Abstract
The groundwater in the Timahdite–Almis Guigou area flows through fluvio-lacustrine and volcanic formations of Plio-Quaternary age and Liassic limestone. The groundwater resources in this area are used for drinking water and irrigation of agricultural plots. 18 groundwater samples were collected for this study. The physico-chemical and bacteriological parameters analysed, such as temperature, electrical conductivity, pH, dissolved oxygen, Na+, K+, Ca2+, Mg2+, Cl−, HCO3−, SO42−, NO2−, NO3−, faecal Coliforms, total Coliforms and faecal Streptococci, are used to characterise the quality of the groundwater and its suitability for drinking and for irrigation. The Piper and Gibbs diagrams and the saturation index were used to study the hydrogeochemical characteristics of groundwater. The quality of these was assessed on the basis of bacteriological quantification and water quality index (WQI) for drinking, and calculation of sodium percentage (Na%), sodium adsorption ratio (SAR) and permeability index (PI) for irrigation. The mineral saturation index in groundwater indicates that only carbonate minerals tend to precipitate, especially in the form of dolomite. On the other hand, the evaporative minerals are still undersaturated. The bacteriological quality and the water quality index (WQI) of this area are considered to be generally good to poor quality, with the exception of a few points, near the public dump of Almis Guigou, plateau of Lamrijate (Timahdite), Aït Hamza and Aït Ghanem, that show significant bacterial contamination and high concentrations of sodium, chlorides and nitrates. According to the calculation of the (Na%), the (SAR) and (PI), the groundwater samples are suitable for irrigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing population growth and agricultural activities threaten the groundwater and surface water resources of the Timahdite–Almis Guigou area by modifying its quantity and quality for drinking and agriculture.
In this area, the risk of pollution is linked to agricultural factors caused by the use of agricultural fertilizers and pesticides, and anthropogenic factors such as the discharge of waste and the use of septic tanks (Amrani 2016; Amrani et al. 2019). The objective of this study was to assess the groundwater quality of the fluvio-lacustrine and volcanic aquifer in the study area for drinking water and irrigation.
Many factors control the chemical quality of groundwater, including water–rock interaction, recharge, geological structure, lithology and geochemical processes in the aquifer. This interaction could lead to the degradation of groundwater from undesirable components that affect their quality (Drever 1997; Salama et al. 1999; Subramani et al. 2010; Hassen et al. 2016). Groundwater is also affected by human activities that introduce contaminants into the environment (Amrani et al. 2019; Kadaoui et al. 2019; Zakaria et al. 2020).
Understanding hydrochemistry and groundwater quality is important for effective management and protection of water resources. The hydrochemistry of groundwater has been used to identify and interpret their quality, the impact of anthropogenic activities and to determine their hydrochemistry (Jalali 2007; Subramani et al. 2010; Belkhiri and Mouni 2013; Sheikhy Narany 2014; Li et al. 2020).
In this work, we focus on the superficial aquifer of Timahdite–Almis Guigou which is considered with the liasic aquifer as the main reservoirs of water resources in this area. The evaluation of the quality of these resources is based on physical, chemical and bacteriological analyses.
Study area
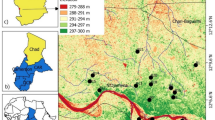
The study area located between the villages of Timahdite and Almis Guigou is part of the transition zone between the tabular Middle Atlas and the folded Middle Atlas. It is located west of the meridian of Boulemane, between the coordinates Lambert X (518 and 559 km) and Y (270 and 330 km). Corresponds to a collapsed area bordered on the SE side by the reliefs of the crossing zone of the Atlasic Middle North Accident. It is limited to the north by the Sefrou and Anoceur limestone plateaus, to the east by the folded Middle Atlas, to the south by the Baqrit Syncline and to the west by the Azrou-Ifrane Causse (Fig. 1).
Geology of the study area
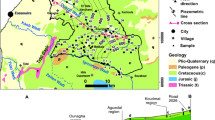
The Meso-Cenozoic lithostratigraphic series in the study area ranges from the upper Triassic to the Quaternary. This series is presented as follows (Fig. 2):
-
Red clay geological formations with evaporite traces, where a median basalt complex is inserted, whose age is attributed to the Upper Triassic—Lower Lias (Termier 1936; Salvan 1984; Ouarhache 1987; Fedan 1988; Hinaje 2004; Ouarhache et al. 2012);
-
These clay and basaltic formations are surmounted by liasic carbonates, first dolomitic and then calcareous (Colo 1961; Michard 1976; Martin 1981; El Arabi, 1987; Fedan 1988; Hinaje 2004);
-
The Dogger is composed of two formations; the Boulemane marls and the Bajocian cornice-limestones (Michard 1976; Fedan 1988; Fedan et al. 1989; Aït Slimane 1989; Charroud 1990; Akasbi et al. 2001);
-
The Cretaceous is formed of limestone and marl (Chbani 1984; Charroud 1990);
-
The Eocene formations are composed of pink limestones, gypseous marls and lacustrine limestones with Flint (Choubert et al. 1952; Du Dresnay 1969; Chbani 1984; Fedan 1988; Aït Slimane 1989; Charroud 1990);
-
The Plio-Quaternary is characterised by fluvio-lacustrine, travertine and volcanic continental formations (Moukadiri 1983; Harmand and Cantagrel 1984; Harmand and Moukadiri 1986; Fedan 1988; Aït Slimane 1989; Hinaje et al. 2001; Hinaje 2004; El Azzouzi et al. 2010; Amrani 2016).
