Abstract
The Garmez area is located in a semi-arid region with limited water resources. Therefore, maintaining the quality of groundwater and preventing it to be contaminated is of particular importance. In this research, five wells were sampled in the study area to investigate the quality of groundwater for drinking and agricultural purposes. Anions and cations of Cl−, SO42−, HCO3−, CO32−, NO3−, Ca2+, Mg2+, Na+ and K+, as well as important water quality factors including pH, EC and TDS were measured using titration, optical absorption spectroscopy, flame atomic emission spectrometry and drying methods. The analyzed data are compared with guidelines of the World Health Organization (WHO) and Iranian standard for drinking water, and with standard of the Food and Agricultural Organization of United Nations (FAO) for irrigation water. Mean values of anion and cation concentrations reveal that the groundwater in the study area is dominated by sulfate and calcium ions. The high correlation coefficients between TDS and dissolved ions, as well as the Gibbs’s diagram, suggest that rock weathering and evaporation–precipitation processes govern dominantly the chemistry of groundwater. The concentrations of major ions are higher than the permitted levels for drinking water, except for pH, chlorine, sodium and nitrate, indicating that most of the groundwater samples do not have a good quality for drinking purpose. All groundwater samples belong to the “Very hard” water class. According to the Schoeller's diagram, groundwater samples belong to a wide range of water quality classes from “Good” to “Unpleasant”. Most of the groundwater samples are of good quality when compared with the FAO standard for irrigation water, except that their calcium and potassium contents exceed the allowed limits. Based on the analysis of water quality indicators including SAR, PI, SSP, MAR, KR and RSC, most of the groundwater samples from the study area are of “Excellent” to “Good” quality classes, thus suitable for irrigation purpose. According to the Wilcox's diagram, groundwater samples fall in the C4S1 and C3S1 categories, reflecting that they are “Saline-suitable for irrigation with appropriate treatment”.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is the most valuable natural resources, as it is essential for the biological processes of human and other organisms. Furthermore, human activities such as agriculture, livestock breeding and the industry, become impossible without water (Soroush et al. 2016). Given the current population growth in the world, the need for water at the global level is increasing, while freshwater resources are limited and heterogeneously distributed. In some countries, including Iran, water scarcity is an obstacle to economic, social and cultural development. Groundwater is considered as the most important water supply source for drinking, irrigation and industrial uses, due to its proper quality, less seasonal and perennial fluctuations, better protected from possible pollution, uniformly spreading over large regions, and easy extraction (Zektser and Everett 2004). It includes a variety of chemical compounds, which are mainly sourced from the geological formations. However, the chemical composition of groundwater is also affected by a wide range of environmental processes, including human activities. Among these factors, increased water withdrawals, chemical additives in agriculture, and waste disposal are seriously effective (Ehya and Marbouti 2016; Rahman et al. 2017; Ehya and Mosleh 2018).
Due to the vast agricultural activities, as well as the diversity of rock units in the study area, there is a possibility of changing the chemical composition, and reducing the quality of groundwater resources. Geological formations such as carbonate and evaporitic ones, which have a high solubility in water, can reduce groundwater quality and restrict its use. Human activities, including excessive use of groundwater resources, the development of agricultural and livestock activities, chemical fertilizer use, urban and industrial sewage disposal, also could contaminate groundwater in the area (Annapoorna and Janardhana 2015; Abdullah-Al-Mamun et al. 2017; Alexander et al. 2017; Ehya and Marbouti 2018). In this research, we assess the quality of groundwater in the Garmez region, SW Iran, for drinking and agricultural purposes. The groundwater samples were analyzed for major physicochemical parameters; the results were then compared with national and international standards and guidelines for drinking and irrigation uses. Since the groundwater quality (for both drinking and irrigation purposes) is not monitored regularly by a specific local or governmental entity (water authority), this research is greatly important in assessing the water quality of the study area.
