elevated abundances of contaminant heavy elements are major limitations. Similarly, in paper, plastic, paint, metallurgy and pharmaceutics, the rocks are unsuitable due to the constituent minerals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbonates are rocks that are composed of predominantly (> 50%) modal content of calcite and dolomite minerals, both of which constitute the main bearer of their engineering behaviour and detect potential economic applications as construction and industrial material (Ames and Cutcliffe 1983; Bell 2007). The variations in the quantity and type of the principal mineral content of carbonate rocks as well as the associated subordinate minerals will depend on the environment of deposition (Nicolas 2009; Paige-Green 2007; Tuğrul and Yılmaz 2012), which unwittingly configure their engineering properties that vary widely. In addition, and contrasting with silicates, the major carbonate minerals are susceptible to physiochemical modifications in response to changing climatic factors and thus can be very unstable owing to high crystal plasticity even at very low conditions in climatic factors such as temperature and pressure (Tuğrul and Yılmaz 2012). The environment of deposition and post-depositional diagenesis impact textural features and mineral and chemical compositional changes on carbonate rocks. It follows, therefore, that these factors are of important considerations in the evaluation of the economic potentials of carbonate rocks that overtly depend on the constituent mineral, chemical and textural indexes (Tang et al 1994; Tuğrul and Zarif 1999; Wyllie and Mah 2004). These indexes are prerequisite determinants for assessment of the suitability of the carbonate rocks for use in structural members or aggregates in geotechnical constructions and for application in industrial process systems.
All over the world, carbonate rocks are utilized as a major component of many geotechnical constructions as dimensional or decorative stones and as crushed rock aggregates in concrete and bituminous mixes (Přikryl 2021). Similarly, in industrial process systems, carbonate rocks are invaluable as refractory agent, fusion catalyst in manufacture of fertilizers, lime, glass, paper, plastic, cement and rubber (Varela et al. 2006; Rabaha and Ewais 2009). The strength and durability of built environment as well as the performance of industrial production systems will depend on the compositional, textural and diagenetic features of the carbonate source rocks (Bell 1993; Bektas et al. 2015; Mathur et al. 2016). However, due to potential variations on the nature of source rock characteristics occasioned by the environment of deposition and geomorphic effects, the use of carbonate rocks in any of the engineering objectives necessitates adequate evaluation to ensure the safety and durability of geotechnical constructions and optimal performance of industrial systems.
The designated Ezeaku Group of lithologies which consists of shale, sandstone and carbonate rocks is a prominent lithostratigraphic unit that are tied to regression and transgression eustatic cycles which inundated Abakaliki and other rift systems that make up the Benue Trough of Nigeria during the Turonian Age (Ojoh 1992; Nwajide 2013). As such, many studies have been undertaken by several researchers and agencies to unravel mostly the tectonics, stratigraphy and sedimentation architecture of the Benue Trough (e.g. Agagu and Adighije 1983; Amajor 1987, 1992; Benerjee 1980, 1982; Umeji1993; Lawal 1991 and many others) and with much reference to petroleum and solid mineral potentials (e.g. Cratchley and Jones 1965; Whiteman1982; Olade 1979; Akande 1999; Ene and Okogbue 2012) as well as the invasion and metamorphic effects of associated intrusives, pyroclastics and dorelites (eg. Uzuakpunwa 1986; Obiora and Umeji 2004, 2005; Obiora and Charan 2010,). Although these groups of rocks are fairly well known in terms of mineralogy and sedimentary facies relative to hydrocarbon and solid mineral potentials and igneous invasion, the potential effect of the mineralogic attribute and pattern of sedimentation on the construction and industrial material quality have received little or no attention. This paper highlights the mineralogic characteristics of some carbonates from Abakaliki Basin and attempts to classify the depositional environment as well as to evaluate the suitability of the rocks for potential industrial and construction applications. The implications of varying carbonate rock classification and material specification schemes are highlighted.
Regional geologic setting
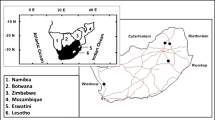
The study area covers the major mining localities of Amananta, Amechi, Ntezi, Ezza and Ezzamgbo, all in Ohaukwu, Ishielu and Ezza North Local Government Areas of Ebonyi State in the south-eastern Nigeria (Fig. 1). The landform is generally a low-lying plain. The area underlain by less resistant rocks has low relief with elevation values ranging between 60 and 150 m. The valleys between the sandstone ridges are mainly characterized by streams and rivers, which are mainly potential places for outcrop location.