Materials and methods
Groundwater sampling
The analysis data used in this work come from a water sampling campaign during the high water period. A set of 18 groundwater samples (16 wells and 2 springs) was chosen so that they were distributed along the study area (Fig. 3). These wells and springs are intended for irrigation of agricultural plots, for drinking water and for domestic use.
The analyses were carried out within 24 h of sampling. The parameters such as electrical conductivity (EC), temperature, hydrogen potential (pH) and dissolved oxygen (DO) were measured in the field immediately after sampling using a HANNA HI 9828 portable multi-parameter device. The bottles were rinsed with the groundwater to be sampled. Four samples were taken for bacteriological analysis in sterile glass bottles.
All water samples collected in the bottles were transported to a laboratory for hydrochemical and bacteriological analysis (laboratory of the Polydisciplinary Faculty of Taza). The physico-chemical parameters were analysed according to the methods recommended by Rodier et al. (2009). Bacteriological analysis, including Total Coliforms (TC), Faecal Coliforms (FC) and Faecal Streptococci (FS), was carried out according to the Moroccan standard methods ISO 9308-1 (2000) and ISO 7899-2, respectively (2000).
Groundwater hydrogeochemistry
For the description of the chemical composition of groundwater in the study area, we chose to represent the results of the chemical analyses on the Piper triangular diagram and the Gibbs diagram. In addition, the saturation index (SI) was also used to assess the hydrochemical characterization of groundwater.
- The trilinear diagram of Piper (1944) was drawn using the program DIAGRAMS version 6.5 (Simler 2009) to identify the hydrogeochemical facies of groundwater. In the Piper diagram, major ions are represented in the two base triangles as milliequivalent percentages of cation and anion. Total cations and total anions are each considered as 100%. The respective locations of cations and anions for analysis are projected into the diagonal field, which represents the total ionic relationship (Chadha 1999).
- The Gibbs diagram was used to study the relationship between water chemistry and aquifer lithology. Gibbs (1970) proposed two diagrams to illustrate hydrogeochemical processes in the geochemistry of groundwater, including water–rock interaction, precipitation and evaporation. For this purpose, the diagrams were plotted according to the concentrations of TDS as a function of the weight ratios of Na+/(Na+ + Ca2+) for the cations and Cl−/(Cl− + HCO3−) for the anions.
- The saturation index (SI) was calculated using the hydrogeochemical program PHREEQC (Parkhurst and Appelo 1999). The equilibrium state of water with respect to a mineral phase can be determined by calculating the saturation index (SI) using analytical data (Garrels and Mackenzie 1967). The saturation index expresses the degree of chemical equilibrium between the water and the mineral in the matrix of the aquifer considered as a measure of the process of dissolution and/or precipitation concerning the water–rock interaction (Drever 1997). Using this program, we were able to calculate the saturation index of anhydrite, aragonite, calcite, dolomite, gypsum and halite. The groundwater saturation index is expressed as follows (Garrels and Mackenzie 1967):
where IS: Saturation index. IAP: is the product of the ionic activity of the species that make up the mineralization. Kt: The equilibrium solubility constant.
Groundwater quality for drinking
To assess the quality of groundwater in the study area for drinking water supply, we based ourselves on the water quality index (WQI) and the quantification of parameters of faecal origin.
Water quality index (WQI)
To assess the water quality and its suitability for consumption, the water quality index (WQI) was calculated using the weighted arithmetic index method (Horton 1965; Brown et al. 1970). The human consumption standards suggested by WHO (2011) were taken for the estimation of the WQI. Ten (10) parameters were taken into account for the calculation of the water quality index, namely TDS, Na+, K+, Ca2+, Mg2+, Cl−, HCO3−, SO42−, NO2− and NO3−.
The water quality index (WQI) was calculated in three steps:
-
1.
A weight (wi) was attributed to each of the physico-chemical parameters according to its major importance in the evaluation of the quality of drinking water. Weights between 1 and 5 were assigned to each water parameter (Table 3).
-
2.
The relative weight (Wi) calculated using the following equation:
$$ {\varvec{Wi}} = \frac{{{\varvec{wi}}}}{{\mathop \sum \nolimits_{{{\varvec{i}} = 1}}^{{\varvec{n}}} {\varvec{wi}}}} $$(2)where Wi: the relative weight; wi: the weight of each parameter; n: number of parameters.