Study area
The Garmez region is the southeastern part of the Behbahan Plain, located in Khuzestan province, SW Iran (Fig. 1a). Garmez and Dowlatabad villages are located adjacent to Kheyrabad River, having fertile alluvial lands for agriculture (Fig. 1b). These two villages have settled more than 800 people, whose main occupation is agriculture and animal husbandry. This area—with a surface of 60 km2—is neighbored by the Kohgiluyeh-va-Boyer Ahmad and Bushehr provinces. The region is limited, from north and northeast, to the Kohgiluyeh Mountains, from east and south east to Gachsaran city in Kohgiluyeh-va-Boyer Ahmad province, from south to Deylam port in Bushehr province, and from west and northwest to Behbahan city. The coordinates of the study area comprise north latitudes 30°30′–30°39′ and east longitudes 50°09′–50°27′. The Garmez area elevates 313 m in average above the sea level. It has a semi-arid climate with severe hot in seven months from April to October. The average annual temperature is 25.3 °C, with the maximum temperature up to 50 °C in July and August, and minimum − 3.0 °C in January. The average annual rainfall and humidity are 304 mm and 46%, respectively, for the observation period from 2010 to 2017.

(as modified by Macleod and Akbari 1970)
a Geographical location of the Garmez area; and b geological map of the study area
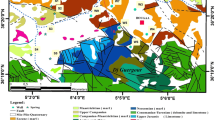
The Garmez area is geologically located in the Zagros Simply Folded Belt, based on the Stöcklin’s classification (1968). The geological formations in the study area range in age from Paleocene–Oligocene to the present. Marls and shales of the Pabdeh Formation are the oldest rock units cropping out in the area, which are overlain by, from old to new, limestones of the Asmari Formation, gypsum and marl layers of the Gachsaran Formation, shellfish limestones and gray marls of the Mishan Formation, calcareous sandstones and red marls of the Aghajari Formation, weathered siltstones of the Lahbari Member, conglomerates of the Bakhtyari Formation and alluvial deposits (Fig. 1b). The alluvial sediments composing the aquifers of the Garmez area vary in grain size from gravel to clay. These deposits are derived dominantly from Asmari, Gachsaran, Mishan and Aghajari formations, and its groundwater quality is not expected to be desirable. Structurally, the presence of two anticlines including Khaviz and Ramechar in the study area is worthy to be noted (Fig. 1b). The Khaviz anticline, with a northwest–southeast trending axis, locates to the northeastern part of the area. It encompasses, in its core, the oldest rock unit in the region—Pabdeh Formation. The Ramechar anticline lies to the eastern and southeastern segments of the region, consisting of younger formations Mishan and Aghajari. Its axis also trends northwest–southeasterly, following the main trend of the Zagros Mountains. These two anticlines have an important role in recharging of the Behbahan Plain’s aquifer (Ehya and Marbouti 2016, 2018).
The study area (Garmez region) comprises two distinct aquifers; one is the southeastern extreme of the Behbahan Plain’s aquifer, and the other is the Garmez’s aquifer (Fig. 2). Both aquifers are from the phreatic type, from the hydrogeological point of view. Under the influence of the Ramechar anticline, the overall direction of groundwater flow in the southeastern sector of the Behbahan Plain’s aquifer is from eastern and southeastern margins to the west and northwest, towards the center of the Behbahan Plain (Ehya and Marbouti 2016, 2018). In the Garmez’s aquifer, the direction of groundwater flow is from both sides of the Kheyrabad River towards this river (Fig. 2). A total of ten wells have been drilled in the Garmez region, draining about 1 million m3 of groundwater from the aquifers, annually. Only 10% of the water extracted from these wells is used for agriculture and the rest is consumed for drinking use. Ninety percent of the water used in agricultural activities is supplied from the Kheyrabad River.