The Albian to the Santonian tectonic episodes controlled the deposition of the stratigraphic sequences in the Abakaliki Basin (Fig. 2), forming a rift structure which constitutes the lower Benue Trough (Nwajide 2013). The NE–SW trending depositional basin was formed during the early Cretaceous rifting processes of the Godwana supercontinent. The rift structure is flanked by Anambra platform to the east and the Ikpe platform to the southwest. The basal unit in the basin consists of arkosic, non-fossiliferous conglomerates that have been acknowledged from outcrops and geophysical data at the Ogoja and Abakaliki areas (Ojoh 1992, Table 1). This unit is attributed to pre-middle Albian in age that is probably related to the earliest stages of basin formation. The Albian sediments, which are associated with the first marine transgression in the basin, were earlier described by Reyment (1965) as the Asu River Group consisting of shale, pyroclastics, intrusive bodies with sandy limestones and sandstones. Ojoh (1992) has divided the group into three formations that range from the middle Albian to lower Cenomanian. The middle Albian Ekebeligwe Formation was interpreted as deep marine from the presence of mega slumps and turbidites and from the foraminifera and ammonite assemblages found in the formation (Ojoh 1992). In the upper Albian, the Ngbo Formation units were deposited.
The occurrence of the Cenomanian units was characterized by the shallow marine Odukpani Formation, which consists of sandstone, shale and limestone that cropped out in the south-eastern margin of the basin (Reyment 1965; Kogbe 1989). The absence of Cenomanian in the Abakaliki Basin had been attributed to a possibly slight folding phase (Nwachukwu 1972). However, Ojoh (1992) noted the presence of some characteristic pollen and spores and established the presence of the Cenomanian in the Abakaliki Basin. He placed the upper sandstones of the Asu River Group in the lower Cenomanian (Ibri and Agila sandstones), while the marine shales outcropping at Ezillo originally classified as part of the Eze-aku shales was also discovered to be of upper Cenomanian age. Turonian deposits have been designated as the Eze-aku Group (Reyment 1965; Amajor 1987; Kogbe 1989). The units were deposited from the lower Turonian by one of the most widespread transgressions during the first transcontinental connection of the Tethys Ocean (present day Mediterranean Sea) with the Atlantic. In the Abakaliki Basin, black shales, limestone and calcareous sandstones constitute the Ezeaku Group. Another transgression in the Coniacian led to renewed limestone deposition on the platforms areas (Nkalagu) and the deposition of bluish grey shales with limestones and calcareous sandstones classified as the Agwu Group (Reyment 1965) and the Nkalagu Formation (Ojoh 1992). The Coniacian stage records the beginning of the regression that culminated in the uplift that ended the first tectonic phase and deposition was transferred to the adjoining Anambra Basin.
In the Santonian period, the deposits in the Abakaliki Basin were folded into an anticlinorium with the subsidence of the platform area. Magmatic activity also occurred with the emplacement of intrusions in some localities, especially in the core of the anticlinorium. Ojoh (1992) has found the sandstones in the Ugep area to contain fossil assemblages indicative of Santonian to possibly Campanian age implying possible deposition in some areas during the Santonian folding episode.
Method of study
The detailed field mapping involved traversing along mine faces, road cuts, rivers and streams channels, with the aid of the GPS coordinates and elevations of locations (Fig. 1). The mapping not only involved location of outcrops, description and sampling of encountered lithologies, but also taking note of salient features such as texture and structures. Ten mine clusters, each identified by the central host town as shown in Fig. 1, were studied and two different sets of samples were collected for petrography and geochemistry analyses, respectively. Brunton compasses were used to measure the attitude of beds, trend of fractures and joints. Logging of outcrop sections was performed where required and samples of rock units were collected and labelled. Although 50 thin sections were cut, 5 per mine cluster, 10 representative samples were selected for detailed petrographic study due to the observed high rate of similarity in rock physical properties and the slides. The thin sections were prepared and analysed for the selected samples at the LogiTech laboratory of the Department of Geology, University of Nigeria, Nsukka. The prepared thin sections were studied on a 100-point count basis under a polarizing microscope. Following standard sample preparation, powdered specimens were subjected to elemental analyses using energy dispersive X-ray fluorescence (EDXRF) spectrometer of model “Minipal 4” for the analysis. The analysis was conducted at the Nigerian Geological Survey Agency, National Geosciences Research Laboratory (NGRL), Kaduna. The pellets were carefully placed in the respective measuring positions on a sample changer of the machine. The following condition sets were used as the machine was switched on: elemental composition determination; nature of the samples to be analysed as pressed powder (pellet); the current 14kv for major oxides, 20kv for trace elements/rare earth metals; selected filters were “kapton” for major oxides, Ag/Al-thin for trace elements/rare earth metals. The selection of filters was guided by a given periodic table used for elemental analysis. Time of measurement for each sample was 100 s and the medium used was air throughout. Loss on ignition (LOI) was determined gravimetrically by heating 1 g of the powdered sample in a clean weighed crucible at 1000 °C, after which the crucible and the content were weighed to get the difference in weight before and after heating. Few other replicate samples for each of the ten selected representative sample groups were tested with the same machine condition to check for accuracy and precision and the differences in the obtained results varied by less than 5% in all.