-
3.
A quality score (qi) for each parameter is determined by dividing its concentration in each water sample by its limit values given by the WHO and the result is multiplied by 100:
$$ \user2{qi } = \user2{ }\frac{{{\varvec{Ci}}}}{{{\varvec{Si}}}}\user2{ } \times 100\user2{ } $$(3)where.
qi: the quality score; Ci: the quantity of each physico-chemical parameter in each water sample (mg/l); Si: the drinking water standard for each chemical parameter (mg/l) as indicated by the WHO (2011).
The water quality index (WQI) is then calculated from the sum of the sub-indices (Sl) of each chemical parameter using the following equations:
The calculated values of the water quality index (WQI) are generally classified into five categories (Table 1): excellent water quality, good water quality, poor water quality, very poor water quality and unsuitable water for drinking (Brown et al. 1972; Chatterji and Raziuddin 2002).
From a bacteriological point of view, the study was based on the quantification of parameters of faecal origin: Faecal Streptococci (FS), Faecal Coliforms (FC) and Total Coliforms (TC). These parameters show the existence of organic pollution due to the presence of certain pathogenic germs. Faecal Streptococci and Faecal Coliforms are important because they live in abundance in the feces of warm-blooded animals and humans, and are good faecal indicators.
Groundwater quality for irrigation
To assess the suitability of groundwater for irrigation in the study area, salinity indices such as sodium absorption ratio (SAR), sodium percentage (Na%) and permeability index (PI) were calculated using the following equations:
-
Sodium Adsorption Ratio (SAR) (Richards 1954):
$$ {\mathbf{SAR}} \left( {\frac{{{\mathbf{meq}}}}{{\mathbf{l}}}} \right) = \frac{{{\mathbf{Na}}^{ + } + {\mathbf{K}}^{ + } }}{{\sqrt {\frac{{\left( {{\mathbf{Ca}}^{2 + } + {\mathbf{Mg}}^{2 + } } \right)}}{2}} }} $$(6) -
Percentage of sodium (Na%) (Wilcox 1955):
$$ {\mathbf{Na}}^{ + } \left( \% \right) = \frac{{\left( {{\mathbf{Na}}^{ + } + {\mathbf{K}}^{ + } } \right) \times 100}}{{\left( {{\mathbf{Ca}}^{2 + } + {\mathbf{Mg}}^{2 + } + {\mathbf{Na}}^{ + } + {\mathbf{K}}^{ + } } \right) }} $$(7) -
Permeability Index (PI) (Doneen 1964):
$$ {\mathbf{IP}} \left( \% \right) = \frac{{{\mathbf{Na}}^{ + } + \sqrt {{\mathbf{HCO}}_{3}^{ - } } \times 100 }}{{{\mathbf{Ca}}^{2 + } + {\mathbf{Mg}}^{2 + } + {\mathbf{Na}}^{ + } }} $$(8)
The Richards (Riverside), Wilcox and Doneen diagrams were used to assess the suitability of groundwater in the study area for irrigation.
The Richards (Riverside) diagram was used using DIAGRAMS program version 6.5 (Simler 2009) to determine the common effect of the risk of EC salinity versus the corresponding sodium SAR risk.
The Wilcox diagram was used using DIAGRAMS program version 6.5 (Simler 2009) to examine the common effect of the hazard of sodium Na+ (%) versus the hazard of corresponding EC salinity.
The Doneen diagram was generated from the Microsoft Excel program by plotting the values of the total concentration of ions against their corresponding IP values.
Results
Hydrogeochemical facies and water type
The representation of the major ion contents of the waters of the fluvio-lacustrine and volcanic aquifer on the triangular diagram of Piper (1944) (Fig. 4) shows that the majority of the samples have cations predominantly calcium and magnesium, while the dominant anions are bicarbonate and sulphates. By analysing the graph, we can distinguish two groups of water or two facies: the bicarbonates, calcium and magnesium facies; and the chlorinated, sulphate, calcium and magnesium facies.
The bicarbonates, calcium and magnesium facies represent 66.66% of the water analysed; this is the most dominant facies in the study area. It generally represents low mineralized waters. The waters of this facies are derived from infiltrations from liasic carbonate rocks.
The facies chlorinated, sulphate, calcium and magnesium represent 33.33% of the waters analysed; it is the less dominant facies. It generally represents the most mineralized waters. The electrical conductivities of the waters range from 1160 to 1770 μs/cm. This facies are located near the fault passage zones, marked by intense grinding and by injections of red Triassic clays rich in evaporatites. The presence of this facies is due to the infiltration and dissolution of the clay evaporatitic rocks of the Upper Triassic—Infra-Lias near the faults.