Samples and methods
Groundwater samples were collected during March 2017 from five wells drilled in two aquifers in the study area. The location of the selected samples relative to the groundwater aquifers is shown in Fig. 2. Water samples contained in 250 ml polyethylene bottles which were washed before with acid solution and distilled water. At the time of sampling, the bottles were also thoroughly rinsed two times with the well water. Before sampling, the pump was allowed to work for 15 min and once the values of water characteristics (T, EC, and pH) reached a constant quantity, then the samples were taken. The water samples were filtered to separate possible suspended solids. The cation samples were acidified using ultra pure 65% nitric acid (0.5 ml per 100 ml of water) to avoid possible changes in the concentration of dissolved compounds. Further, to prevent unpredictable variations in the water composition, samples were stored in a refrigerator below 4 °C (APHA 2005). The electrical conductivity (EC) and pH values of the samples were determined at the site of sampling using a Hach-SensION5 conductivity meter (EC meter) and a Jenway pH meter. The geographic locations of the sampling sites were measured and recorded using a Trimble GeoExplorer 2008 Series GPS apparatus. The nitrate concentration in samples was measured using optical absorption spectroscopy (OAS) within 24 h of sampling. The concentrations of Cl−, SO42−, HCO3−, CO32−, Ca2+ and Mg2+ were determined by titration, K+ and Na+ by flame atomic emission spectrometry (FAES), and total dissolved solids (TDS) by drying methods (Nollet 2007). All chemical analyzes were performed in the Arvand Khak Azma Laboratory (Ahvaz, Iran). The values of physicochemical parameters of the groundwater samples were compared with the guidelines of the World Health Organization (WHO, 2008) and the Iranian standard (ISIRI 2010) for drinking water, and the standard of Food and Agriculture Organization of the United Nations (FAO; Ayers and Westcot 1994) for irrigation water (Table 1).
Results and discussion
Hydrochemistry of groundwater
The data for main parameters analyzed in groundwater from the Garmez area are given in Table 2. According to the mean values of anion and cation concentrations, the groundwater in the study area is dominated by SO42− and Ca2+ which account for more than 69% of the total anions and cations, followed by HCO3−, Cl−, Na+ and Mg2+ ions. Nitrate and K+ have the least share in total concentrations of anions and cations, as their contents comprise less than 1% of the ions concentration sum. The dominance of sulfate and calcium in groundwater is obviously due to the widespread evaporitic (gypsum and anhydrite) and carbonate rock types in the study area. Except for the sample S1 with a slightly acidic nature, pH values for other samples indicate a neutral to slightly alkaline essence for groundwater. Although the studied samples are selected from two distinct aquifers, with the exception of the values for NO3−, SO42−, Ca2+ and Mg2+ in S4 and S5 samples which are less than those in other samples, the remaining parameters do not show a significant difference between the samples. Samples S4 and S5 are taken from the Garmez’s aquifer which is adjacent to the Kheyrabad River (Fig. 2). In the absence of data on the chemical composition of the Kheyrabad River in the study area, it is not possible to comment on the impact of the river's water on the hydrochemistry of the Garmez’s aquifer. However, the chemical effect of the river’s water on groundwater is not far from the mind. The values for all analyzed parameters in all samples fall within the reported ranges in groundwater from the Behbahan Plain by Ehya and Marbouti (2016, 2018).
The interrelations between physicochemical parameters in groundwater were investigated using the Pearson’s correlation matrix (Table 3) (Gorsuch 1983). The highest positive correlation (r = 0.95) exists between SO42− and TDS, while the lowest positive (r = 0.12) between NO3− and Cl−. An interesting result is that pH displays a negative correlation with all water parameters. As it is expected, TDS and EC display strong–moderate positive correlations with all ions (r ≥ 0.25), except for HCO3− with which TDS has negative correlation, indicating that the origin of dissolved minerals in groundwater might be the dissolution of evaporitic minerals halite and gypsum. Among cations, only Ca2+ and K+ show a weak negative correlation (r = − 0.04) which can be interpreted as they derived from absolutely different sources. In the case of anions, HCO3− represents weak or negative correlations with Cl−, SO42− and NO3−. On the other hand, chlorine shows a weak correlation (r = 0.12) with NO3−. The correlation coefficients between cations and anions indicate that Na+ has weak correlations with NO3− and HCO3−, while Ca2+ with HCO3−, and K+ with NO3− have negative correlations. These weak or negative correlations also suggest non-identical source for relating ions. An alternative source for some ions (e.g. K+, NO3− and HCO3−) could be anthropogenic activities including chemical fertilizers and animal husbandry.