Results and discussion
Petrography
The lithologic units and representative outcrop sequences were interpreted cartographically and the result is illustrated in Fig. 3. It can be seen from the figure that two rock units, namely, shale and sandstone, associate with the carbonates in all the studied mine clusters (Fig. 1). Figures 4A, B, 5A–E and 6A, B show perspective views of the examined mine cuts outcrops that show the vertical contacts, structural relationships and stratigraphic placement of the mined carbonates in relation to nearby linked sequences of rock units. The carbonate rock being exploited in the mine clusters display three main lithofacies. The first facies (Fig. 4A) are graded from the underlying shale unit with extensive, infilled fractures and lack significant body fossils. The second facies, at the middle of the entire carbonate units, contain ubiquitous fossils and readily disintegrates on direct impact (Figs. 5). This unit is less indurated with fewer joints compared with the first underlying facies. The third carbonate rock facies that top the unit has the highest compaction and hardness relative to the lower facies. Although severely fractured with lesser number of recoverable fossils, stratigraphic bedding is very pronounced and is graded into the sandstone units (Figs. 4A, 6). The sedimentologic and stratigraphic description of the sequences is similar to that given by other researchers (Reyment 1965; Amajor 1987; Kogbe 1989; Ojoh1992) for the Ezeaku Group and is similarly interpreted. Banerjee (1980) reported that the sequences were deposited in a subtidal environmental setting where deposition energy and sediment supply fluctuated erratically. Such fluctuation may have led to the deposition of sandstones during high energy events, followed by carbonates and shales during ebbs. Similarly, Amajor (1987) analysed the lithofacies association based on their specific sedimentary features and concluded that the sequences were formed during the Albian regression, and in the Turonian, a storm-dominated transgression left the observed sedimentary imprints.
The results of the petrographic investigations of the thin sections from the studied carbonates of the mine clusters are shown as photomicrographs (Figs. 7, 8, 9, and 10) and summarized in Table 2. The studied carbonates have samples that exhibit heterogranular textures and display poorly sorted grains that are dispersed in a drusy mosaic groundmass. Also, the figures and tables show carbonate minerals ranging from microcrystalline to coarsely crystalline calcite grains. As observed from the photomicrographs, the bivalves (body fossils of ammonites and gastropods) are randomly oriented with elongate, circular to semicircular shapes. The samples are predominantly grain supported with grain size ranging from 0.02 to 1.0 mm in diameter and displaying subangular to well-rounded shapes, well to poorly sorted texture, brown colour and moderate to high relief (Figs. 7, 8). In addition, the grains are accompanied by organic materials that are associated with trace microbial borings (the small, dark brown patches visible in many grains). In some cases, the rocks consist of angular to sub-rounded ooids and pelloids grains hosted in a micrite matrix (Figs. 7A–D, 8A,B). The ooids have grain diameter that vary between 0.001 and 0.08 mm, and are well sorted, with grey, brown to dark colour, all of which are dispersed in moderate to strong relief. As indicated in Figs. 9 and 10, there are also fine-grained (< 0.02 mm) quartz and brown muscovite. The microstructure of small ooids is completely or highly altered by crystal diminution (Fig. 9). Some ooid grains still have preserved relics of the original structure, but most grains exhibit the characteristic criteria of pelloids (structureless round micrite grains). Genetically, these pelloids are altered faecal pellets, micritized and recrystallized ooids or bioclasts (Fig. 10). The petrographic characteristics of the carbonates are characteristic of deposition in varying environments from brackish water to open, shallow storm and tide-dominated sea (Banerjee 1981). Peters (1978) and Banerjee (1981) believed that the bioclastic carbonates were formed in a mixed depositional setting where storms (high energy system as evidenced by the existence of large mineral grains, partially intact and completely preserved body fossils as well as large lithic clasts) interact and alternate with quiet flow events.