Gibbs diagram
The analytical data plotted on the Gibbs diagrams (Fig. 5) reveal that all the water samples are in the water–rock interaction field. Therefore, water–rock interaction is the major geochemical process controlling groundwater mineralisation in the study area.
Saturation index
The saturation state of the groundwater was determined using the hydrogeochemical model PHREEQC (Parkhurst and Appelo 1999). The significant results of all the samples analysed (Table 2) show that the waters are saturated with respect to aragonite, calcite and dolomite, but they are rather undersaturated with regard to anhydrite, gypsum and halite. The calculation of the mineral saturation index in water indicates that only carbonate minerals tend to precipitate. However, the evaporative minerals are always in an undersaturated state.
Groundwater quality for drinking
Water quality index (WQI)
The water quality index (WQI) was used to assess the quality of groundwater for drinking in the hydrogeological basin of Timahdite-Almis Guigou. The results of the chemical analyses of the groundwater in this area, in addition to the comparison with the human consumption standards suggested by the WHO (2011) and the weights attributed to the different physico-chemical parameters according to their relative importance in the overall quality of the drinking water, are represented in Table 3.
The weights were attributed according to their major importance in assessing water quality, and their implications for health when they have a high concentration in water (Sadat-Noori et al. 2013 and Hosseini-Moghari et al. 2015).
The calculated values of the (WQI) for the study area vary between 23.94 and 58.88. The area presents three types of groundwater quality, as shown in Table 4.
From a bacteriological point of view, the study was based on the quantification of parameters of faecal origin: Faecal Streptococci (FS), Faecal Coliforms (FC) and Total Coliforms (TC). The results of the bacteriological analyses of the groundwater sampling points in the study area are illustrated in Table 5.
The water from wells P9, P12, P3, P4 and Tit Zill are found to be very rich in pathogenic germs. The number of faecal coliforms is highest in well P12. Indeed, this well captures the water table near the public landfill of Almis Guigou. As for wells P9, P3 and P4 on the Lamrijate plateau (Timahdite), Aït Hamza and Aït Ghanem, they are linked to the existence of septic tanks and the leaching of animal waste. Concerning the Tit Zill spring is also slightly contaminated by the infiltration of septic tanks, the infiltrations from Oued Guigou which is slightly polluted from Timahdite and the leaching of animal waste from Aït Ghanem.
Groundwater quality for irrigation
To assess and classify the quality of groundwater for irrigation in the study area, different parameters were calculated and interpreted, such as sodium adsorption ratio (SAR), percentage of sodium ( Na %) and permeability index (PI). The calculated values were represented on the Richards diagram (1954), the Wilcox diagram (1955) and the Doneen diagram (1964).
Sodium adsorption ratio (SAR)
The diagram from the US Salinity Laboratory (Richards 1954) is widely used to determine the common effect of EC salinity risk versus corresponding SAR sodium risk. The risk of soil salinization can be assessed by the electrical conductivity and the rate of alkalinization. Taking into account these two parameters, this author proposed a class diagram with sixteen classes corresponding to the saline-alkaline risks. This classification was used widely to characterize the water for irrigation.
Taking into account this classification, and after positioning the water points of the different campaigns on the Richards diagram according to the electrical conductivity and the SAR value, we were able to observe the presence of two groups (Fig. 6), C3.S1 and C4.S1.
Classification of irrigation waters on the Riverside diagram (Richards 1954)
The calculated SAR values of the groundwater samples in the study area vary between 0.28 and 6.63 with an average value of 3. Therefore, the SAR values are less than 10 and classified as excellent for the irrigation.
Percentage of sodium (Na%)
The calculation of Na% of the groundwater samples in the study area shows values between 5.65 and 20.07% with an average of 12.37%. The maximum Na% content for irrigation was fixed at 60% by the WHO. The groundwater samples are good for irrigation compared to the percentage of Na. According to the Wilcox classification (1955) based on the values of Na% and electrical conductivity (EC) (Fig. 7), all samples fell into the good and excellent class.
Permeability index (IP)
The permeability index calculated for the groundwater samples in the hydrogeological basin of Timahdite-Almis Guigou is between 30.09 and 76.03% with an average of 48.50%. The representation of the permeability index values on the Doneen diagram (1964), based on the total ionic concentration (TDS) and the permeability index (PI) (Fig. 8), showed that all groundwater samples are distributed in class I. This indicates that the groundwater is good for irrigation.
Discussion
The hydrogeochemical study of the waters of the surface aquifer of the Timahdite–Almis Guigou basin has shown that the waters are moderately mineralized. The representation of the major ion contents of the waters of the fluvio-lacustrine and volcanic aquifer of Plio-quaternary age on the triangular diagram of Piper allows to distinguish two groups of waters or two facies: bicarbonate, calcium and magnesium facies and chlorinated, sulphated, calcium and magnesium facies.