Groundwater facies
A Piper (1944) diagram has been used to determine the hydrochemical facies of groundwater in the Garmez area (Fig. 3). The groundwater facies is sulfate–calcium, which could be due to the dissolution of gypsum and carbonate rocks from the nearby sedimentary sequences, especially the Gachsaran and Mishan formations. These sediments played an important role in providing material to form alluvium in the study area. Table 4 shows the classification of water samples according to the importance of anions and cations, proposed by Schoeller (1962). Despite the fact that sample S2 appears in the same group as samples S4 and S5, the sulfate load of sample S2 is three times higher than those of the other two samples, making a real difference between sample S2 and the group of S4 and S5 samples. The Piper triangular diagram is furthermore showing this (Fig. 3).
Source of dissolved salts
Based on TDS, Na/(Na + Ca) and Cl/(Cl + HCO3) parameters, Gibbs (1970) provided a dual diagram to determine the mechanisms controlling water’s chemistry, and to identify the hydrochemical evolution of water. These diagrams are widely used to study the effects of processes such as evaporation, precipitation, rock weathering and sea water on the chemistry of water (Eby 2004). In the Gibbs’s diagram for analyzed samples, the data points are located along the boundary between rock weathering and evaporation–precipitation dominated areas, indicating that the chemistry of groundwater is governed dominantly by these two processes (Fig. 4a, b). This conclusion is further supported by the semiarid climate of the study area, as well as the presence of extensive soluble rock materials (Gachsaran, Mishan and Aghajari formations).
Water quality for drinking use
The values of pH, chlorine and nitrate in all groundwater samples fall within the maximum permissible limits, compared with the WHO guidelines and ISIRI standard for drinking water. In the case of sodium, its content is below the maximum permissible quantities, except for the sample S3 in which sodium content is above the permitted value. The quantities of EC, TDS and sulfate in all samples are above the permitted levels. The concentration of calcium in all samples is higher than the maximum permissible limits, except for the sample S5 in which calcium value is below the allowed limit. The magnesium concentrations in all samples are above the maximum desirable limit of the ISIRI standard, while only the samples S1 and S3 are above the maximum permissible level of the WHO guideline. Regardless of pH, chlorine, sodium and nitrate parameters, most of the samples studied do not have good quality for drinking regarding the other analyzed parameters.
Hem (1970) classifies groundwater based on its TDS value (Table 5). All groundwater samples are classified in the “Partially salty” category, according to the Hem's classification. The hardness of water is also an important parameter in assessing its quality for drinking purpose. The water’s hardness is related to its content of calcium, magnesium and some other two valence cations. It is calculated using Eq. 1 and is called the total hardness (TH), expressed in mg-CaCO3/l (Todd 1980):
The TH values for groundwater samples are given in Table 6. Water is classified in terms of TH (Hem 1970; Table 5). Soft water is considered as harmful because of increasing the risk of atherosclerosis (Hem 1989). In terms of TH, all groundwater samples belong to the “Very hard” water class.
Schoeller's diagram
The Schoeller (1962) diagram is also used for assessing the quality of drinking water. In this chart, based on eight chemical parameters including sodium, chlorine, sulfate, calcium, magnesium, carbonate (plus bicarbonate), total dissolved solids (TDS) and total hardness, water is classified into six classes including good, permissible, intermediate, unpleasant, very unpleasant and non-potable (Schoeller 1962). According to the Schoeller quality chart (Fig. 5), groundwater samples from the Garmez area fall within a wide range from “Good” to “Unpleasant” water categories.
Water quality for irrigation purpose
The presence and concentration of some ions are important in irrigation water. The content of dissolved salts in irrigation water affects the plants, as well as the physical and chemical properties of the agricultural soil (Ehya and Firouzeh Moghadam 2017; Guan et al. 2017). The study area comprises erosion-sensitive evaporitic formations (i.e. Gachsaran and Aghajari sequences), having high potential for dissolution and sediment transport. The Gachsaran Formation has thick layers of gypsum and anhydrite, both of which are soluble in water, thus may increase the amount of solutes in groundwater of the study area.