The compositional analysis (Table 2) of the rock contents shows three main constituents of micrite, allochems and sparite calcites in an increasing order of abundance. The micrite, which is the carbonate mud, appears greyish to brownish under a polarizing microscope, and less translucent than the sparite. The average grain size of the micrite is about 0.03–0.04 µm in diameter and constitutes about 45–50% of the rocks. The allochem constitutes about 40% of the rock. Body fossil fragments were observed and constitute about 75% of the entire allochem constituents, which also includes lithoclasts and intraclasts. In the thin section slides, the allochems are composed of skeletal bioclasts of mainly shells of molluscs and moulds of ammonites, gastropods, pelecypods and ostracods (Figs. 5, 8). The rock fragments include euhedral quartz grains, which constitute about 10% of the allochem contents and are somehow rounded with very small sizes (< 0.02 mm in diameter). The intraclasts are composed of reworked carbonate rocks, which originated within the depositional basin. They constitute approximately 10% of the allochem fragments. The sparry calcite is a pore filling cement, which grows within the carbonate rock fabric during diagenesis. Presumably, the sparry calcite forms during the dissolution of shells and subsequent crystallization filling all the void spaces. Sparry calcite constitutes about 7–10% of the rock unit. Based on the constituents of the rocks, which averages to 50% micrite, 40% allochem and 10%sparry calcite, the carbonates can be classified into type (2) carbonate rock, micritic allochemical limestone (Folk 1962). The relative proportions of the allochem contents of 75% fossil fragments, 10% lithoclast and 10% intraclasts qualify the carbonates as biomicrite using the classification scheme of Folk (1962). The presence of clastic materials such as quartz and the range of the sizes of transported constituents (clasts) from 0.02 to 1 mm qualifies the studied rocks as calcarenite, based on the classification scheme for carbonate rocks outlined in Pettijohn (1975). Since the grains are “floating” in mud and the rock is mud supported with the relative percentages of the mud being about 10%, the studied rock qualifies as wackestone using the Dunham classification of 1962. In addition, the presence of quartz grains in the carbonates indicates vicinity to the shoreline. The intact nature of the fossil materials also reflects a low energy setting.
The studied thin sections show significant features of diagenesis (Figs. 7, 8, 9, 10) which typically involves a variety of physical and chemical processes, the most common being cementation, compaction, neomorphism and dissolution. From the figures, the contacts between the individual grains or clasts show both floating, sutured and line types of grain contacts which are clear pointers to post-depositional diagenetic features (Ahmad et al. 2021). For instance, calcite cement binds the bivalve shells together and displays a low grade of cementation because of the floating contact the sparry calcite has with the skeletal or fossil fragments (Figs. 9, 10). The line type of grain contact indicates a low degree of cementation. Compaction is indicated by the presence of cracks in the studied thin sections. The line contact between the grains and each other shows that the overburden pressure or depth of burial is shallow. The studied rockslides indicate some evidence of neomorphism, which is the replacement of one mineral by another or of one polymorph of a mineral by another. For instance, transformation is shown by the replacement of calcite in the bivalve shells by sparry calcite. The thin section samples also display aggrading neomorphism, since the sparry calcite cement shows crystals normal to the grain surface and increasing grain size away from the grain surface. Dissolution is indicated by the failure/collapse of micrite enveloping edges. The preferred orientation of skeletal fragments and minerals reflects compaction and tectonic stress that occurred within the basin, perhaps during the Santonian. The intact nature of the fossil materials also reflects a low energy setting, the less amount of transport and no reworking of the sediment. Indeed, the limestone was deposited in quiet or sheltered water where fine-grained carbonate mud could settle and organic remains were buried in an unbroken condition without any consideration for size or fragility (Reineck and Singh 1973). In addition, the carbonates grade from lime to wackestone, which demonstrates the low energy, lagoonal or outer-shelf/ramp environment (Ahmad et al. 2021).
Geochemistry