According to these results, it can be said that water flows in the plio-quaternary system through the multiple-play boundary faults of the collapsed area Timahdite–Almis Guigou oriented N30. These faults favour the flow of groundwater from the carbonate liasic boundary zones to the subsident area at fluvio-lacustrine and volcanic fillings. During their journey at depth, the water resulting from infiltrations from carbonate rocks has a carbonate calcium and magnesium facies, while the waters that flowing near the fault planes with Triassic clay evaporite injections have a chlorinated, sulphated, calcium and magnesium facies (Amrani and Hinaje 2014a).
The Gibbs diagram clearly shows that the groundwater samples are in the area of rock alteration dominance or water–rock interaction. This confirms the idea that the dissolution of the Liassic carbonate and Triassic clay-evaporite formations is the process controlling the main chemical composition of the groundwater in the area of Timahdite–Almis Guigou. These results are consistent with those of Aghazadeh and Mogaddam (2010), Hassen et al. (2016) and Qian et al. (2016), respectively, in the Oshnavieh Area (Iran), in the case of Oum Ali-Thelepte aquifer, (Tunisia) and in an agro-pastoral area, Ordos Basin (China).
The calculation of the mineral saturation index in water indicates that only carbonate minerals tend to precipitate mainly in the form of dolomite (Amrani and Hinaje 2014b). The precipitation of these minerals results in equilibrium Ca2+ concentrations and dissolution of evaporated materials (Hamzaoui-Azaza et al. 2011; Hassen et al. 2016). On the other hand, evaporitic minerals are always in the state of undersaturation, suggesting that their concentrations of soluble components Na+, Cl−, Ca2+, and SO42− are not limited by mineral equilibrium (Belkhiri et al. 2012; Hassen et al. 2016).
The results of the bacteriological contamination by faecal Streptococci, faecal Coliforms and total Coliforms show that the waters of the studied wells are very rich in pathogenic germs. Our results are comparable with those obtained by Kadaoui et al. (2019) concerning the assessment of physico-chemical and bacteriological groundwater quality in irrigated Triffa Plain, North-East of Morocco. This contamination is linked to the existence of septic tanks and the leaching of animal waste.
The Water Quality Index (WQI) values of the groundwater samples calculated in the study area are considered to be of generally good quality. The "poor quality" samples are characterised by high concentrations of sodium, chlorides and nitrates. Based on the distribution of the points, the human activities (domestic discharges and agricultural activities) are the main sources of the deterioration of the quality of the groundwater in these sampling sites.
The concentration of sodium, chlorides and nitrates decreases with the direction of groundwater flow and at the depth of the water table; suggesting dilution of these ions by self-purification. These observations are similar to those of Sadat-Noori et al. (2013) in the study of Groundwater quality assessment using the Water Quality Index and GIS in Saveh-Nobaran aquifer in Iran and Chen et al. (2019) in the study of Hydrogeochemical Characteristics and Quality Assessment of Groundwater in an Irrigated area, Northwest China.
The results of the analytical data plotted on the salinity diagram (Richards 1954) taking into account the conductivity values to the SAR show that the waters are represented mainly in classes C3. S1 and C4. S1. Class C3S1 indicates water with high salinity and low sodium content, which can be used for irrigation of almost all types of soil with low risk of exchangeable sodium (Aghazadeh and Mogaddam 2010), and class C4S1 indicates water with very high salinity and low sodium content; suitable for plants with good salt tolerance but unsuitable for irrigation in soils with restricted drainage (Yang et al. 2020). Overall, these waters are suitable for irrigation in the study area.
The quality of groundwater for irrigation generally depends on the relative values of EC, Na+ and other cations and anions (Todd 1980). The representation of groundwater samples on the Wilcox diagram (1955), based on Na% and electrical conductivity (EC) values, showed that all samples fell into the good and excellent class for agriculture. The studies by Aghazadeh and Mogaddam (2010), and Hassen et al. (2016) also found similar results to those in the Oshnavieh Area of northwest Iran and in the Oum Ali-Thelepte aquifer in central Tunisia.
The permeability index (PI) is used to assess the quality of groundwater for irrigation. The representation of the groundwater samples on the Doneen diagram (1964), based on total ionic concentration (TDS) and permeability index (PI), shows that all groundwater samples fell into class I. Therefore, the groundwater in the study area is good for irrigation. Similar results were found in the Ganges river basin areas of Bangladesh by Islam and Mostafa (2021).
Conclusion
In the present study, the groundwater of the hydrogeological basin of Timahdite–Almis Guigou was analysed to determine their physico-chemical and bacteriological characteristics, with the aim of assessing the quality of groundwater for drinking and for agriculture.
The representation of major ion contents of the Piper diagram shows that the hydrogeochemical facies of the groundwater are the chlorinated, sulphated, calcium and magnesium facies, and the bicarbonate, calcium and magnesium facies.