The values of pH, HCO3−, Cl−, Mg2+ and Na+in all samples are below the maximum acceptable limits of the FAO standard for irrigation water, while concentrations of Ca2+ and K+ in all samples are above the permitted limits. The EC values in all samples are below the allowed limit, except for sample S3 in which it is high. The TDS quantities in samples S1 and S3 is higher than the acceptable level, while in other samples it is low. The contents of SO42− and NO3− in samples S4 and S5 are lower than the permitted amounts, but in other samples, they are greater than the admissible limits. Altogether, the quality of most samples is suitable for irrigation, except that their calcium and potassium contents exceed the allowed limits. To evaluate the quality of water for irrigation use, water quality parameters and indices including EC, soluble sodium percentage (SSP), sodium adsorption ratio (SAR), residual sodium carbonate (RSC), permeability index (PI), magnesium adsorption ratio (MAR), and the Kelly's ratio (KR) were also calculated (Table 6).
Electrical conductivity
The electrical conductivity (EC) of water represents its salinity hazard. This parameter is considered important in the classification of irrigation water, because high EC leads to the formation of saline soil (Esmaeili and Moore 2012). The classification of irrigation water based on EC is given in Table 5. Accordingly, sample S5 belongs to the “Permissible” class, while S1, S2, S3 and S4 to the “Doubtful” category.
Soluble sodium percentage
Sodium content is very important in irrigation water, as it decreases soil permeability by damaging its structure. After irrigation, the water goes down from soil surface to the lower horizons, resulting in hardening of the soil in the upper parts. This, in turn, prevents aeration in the roots of the plants. The soluble sodium percentage (SSP), also known as %Na, is calculated using Eq. 2 (Wilcox 1955), where all ion concentrations are in meq/l:
The SSP values vary from 13.0 to 34.8% in groundwater samples (Table 6). Samples S1, S2 and S4 are of “Excellent”, while S3 and S5 of “Good” qualities as irrigating water (Table 5).
Sodium adsorption ratio
The sodium adsorption ratio (SAR) of irrigation water can be used as a criterion for determining the risk of soil alkalization, because after approaching equilibration between irrigation water and the soil, the irrigation water's SAR can be related to the soluble sodium adsorption ratio in the soil. Therefore, the excess amount of SAR in irrigation water reduces the permeability of the soil, inhibiting the water needed for agricultural crops (Singh et al. 2014). The SAR index is calculated using Eq. 3 (Karanth 1987), where all ion concentrations are in meq/l:
The amount of SAR varies from 1.2 to 2.6 in groundwater (Table 6), rendering all the water samples “Excellent” for irrigation (Table 5).
To determine the quality of groundwater for irrigation purpose, Wilcox (1955) diagram is one of the most important categorizations in this field. In the Wilcox chart, the horizontal axis is allocated to water salinity, while the vertical axis to the SAR. The coordinates of each sample is placed in a region enclosed with C in terms of salinity, and S regarding the SAR (Wilcox 1955). As shown in Fig. 6, the samples S1, S2, S3 and S4 are in the C4S1 class, while S5 is in the C3S1 category, meaning “Saline-suitable for irrigation with appropriate treatment” (Table 7).
Residual sodium carbonate
Irrigation water quality is assessed with respect to the risk of residual sodium carbonate (RSC). High concentrations of bicarbonate in water can disrupt plant growth through deposition of carbonate, reducing soil permeability, lowering water penetration rate and increasing soil erosion. The RSC is calculated using Eq. 4, in which all ion concentrations are in meq/l (Eaton 1950):
The amount of RSC in groundwater samples varies from − 38.1 to − 8.8 meq/l (Table 6). Accordingly, groundwater of the study area is suitable for irrigation (Table 5).
Permeability index
Soil permeability is reduced after prolonged use of irrigation water due to deposition of Na+, Ca2+, Mg2+ and HCO3− chemical compounds. An appropriate parameter for evaluating this effect is the permeability index (PI), which is calculated using Eq. 5, where all ion concentrations are in meq/l (Subramani et al. 2005):
The PI values in the samples fall in the span from 17.4 to 43.9% (Table 6), indicating that the water samples S1 and S2 are “Unsuitable”, while S3, S4 and S5 are of “Good” quality for irrigation (Table 5).
Magnesium adsorption ratio
High concentration of magnesium in water affects adversely on the soil quality, because it leads the soil to become alkaline; therefore, the yield of the crops is reduced (Kumar et al. 2007; Song et al. 2017). The magnesium adsorption ratio (MAR) of more than 50% can poison the water. The MAR is calculated from Eq. 6, where all ion concentrations are in meq/l (Paliwal 1972):
The MAR amount in groundwater samples varies from 14.7 to 49.5% (Table 6). All samples have “Good” quality for irrigation regarding the MAR (Table 5).