The major and trace element composition of the studied carbonates are presented in Tables 3 and 4. The relative abundance of chemical facies is depicted in Fig. 11A, B. The table and the figure reveal that the studied rocks have more siliceous mineral content in Samples 1, 2, 3, 4 and 5 since the measured SiO2 concentration is above 40wt% in the samples, while other samples recorded comparatively lower concentrations. Also, Samples 4, 9 and 10 have determined MgO concentration that are above 4wt%, which is higher compared with the concentration values in the other samples. This suggests that there is higher quantity of dolomite and other minerals which bear MgO in the samples compared with the other samples. Similarly, CaO concentration is higher in Samples 6, 7, 8 and 10, which indicates the presence of a higher percentage of calcite mineral in the samples. While the Fe2O3 concentration value is higher in Samples 2, 4, 5, 9 and10, the molarity of Al2O3 is elevated in Samples 2, 4, 5, 9 and 10, both of which imply that the samples are richer in the proportion of aluminium- and iron-containing minerals respectively, relative to the other samples. Again, the total alkali concentration in the studied rocks is higher in Samples 2, 4, 9 and 10, with all the measured values being above 5wt%, which suggests that the rocks of these samples contain more minerals that have alkalis compared with the other samples. However, the measured concentration of TiO2, SO4, Cl and P2O5 are notably low, except for TiO2 proportion in Samples 4 and 5, SO4 content in Samples 5 and 9, and Cl percent concentration in Samples 5, 9 and 10, where the values are up to 2wt % on average. The same applies to the concentration of Na2O that recorded significant values above 2wt% only in Samples 1, 2 and 3. The lower content of these chemical parameters points to the fact that the studied rocks lack minerals that are rich in the content of these elements. The LOI, which is an important measure of carbon content, is higher in Samples 5, 6, 7, 8, 9 and 10 where the proportions are above 14 wt% and this indicates that the samples contain more carbonaceous materials or organics relative to the others. The implication of the observed trends in the variation of rock chemical composition illustrates the existence of different grades of materials from the studied mine clusters. In construction application which has been receiving the attention of artisanal and small-scale miners for some decades now, the presence of siliceous mineral such as quartz, and carbonaceous matter such as organics and dolomites are viewed with disdain. This is owing to the fact that they are susceptible to climatic modification as well as detrimental reactions with alkalis contained in Portland cement. The samples with significant percentage of the deleterious materials will, therefore, not perform satisfactorily when involved in geotechnical constructions. In view of this, samples with higher MgO, SiO2 and LOI will likely perform unacceptably in construction projects. Conversely, samples with higher concentration of CaO will be more suitable in construction. Similarly, the sample with higher CaO will be more acceptable in industrial applications. This is even more so if such samples are with low concentrations of volatile and contaminants such as P2O5, SO4, Cl and TiO2, all of which will interfere with the fusion temperature of CaO. However, for the same reason of elevated heating temperature, the higher concentrations of SiO2, above 2wt%, will likely render doubtful performance of some samples in industrial process systems.
The concentrations of trace elements are significantly high (> 100 ppm) for V, Cr, Sr, Zr, Cu, Ba, Pb and Zn and comparatively low for Sb, Ge, Ce, Y, As, Hg, Ti and Au. Regression analyses of some of the elemental concentrations from the rock are presented in Fig. 12. According to Fig. 12A–C, the CaO represents a moderate negative correlation with TiO2 and SiO2 and a weak to moderate negative correlation with Na2O + K2O.The correlation between the chemical facies in the cross plots shows significant and varied relationship among the measured chemical indexes, which demonstrates contributions from many and varied source and correlative abundance for the elements. The nature of the correlative abundances of the elements stem from the depositional and post-depositional processes that are prevalent in low energy/water environment with the usual inputs of sediments from continental and/or deep setting. The predominance of SiO2 and CaO lend support to the observed petrography of the rocks in which calcite dominates with the pronounced presence of quartz. The low contents of iron, aluminium and alkalis are attributed to the paucity of minerals that contain those elements.
Geochemical classification of carbonates used some standard plots, which include a classical (rapid) rectangular diagram, Martinet and Sougy (1961) diagram, tectonic discriminant diagram and ternary plots. In the carbonate classification (Fig. 13), the studied samples plotted within a wide range from dolomitic limestone through magnesium limestone to calcareous limestone. Similarly, from the Martinet and Sougy plots of CaO and MgO (Fig. 14), samples are plotted within a wide range from dolomitic limestone, through siliceous limestone, to calcareous limestone. Also, Fig. 15 which presents the tectonic discriminant plot (Roser and Korsch 1986) for the referenced samples indicates that the studied rocks were formed in a wide range of tectonic setting from oceanic island arc to the passive margin. In addition, two separated domains exist in the cross plot, in which the former oceanic island arc setting predominates the later passive margin conditions. From the ternary diagram (Fig. 16), which is based on alkali, potassium and iron ratios, it is evident that the rock classified as carbonaceous to carbonaceous ferrite was formed in the marine to transitional zone (Helene et al. 2013). Besides, the rock sequence ranges from deep to shallow marine shales, carbonates to transitional and continental sandstones (Reyment 1965; Amajor 1987; Ojoh 1992).