The result of the saturation index calculation shows that the water samples are saturated with carbonate minerals and undersaturated with evaporite minerals. According to Gibbs diagrams, the samples fall within the weathering domain of rocks and the chemical quality of the water is related to the lithology of the study area.
The high presence of germs is an indicator of faecal contamination which poses a threat to the inhabitants. These results confirm the impact of agricultural intensification and domestic wastewater discharges. The calculated water quality index shows that the samples are generally considered to be of good quality for drinking.
On the basis of the sodium adsorption ratio (SAR), the evaluation of the EC, the percentage of sodium (Na%) and the permeability index (PI), the groundwater samples are suitable for irrigation.
From this study, it can be deduced that the groundwater in the Timahdite–Almis Guigou area can be used for drinking water and for irrigation of agricultural plots.
References
Aghazadeh N, Mogaddam AA (2010) Assessment of groundwater quality and its suitability for dinking and agriculture uses in the Oshnavieh area, northwest of Iran. J Environ Prot 1:30–40. https://doi.org/10.4236/jep.2010.11005
Aït Slimane A (1989) Evolution structuro-sédimentaire paléogène de la partie Sud-Ouest du Moyen Atlas (Baqrit-Timahdite-Guigou, Bou Anguer, Ain Nokra, Oudiksou). Thèse 3° cycle, Fac. Sc. Marrakech, p 179
Akasbi A, Sadki S, Akhessas A, Fedan B (2001) Le ‹‹ niveau brun›› du Toarcien inférieur dans le Moyen Atlas septentrional: caractérisation, processus de mise en place et cadre géodynamique régional. Comun. Inst. Geol. e Mineiro, vol 88, pp 265–276
Amrani S, Hinaje S (2014a) Utilisation des analyses hydro-géochimiques et des analyses en composantes principales (A.C.P) dans l’explication du chimisme des eaux souterraines de la nappe plio-quaternaire entre Timahdite et Almis Guigou (Moyen Atlas, Maroc). ScienceLib Edit Mersenne 6:14
Amrani S, Hinaje S (2014b) Hydrodynamisme et minéralisation des eaux souterraines de la nappe phréatique plio-quaternaire du plateau Timahdite—Almis Guigou (Moyen Atlas, Maroc). Eur Sci J 10:174–189
Amrani S, Hinaje S (2019) Gharmane Y (2019) Application des méthodes paramétriques (drastic et si) pour l’étude de la vulnérabilité à la pollution potentielle par les nitrates de la nappe d’eau superficielle de Timahdite-Almis Guigou (Moyen Atlas, Maroc). Rev Sci Eau 32(3):237–252. https://doi.org/10.7202/1067307ar
Amrani S (2016) Hydrodynamisme, hydrogéochimie et vulnérabilité de la nappe d’eau superficielle et leur relation avec la tectonique cassante dans la zone effondrée Timahdite—Almis Guigou (Moyen Atlas, Maroc). Thèse de Doct. Nat., Fac. Sci. et Tech., Université Fès, p 178
Belkhiri L, Mouni L (2013) Geochemical modeling of groundwater in the El Eulma area, Algeria. Desalin Water Treat 51(7–9):1468–1476. https://doi.org/10.1080/19443994.2012.699350
Belkhiri L, Mouni L, Boudoukha A (2012) Geochemical evolution of groundwater in an alluvial aquifer: case of El Eulma aquifer, East Algeria. Enviro Earth Sci 67:46–55. https://doi.org/10.1016/j.jafrearsci.2012.03.001
Brown RM, McClelland NI, Deininger RA, Tozer RG (1970) A water quality index: do we dare? Water Sew Works 117(10):339–343
Brown RM, McCleiland NJ, Deiniger RA, O’Connor MFA(1972) Water quality index—crossing the physical barrier. In: Jenkis SH (eds) Proceedings in international conference on water pollution research Jerusalem, vol 6, pp 787–797
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7:431–439. https://doi.org/10.1007/s100400050216
Charroud M (1990) Evolution géodynamique de la partie sud-ouest du moyen atlas durant le passage jurassique-crétacé, le crétacé supérieur et le paléogène: un exemple d’évolution intraplaque. Thèse 3° cycle, Rabat p 234
Chatterji C, Raziuddin M (2002) Determination of water quality index of a degraded river in Asanol Industrial area Raniganj, Burdwan, West Bengal. Nat Environ Pollut Technol 1(2):181–189
Chbani B (1984) Sédimentologie du Crétacé et de l’éocène de Timahdit (Boulemane) C.E.A. Sciences, Trv. Dep. Géol. Fac. Sci. Marrakkech 3
Chen J, Huang Q, Lin Y, Fang Y, Qian H, Liu R, Ma H (2019) Hydrogeochemical characteristics and quality assessment of groundwater in an irrigated region, Northwest China. Water 11(1):96. https://doi.org/10.3390/w11010096