Kelly's ratio
The classification of groundwater quality for irrigation was also carried out based on the Kelly's ratio (KR). The KR quantity is calculated using Eq. 7, where all ion concentrations are in meq/l (Kelly 1940):
If the KR value is greater than one, the amount of sodium in water is excessive, while water with a KR of less than one is suitable for irrigation. The KR amounts in water samples from the study area range from 0.1 to 0.5 (Table 6). Therefore, groundwater is suitable for irrigation (Table 5).
Conclusions
Groundwater samples from the Garmez area, SW Iran, were analyzed to assess their quality for drinking and agricultural uses. Results show that groundwater in the study area is dominated by sulfate and calcium components. The high correlation coefficients between TDS and most of the dissolved ions, as well as the Gibbs’s diagram suggest that the chemistry of groundwater is governed dominantly by rock weathering and evaporation–precipitation processes. However, the negative correlations existing between some ions indicate an anthropogenic source for them, as well.
Considering the values of analyzed parameters, except for pH, chlorine, sodium and nitrate, most of the groundwater samples do not have a good quality for drinking purpose, when compared with the WHO guidelines and Iranian standard for drinking water. Given that groundwater is the main source of drinking water supply for the people of the region, water authorities must take necessary measures to improve the water quality through establishing a monitoring protocol, including parameters of interest and sampling frequency.
Compared with the FAO standard for irrigation water, most of the groundwater samples are of good quality, except that their calcium and potassium contents exceed the permissible limits. The SSP, SAR, RSC, PI, MAR and KR water quality indicators, also suggest that groundwater from the studied area has a suitable quality for irrigation purpose. However, the Wilcox's diagram reflects that groundwater is suitable for irrigation with appropriate treatment, attracting the attention of the water authorities to improve the water quality in this sense, too.
In this study, groundwater samples were taken at the end of the wet season. In previous studies on groundwater from the Behbahan Plain, sampling was carried out at the end of the dry season. Considering that the study area is close to the Behbahan Plain—in fact, its southeastern extreme—it can be said that these studies totally assessed the water quality of the Behbahan Plain in both wet and dry seasons, and their results can be of interest to local water authorities.
References
Abdullah-Al-Mamun MM, Masum KM, Sarker AHMR, Mansor A (2017) Ecosystem services assessment using a valuation framework for the Bangladesh Sundarbans: livelihood contribution and degradation analysis. J Forestry Res 28:1–13
Alexander AC, Ndambuki J, Salim R, Manda A (2017) Assessment of spatial variation of groundwater quality in a mining basin. Sustainability 9:823. https://doi.org/10.3390/su9050823
Annapoorna H, Janardhana MR (2015) Assessment of groundwater quality for drinking purpose in rural areas surrounding a defunct copper mine. Aquat Procedia 4:685–692
APHA (American Public Health Association) (2005) Standard method for examination of water and wastewater [M], 21st edn. APHA, AWWA, WPCF, Washington, DC
Ayers RS, Westcot DW (1994) Food and Agriculture Organization of the United Nations (FAO), Water Quality for Agriculture, Irrigation and Drainage, Rome, Italy. Paper no. 29. Rev. 1, M-56
Doneen L (1962) The influence of crop and soil on percolating water. In: Proceeding 1961 biennial conference on groundwater recharge, pp 156–163
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 69:123–134
Eby GN (2004) Principles of environmental geochemistry. Thomson Brooks/Cole, Pacific Grove, p 514
Ehya F, Firouzeh Moghadam Z (2017) Hydrochemistry and water quality assessment of the Maroon River in Behbahan area, SW Iran. Water Pract Technol 12:818–831
Ehya F, Marbouti Z (2016) Hydrochemistry and contamination of groundwater resources in the Behbahan plain, SW Iran. Environ Earth Sci 75:455