Plot of the rapid method for classifying carbonate rocks from their chemical analysis. Note: A: dolomitic limestones; B: magnesian limestones; C: limestone sensu lato; C1: slightly dolomitic limestones; C2: dolomitic limestones sensu stricto; D: calcareous dolomites sensu lato; D1: calcareous dolomites sensu stricto, D2: slightly calcareous dolomites; E: dolomites
Diagram of Martinet and Sougy for classifying the studied carbonate rocks from their chemical analysis. Note 1: dolomites; 2: siliceous dolomites; 3: dolomitic cherts; 4: calcareous dolomites; 5: calcareous–siliceous dolomites; 6: dolomitic–calcareous cherts; 7: dolomitic limestones; 8: dolomitic–siliceous limestones; 9: calcareous–dolomitic cherts; 10: magnesian limestones; 11: siliceous magnesian limestones; 12: calcareous–magnesian cherts; 13: limestones; 14: siliceous limestones; 15: calcareous cherts; 16: impure cherts; 17: cherts
Tectonic discriminant diagram for the studied carbonate sediments (after Roser and Korsch 1986)
Ternary plot A (alkali), K potassium) and iron (F) ratios for geochemical classification of the sampled rocks. Note: 1 = argillaceous, 2 = carbonaceous argillaceous, 3 = carbonaceous, 4 = carbonaceous ferrite, 5 = ferruginous, 6 = ferruginous argillite, cz = continental zone, tz = transition zone, mz = marine zone
Economic potential
The economic potential in terms of industrial applications of the carbonate rocks is evaluated by comparing the measured geological and geochemical properties with some end use specifications (Table 5) for use in cement production, chemical industrial uses and pharmaceutical uses. The carbonate rocks are not suitable for cement production in their natural state (Ugwuanyi 2017), mainly because of their low CaO (26.38 wt%) composition, high loss on ignition (LOI) due to high organic content and high composition of silica, SiO2 (32.35 wt%). In addition, the significant presence of heavy metal contaminants such as Pb and Ba is also problematic in carbonates used in cement production, since such elements will not only increase the melting point of the clinkers leading to higher input energy requirement, but also result in products with abnormal setting time and low engineering value. Succinctly, the rocks failed to satisfy available end use specification from the three different sources. Perhaps, the low quality of the carbonates may have contributed to the abandonment of the Nkalagu Cement Factory (Nigercem) few years after commissioning. Such low-quality material will not only escalate production costs, but also lead to finished products with prohibitive prizes that will be unable to thrive in competitive Portland cement market (Ames and Cutcliffe 1983). Carbonate rocks to be used for construction purposes should be as cubic as possible with no laminations or cracks. Particle shape is important because cubic particles interlock and press firmly into the asphalt binder. Flat and elongate particles do not interlock and develop voids that lead to premature breakup of the road surface and also grain colour should be white to reflect heat (Danner 1966; Power 1985; Tepordei 1985). The petrographic study of the rocks shows that the grains are angular to subrounded, and the megascopic and microscopic study also shows that it is leucocratic. All these demonstrate that the rocks can be used for construction purposes. However, they should be used with caution due to the presence of diagenetic features and biofacies in the rocks, which may be mobilized during exploitation and usage to entrain lower structural strength and decreased durability depending on the binding media and exposure to present environmental conditions. The rock composition did not meet the limits for metallurgical grade, because of high silica content and low CaO (Table 5). However, the rocks can be used on modification. For carbonate rocks to be used for pharmaceutical purposes, they should contain more than 71 wt% CaO and less than 1wt% trace metals including iron and total alkalis (Power 1985). The rocks have low CaO content (26.3 wt%), more than 1 wt% iron content (6.5 wt%) and total alkali is about 3.2 wt%, all of which disqualify the rock for pharmaceutical application, except on beneficiation. A grade assessment using normalized values of insoluble, dolomite (D1) and calcite (K1) from the ternary plot (Fig. 16) which subdivides carbonates to their various basic end uses is represented in Fig. 17. It is evident from the figure that the carbonate rocks can be used as aggregates, while a few others can be used as agricultural limestone. Furthermore, Fig. 17 shows that the rocks cannot be applied in cement production.
Ternary plot of carbonate rock classification by end use (Hershey and Maher 1985)
Conclusions
Conclusions of the present study are as follows:
-
The lithologic sequences in the studied mine clusters show that the mines exploited or is exploiting carbonate rocks interstratified with sandstones and shales. The carbonate rocks are composed of predominantly micrite, sparite and allochem calcites with subordinate quartz and organics. The carbonate rocks show three distinct facies based on fracture density, degree of induration and fossil contents
-
The mineral assemblages are arranged in a hetereogranular texture style in which there is prominent display of poorly sorted grains that are dispersed diffusely in a drusy mosaic groundmass.
-
There is significant evidence of diagenesis of the textural pattern and constituent minerals through cementation, compaction, neomorphism and dissolution processes that have been occurring since the Turonian deposition of the carbonate rocks.
-
The nature of the mineral content and their modifications by diagenesis have caused the carbonate rocks to contain a significant proportion of SiO2, CaO, Mg2O, Fe2O3, Al2O3, Na2O and K2O with notable composition in trace elements such as V, Cr, Sr, Zr, Cu, Ba, Pb, Zn and high LOI
-
The studied rocks are classified as biomicrite or wackestone. The limestone classes range from dolomitic through siliceous to carbonaceous types. The varied classes are a reflection of the complex environment of deposition from shallow marine to transitional setting in basins that ranged from oceanic island arcs to passive margins.