Choubert G, Salvan H, Termier H, Termier G (1952) Sur l’âge des calcaires de Timahdit (Moyen Atlas). Cent Rech Acad Sci 21:2090–2092
Colo G (1961) Contribution à l’étude du Moyen-Atlas septentrional. Not Mém Serv Géol Maroc Rabat 139:226
Doneen LD (1964) Notes on water quality in agriculture. Water Science and Engineering, University of California. Davis, p 48
Drever JF (1997) The geochemistry of natural waters, 3rd edn. Prentice-Hall Inc., New York p, p 379
Du Dresnay R (1969) Discussions stratigraphiques sur les conditions de gisements de bois fossiles mésozoiques du Maroc, étudiées par Mlles ATTIMIS Y, Cremier F et GAZEAU F. Notes Et Mém Serv Géol Maroc 210:121–178
El Arabi H (1987) Etude stratigraphique et sédimentologique du lias aux confins du causse moyen atlasique et du moyen atlas plissé (Maroc).Thèse 3° cycle, Touleuse, p 192
El Azzouzi M, Maury RC, Bellon H, Youbi N, Cotton J, Kharbouch F (2010) Petrology and K-Ar chronology of the Neogene-Quaternary Middle Atlas basaltic province, Morocco. Bull Soc Géol Fr 181(3):243–257
Fedan B, Laville E, El Mezgueldi A (1989) Le bassin jurassique du Moyen Atlas (Maroc): exemple de bassin sur relais de décrochements. Bull Soc Géol Fr 8(6):1123–1136
Fedan B (1988) Evolution géodynamique d’un bassin intraplaque sur décrouchements : (Moyen-Atlas, Maroc) durant le Méso-Cénozoïque. Thése Doctorat d’Etat univ. Mohammed V, Rabat p 338, 124 fig. Annexe hors-texte
Garrels R, Mackenzie F (1967) Origin of the chemical compositions of some springs and lakes. In: Ground RF (ed) Equilibrium concepts in natural water systems. American Chemical Society Publications, Washington
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090
Hamzaoui-Azaza F, Ketata M, Bouhlila R, Gueddari M (2011) Hydrochemical evolution and evolution of drinking water quality in Zeuss-Koutine aquifer, south-eastern of Tunisia. Environ Monit Assess 174:283–298
Harmand C, Cantagrel JM (1984) Le volcanisme alcalin tertiaire et quaternaire du moyen atlas (Maroc): chronologie K/Ar et cadre géodynamique. J Afric Earth Sc 2(1):595–603
Harmand C, Moukadiri A (1986) Synchronisme entre tectonique compressive et volcanisme alcalin: exemple de la province quaternaire du Moyen-Atlas (Maroc). Bull Soc Géol 4:595–603
Hassen I, Hamzaoui-Azaza F, Bouhlila R (2016) Application of multivariate statistical analysis and hydrochemical and isotopic investigations for evaluation of groundwater quality and its suitability for drinking and agriculture purposes: case of Oum Ali-Thelepte aquifer, central Tunisia. Environ Monit Assess 188:20. https://doi.org/10.1007/s10661-016-5124-7
Hinaje S, Aït Brahim L, Gourari L, Charroud M (2001) Evénements tectoniques et paléocontraintes enregistrées par les dépôts néogènes et quaternaires du Moyen Atlas (Maroc). Comm. Inst. Geol. eMineiro, t.88, pp 255–264.
Hinaje S (2004) Tectonique cassante et paléochamps de contraintes dans le moyen atlas et le haut atlas central (Midelt-Errachidia) depuis le trias jusqu'à l’actuel. Thèse Doc. Etat, Univ. Rabat, p 363
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–306
Hosseini-Moghari SM, Ebrahimi K, Azarnivand A (2015) Groundwater quality assessment with respect to fuzzy water quality index (FWQI): an application of expert systems in environmental monitoring. Environ Earth Sci 74(10):7229–7238. https://doi.org/10.1007/s12665-015-4703-1
Islam MS, Mostafa MG (2021) Hydro-geochemical evaluation of groundwater for irrigation in the Ganges river basin areas of Bangladesh. Res Sq. https://doi.org/10.21203/rs.3.rs-161359/v1
ISO 7899-2 (2000) Water quality—Detection and enumeration of intestinal enterococci—Part 2: membrane filtration method
ISO 9308-1 (2000) Water quality—Detection and enumeration of Escherichia coli and coliform bacteria—Part 1: membrane filtration method
Jalali M (2007) Salinization of groundwater in arid and semi-arid zones: an example from Tajarak, western Iran. Environ Geol 52(6):1133–1149. https://doi.org/10.1007/s00254-006-0551-3
Kadaoui M, Bouali A, Arabi M (2019) Assessment of physicochemical and bacteriological groundwater quality in irrigated Triffa Plain, North-East of Morocco. J Water Land Dev 42:100–109
Li W, Chen X, Xie L, Cheng G, Liu Z, Yi S (2020) Natural and human-induced factors controlling the phreatic groundwater geochemistry of the Longgang River basin, South China. Open Geosci 12(1):203–219. https://doi.org/10.1515/geo-2020-0039