Ehya F, Marbouti Z (2018) Groundwater quality assessment and its suitability for agricultural purposes in the Behbahan Plain, SW Iran. Water Pract Technol 13:62–78
Ehya F, Mosleh A (2018) Hydrochemistry and quality assessment of groundwater in Basht Plain, Kohgiluyeh-va-Boyer Ahmad Province, SW Iran. Environ Earth Sci 77:164
Esmaeili A, Moore F (2012) Hydrogeochemical assessment of groundwater in Isfahan province, Iran. Environ Earth Sci 67:107–120
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Gorsuch RL (1983) Factor analysis, 2nd edn. Lawrence Erlbaum Associates, Hillside
Guan X, Liao S, Bai J, Wang F, Li Z, Wen Q, He J, Chen T (2017) Urban land-use classification by combining high-resolution optical and long-wave infrared images. Geo-spatial Inform Sci 20: 299–308
Hem JD (1970) Study and interpretation of the chemical characteristics of natural water, 2nd edn. Geological Survey Water Supply Paper 1473—United States Government Printing Office, Washington, DC
Hem JD (1989) Study and interpretation of the chemical characteristics of natural waters. Water supply paper, 2254 U.S. Geological Survey. Washington, DC
Institute of Standards and Industrial Research of Iran (ISIRI) (2010) Drinking water—physical and chemical specifications. ICS:13.060.020, 5th revision (in Persian)
Karanth KR (1987) Groundwater assessment, development and management. Tata McGraw Hill, New Delhi
Kelly WP (1940) Permissible composition and concentration of irrigated waters. In: Proceedings of the ASCF, vol 66. p 607
Kumar M, Kumari K, Ramanathan AL, Saxena R (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab, India. Environ Geol 53:553–574
Macleod JH, Akbari Y (1970) Behbahan geological compilation map 1:100000. Iranian oil Operating Companies, Geological and Exploration Division, Tehran
Nollet LML (ed) (2007) Handbook of water analysis, 2nd edn. CRC Press, Boca Raton, p 769
Paliwal KV (1972) Irrigation with saline water. In: Monogram no. 2 (new series). IARI, New Delhi, pp 198
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–928
Rahman MATMT, Saadat AHM, Safiqul Islam MD, Al-Mansur MDA, Ahmed S (2017) Groundwater characterization and selection of suitable water type for irrigation in the western region of Bangladesh. Appl Water Sci 7:233–243
Richards LA (1954) Diagnosis and improvement of saline and alkali soils, Agric Handbook, vol 60. US Department of Agriculture, Washington, DC
Schoeller H (1962) Les eaux souterraines. Masson Cie, Paris
Singh CK, Kumari R, Singh RP, Mukherjee S (2014) Geochemical characterization and heavy metal contamination of groundwater in Satluj River Basin. Environ Earth Sci 71:201–216
Song XP, Huang C, Townshend JR (2017) Improving global land cover characterization through data fusion. Geo-spatial Inform Sci 20:141–150
Soroush M, Ehya F, Maleki S (2016) Major and trace elements in some bottled water brands from Khuzestan Province market, SW Iran, and accordance with national and international standards. Environ Earth Sci 75:1–10
Stöcklin J (1968) Structural history and tectonics of Iran: a review. Am Asso Petrol Geol Bull 52:1229–1258
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110
Todd DK (1980) Groundwater hydrology. Wiley, New York
Wilcox LV (1955) Classification and use of irrigation water. Agric circ 969. USDA, Washington, DC p:19
World Health Organization (WHO) (2008) Guidelines for drinking-water quality: incorporating first and second addenda, recommendations, 3rd edn, vol.1. WHO Press, Geneva, p 668
Zektser IS, Everett LG (eds) (2004) Groundwater resources of the world and their use United Nations Educational, Scientific and Cultural Organization 7, place de Fontenoy, 75352 Paris 07 SP
Acknowledgements
This study was financially supported by the second author at the time when she was studying for a Master’s degree in Behbahan Islamic Azad University. We would like to express our gratitude to the anonymous reviewers for their helpful comments which improved significantly the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ehya, F., Saeedi, F. Assessment of groundwater quality in the Garmez area (Southeastern Khuzestan province, SW Iran) for drinking and irrigation uses. Carbonates Evaporites 34, 1443–1454 (2019). https://doi.org/10.1007/s13146-018-0481-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13146-018-0481-7