-
The chemical composition, which is consequent upon the mineral content, when compared with some reference standards, shows that the studied rocks are marginally suitable for construction purposes because of the presence/proportion of deleterious minerals such as quartz and organic materials. In industrial application, the rocks for the same reason of silica and organic proportion as well as heavy metal contamination are unsuitable in their natural state.
Data availability
Data sets acquired in the course of this study and/or analysed therein will be provided by the corresponding author if requested.
References
Agagu O, Adighije CI (1983) Tectonic and Sedimentation framework of the lower Benue, southeastern Nigeria. J Afr Earth Sci 1:267–327
Akande SO (1999) Fluid flow histories in Cretaceous sediments of the Benue rift basins Nigeria: fluid inclusions, isotopes and alteration mineralogy constrains. Bull NAPE 14:112–129
Amajor LC (1987) The Eze-Aku sandstone (Turonian) Ridges of south-eastern Nigeria: a reinterpretation of their depositional origin. J Min Geol 23:17–26
Amajor LC (1992) Storm-induced turbidite-like deposit: an example from the TuronianThe Eze-Aku Formation at Nkalagu, south-eastern Nigeria. J Min Geol 28:7–17
Ahmad F, Quasim MA, Ahmad AHM (2021) Microfacies and diagenetic overprints in the limestones of Middle Jurassic Fort Member (Jaisalmer Formation), Western Rajasthan, India: Implications for the depositional environment, cyclicity, and reservoir quality. Geol J 56(1):130–151
Banerjee L (1980) A subtidal bar model for the Eze Aku Sandstone. Sedim Geol 25:291–309
Banerjee I (1981) Storm lag and related facies of the bioclastic limestones of the Ezeaku Formation (Turonian) Niveria. Sediment Geol 26:133–147
Banerjee L (1982) Trace fossil of the bioturbated sandstone facies of the Eze Aku Formation Nigeria. Indian J Earth Sci 9:93–98
Ames J, Cutcliffe W (1983) Cement and cement raw materials. In Lefond SJ (ed) Industrial rocks and minerals: New York, American Institute of Mining, Metallurgical, and Petroleum Engineers, Inc., pp 133–159
Bektas F, Wang K, Ren J (2015) Carbonate aggregate in concrete. Final Report submitted to Minnesota Department of Transportation Research Services and Library 395 John Ireland Boulevard, MS 330 St. Paul, Minnesota, pp 55155–1899
Bell FG (2007) Engineering Geology. Elsevier
Bell FG (1993) Durability of carbonate rock as building stone with comments on its preservation. Environ Geol 21:187–200
Cratchley JC, Jones JP (1965) An interpretation of the geology and gravity anomalies of the Benue valley Nigeria. Overseas Geological Survey, London, Geophysics Paper 1, pp 26
Calzia J, Matt J, Gantenbein M (1995) Geology, geochemistry and resource potential of carbonate rocks in the San Bernardino National Forests, California, pp 11–17
Dangote Cement Factory Obajana (2016) Clinker analysis at Dangote Cement Factory Obajana as at 26/08/2016. 8:53 am for Cement production in the Company
Danner W (1966) Limestone Resources of Western Washington: Washington Division of Mines and Geol Bull 52:473–485
Dunham RJ (1962) Classification of carbonate rocks according to their depositional texture. In: Ham WE (ed) Classification of carbonate rocks, Tulsa, OK, AAPG Memoirs, pp 108–121
Ene GE, Okogbue CO (2012) Occurrence and industrial properties of some barite deposits in the Abakaliki Basin, Southeastern Nigeria. Nat Resour Res 21:347–357
Folk R (1962) Spectral subdivision of limestones types. In: Ham WE (ed) Classification of carbonates rocks. Am Assoc Petrol Geol Memoirs 162:62–84
Harben PW (1995) The industrial minerals handbook. A guide to Markets, Specifications and Prices, Ontario, 2nd edn, pp 36–43
Helene M (2013) New computerized method for the geochemical classification of Precambrian carbonate rocks. Case of a set of African Cap carbonates. Int. J. Geol. 4:1–13
Hershey RE, Maher SW (1985) Limestone and dolomite resources of Tennessee. State of Tennessee. Department of conservation, Division of Geology, Nashville, Tennessee
Kogbe CA (1989) Paleogeographic History of Nigeria from Albian Times. In: Kogbe CA (ed) Geology of Nigeria. Elizabethan Publishing Company, Lagos, p 375
Lawal O (1991) Palynological age and correlation of a black shale section in the Ezeaku Formation, lower Benue trough, Nigeria. J Afr Earth Sci (and Middle East) 12(3):473–482