Martin J (1981) Le moyen atlas central étude géomorphologique. Notes Et Mém Serv. Géol Maroc 258:445
Michard A (1976) Eléments de géologie marocaine. Notes Et Mém Serv Géol Maroc 252:408p
Moukadiri A (1983) Les enclaves ultrabasiques associees aux basaltes alcalins dans le district volcaniques d’Azrou-Timahdite (Moyen Atlas, Maroc). Thése 3éme cycle, Clermont-Ferrand, p 150
Ouarhache D (1987) Etude géologique dans le paléozoïque et le Trais de la bordure NW du Causse moyen-atlasique (S et SW de Fès, Maroc). Thése de 3éme cycle. Univ. Paul-Sabatier, Touleuse, p 130
Ouarhache D, Charriere A, Chalot-Pra F, Wartiti M (2012) Triassic to early liassic continental rifting chronology and process at the southwest margin of the Alpine Tethys (Middle Atlas and High Moulouya, Morocco); Correlations with the Atlantic rifting, synchronous and diachronous. Bull Soc Geol Fr 183:233–249. https://doi.org/10.2113/gssgfbull.183.3.233
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (version2). A computer program for speciation, batch-reaction, onedimensional transport, and inverse geochemical calculations. USGS Water-Resources Investigations Report, pp 99–4259
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25:914–923
Qian C, Wu X, Mu WP, Fu RZ, Zhu G, Wang ZR, Wang DD (2016) Hydrogeochemical characterization and suitability assessment of groundwater in an agro pastoral area, Ordos Basin, NW China. Environ Earth Sci 75:16. https://doi.org/10.1007/s12665-016-6123-2
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Agric. Handbook 60, USDA, Washington D.C. 160p.
Rodier J, Legube B, Merlet N (2009) Analyse de l’eau, 9th edn. In: Dunod (eds), Paris, France, p 1579
Sadat-Noori SM, Ebrahimi K, Liaghat AM (2013) Groundwater quality assessment using the Water Quality Index and GIS in Saveh-Nobaran aquifer, Iran. Environ Earth Sci 71(9):3827–3843. https://doi.org/10.1007/s12665-013-2770-8
Salama RB, Otto CJ, Fitzpatrick RW (1999) Contributions of groundwater conditions to soil and water salinization. Hydrogeol J 7(1):46–64. https://doi.org/10.1007/s100400050179
Salvan H (1984) Les formations évaporitiques du Trias marocain. Problèmes stratigraphiques, paléogéographiques et paléoclimatologiques. Quelques réflexions. Rev Géol Dyn Geogr Phys 25:187–203
Sheikhy Narany T, Firuz Ramli M, Zaharin Aris A, Azmin Sulaiman WN, Juahirand Kazem Fakharian H (2014) Identification of the hydrogeochemical processes in groundwater using classic integrated geochemical methods and geostatistical techniques, in Amol-Babol Plain, Iran. Sci World J 2014:15. https://doi.org/10.1155/2014/419058
Simler R (2009) DIAGRAMMES: logiciel d’hydrochimie multilangage en distribution libre. Laboratoire d’Hydrogéologie d’Avignon, France
Subramani T, Rajmohan N, Elango L (2010) Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environ Monit Assess 162(1–4):123–137
Termier H (1936) Etude géologique sur le Maroc central et la Moyen Atlas septentrional. Notes Et Mém. Serv Géol Maroc 33:1566
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, New York, p 315
WHO (2011) Hardness in drinking-water background document for development of who guidelines for drinking—water quality. World Health Organization: Geneva, Switzerland, p 19
Wilcox LV (1955) Classification and use of irrigation water, USDA, circular. Washington, DC, USA, p 969
Yang Q, Li Z, Xie C, Liang J, Ma H (2020) Risk assessment of groundwater hydrochemistry for irrigation suitability in Ordos Basin, China. Nat Hazards 101:309–325. https://doi.org/10.1007/s11069-018-3451-4
Zakaria N, Anornu G, Adomako D, Owusu-Nimo F, Gibrilla A (2020) Evolution of groundwater hydrogeochemistry and assessment of groundwater quality in the Anayari catchment. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2020.100489
Acknowledgements
I would like to express my gratitude, my sincere gratitude and my thanks to my colleagues and to all members of the Laboratory of Intelligent Systems, Georesources and Renewable Energies.
Funding
This research did not receive any specific funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amrani, S., Hinaje, S., El Fartati, M. et al. Assessment of groundwater quality for drinking and irrigation in the Timahdite–Almis Guigou area (Middle Atlas, Morocco). Appl Water Sci 12, 82 (2022). https://doi.org/10.1007/s13201-022-01609-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01609-3