Martinet R, Sougy J (1961) Practical utilization of chemical classification of carbonate rocks. C r Somm Soc Geol 23:81–92
Mathur NK, Jakhar SR, Rajendra M (2016) Calcium carbonate and derived products. Int J Sci Res (IJSR) 5(4):1–6
Nicolas G (2009) Sedimentology and Stratigraphy. Blackwell Scientific Publications, Oxford
Nwachukwu S (1972) The tectonic evolution of the southern portion of the Benue Trough, Nigeria. Geol Mag 109:411–419
Nwajide CS (2013) Geology of Nigeria’s sedimentary basins. CSS Bookshop Ltd, Lagos
Obiora SC, Umeji AC (2005) Petrography of some altered intrusive rocks from the lower Benue Trough. Nigeria J Min Geol 41:1–9
Obiora SC, Charan SN (2010) Geochemical constraints on the origin of some intrusive igneous rocks from the Lower Benue rift, Southeastern Nigeria. J Afr Earth Sci 58:197–210
Obiora SC, Umeji AC (2004) Petrographic evidence for regional burial metamorphism of sedimentary rocks in the Lower Benue rift. J Afr Earth Sci 38:269–277
Ojoh KA (1992) The southern of the benue trough cretaceous stratigraphy, basin analysis, paleo–oceanography and geodynamic evolution in domain of the South Atlantic. Nig Assoc Petrol Explor Bull 7:131–152
Olade MA (1979) On the genesis of lead-zinc deposits in Nigeria Benue rift (Aulacogen): a reinterpretation. J Min Geol 13:20–27
Paige-Green P (2007) Durability of basic crystalline rocks and specification for use as road base aggregate. Bull Eng Geol Environ 66:431–440
Power T (1985) Limestone specifications: industrial minerals, in Mineral facts and problems: U.S. Bureau Mines Bull 6:65–91
Peters SW (1978) Mid-Cretaceous paleoenvironments and biostratigraphy of the Benue Trough, Nigeria. Geol Soc Am Bull 89:151–155
Pettijohn FJ (1975) Sedimentary rocks. Harper and Row publishers, New York
Přikryl (2021) Geomaterials as construction aggregates: a state‑of‑the‑art. Bull Eng Geol Environ 80:8831–8845
Rabaha M, Ewais CMM (2009) Muilti-impregnating pitch-bonded Egyptian dolomite refractory brick for application in ladle furnaces. Ceram Int 35:813–819
Reineck HE, Singh IB (1973) Depositional sedimentary environments. Springer, New York
Reyment R (1965) Aspect of the Geology of Nigeria. Ibadan University Press, Ibadan
Roser B, Korsch R (1986) Determination of tectonic setting of sandstone-mudstone suites using SiO2 Content and K2O/Na2O. J Geol 19:260–278
Tang M, Deng M, Lon X, Han S (1994) Studies on alkali-carbonate reaction. ACI Mater J. American Concrete Institute, Farmington Hills, Michigan, pp 26–29
Tepordei V (1985) Crushed stone: in mineral facts and problems: U.S. Bureau of Mines Bull 675:757–768
Tuğrul A, Yılmaz M (2012) Assessing the quality of sandstones for use as aggregate in concrete. Mag Concr Res 64(12):1067–1078
Tuğrul A, Zarif IH (1999) Correlation of mineralogical and textural characteristics with engineering properties of selected granitic rocks from Turkey. Eng Geol 51(4):303–317
Ugwuanyi M (2017) Suitability of carbonates from Abakaliki fold belt asraw material in cement production. Unpublished Industrial Training Report. Department of Geology, University of Nigeria Nsukka
Umeshwar P (2003) Economic geology. Satish Kumar Jain for C.B.C, New Delhi, pp 204–207
Umeji OP (1993) Hummocky cross-stratification in the Turonian EzeAku Sandstones of southeastern Nigeria: sedimentological significance. J Min Geol 29:71–76
Uzuakpunwa AB (1986) A pre-Albian succession in parts of southeastern Nigeria. Cahiers Geologiques 9:358–362
Varela JJ, Petter CO, Wotruba H (2006) Product quality improvement of Brazilian impure marble. Min Eng 19:233–363
Whiteman AJ (1982) Nigeria: its petroleum resources and potential. Graham and Trotman, London, p 396
Wyllie DC, Mah WC (2004) Rock slope engineering, 4th edn. Taylor & Francis Group, New York
Funding
There was no funding received for the conduct of the study expressed in this article.
Author information
Authors and Affiliations
Contributions
All the authors contributed in the conceptualization and design, data collection and analyses, and data interpretation and drafting of manuscript of the work in the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ene, G.E., Dumbo, M.J. & Ugwu, F.O. Evaluation of the influence of mineralogical attributes on the economic potentials of some carbonate rocks from Abakaliki Fold Belt, south-eastern Nigeria. Carbonates Evaporites 39, 39 (2024). https://doi.org/10.1007/s13146-024-00946-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s13146-024-00946-5