Abstract
Purpose
Serum thyroglobulin (Tg) level is frequently elevated shortly after radioactive iodine (RAI) ablation therapy. The authors studied the relationship between the elevation of serum Tg after RAI therapy and iodine uptake pattern on post-ablation whole body scans (RxWBSs) in patients with papillary thyroid carcinoma (PTC).
Materials and Methods
The study subjects were patients with PTC that had undergone first RAI therapy with thyroid hormone withdrawal after total thyroidectomy. Patients with a high level of serum anti-Tg antibody (TgAb, ≥ 60 U/mL), possible regional or distant metastasis as determined by pre-ablation or post-ablation studies, and negative iodine uptake of the anterior neck on RxWBS were excluded. Serum Tg was checked twice, that is, 7 days after (post-ablation Tg) and on the day of RAI therapy (pre-ablation Tg). Ratio of pre-ablation Tg to post-ablation Tg (Tg ratio) was used to assess changes in serum Tg levels after RAI therapy. Patients were classified into two groups according to the presence of midline uptake above the thyroidectomy bed on RxWBS (negative (group 1) or positive (group 2) midline uptake). Variables were subjected to analysis to identify differences between the two groups.
Results
Two hundred and fifty patients were enrolled in this study; 101 in group 1 and 149 in group 2. Based on univariate analysis, post-ablation Tg (8.12 ± 11.05 vs. 34.12 ± 54.31; P < 0.001) and Tg ratio (7.81 ± 8.98 vs. 20.01 ± 19.84; P < 0.001) were significantly higher in group 2. On the other hand, gender, tumor (T) stage, lymph node (N) stage, size, multiplicity or bilaterality of primary tumor, dose of 131I, serum TgAb and thyroid-stimulating hormone (TSH) level (before or after RAI therapy) were not significantly different in the two groups. Variables with P values of < 0.25 by univariate analysis were subjected to multivariate analysis, which showed post-ablation Tg (OR 1.060, 95 % CI = 1.028–1.092; P < 0.001) and Tg ratio (OR 1.059, 95 % CI = 1.028–1.092; P = 0.001) were significantly higher in group 2.
Conclusion
Serum Tg level after RAI therapy was significantly higher in patients with midline uptake on RxWBS, compared with patients without midline uptake on RxWBS. Further investigations are needed to reveal the correlation between serum Tg elevation and clinical outcome according to the presence of midline uptake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An elevated serum thyroglobulin (Tg) level before radioactive iodine (RAI) therapy is a well-known prognostic factor for the prediction of early therapeutic failure in differentiated thyroid carcinoma (DTC) [1–6]. However, pre-ablation Tg level does not actually reflect therapeutic responsiveness to RAI.
In previous studies, the effectiveness of RAI therapy on remnant thyroid tissue was evaluated by measuring changes in serum Tg levels in patients with DTC [7–9]. In those studies, serum Tg was measured twice, that is, just before the administration of RAI and at 3 or 5 days after RAI therapy. The results showed that serum Tg was generally elevated after RAI therapy and the amounts of Tg elevation yielded better or equal predictions of ablation failure than pre-ablation Tg levels.
Iodine uptake patterns on post-ablation whole body scans (RxWBS) are considered as an important prognostic factor in DTC, especially with respect to the detection of remaining locoregional metastatic disease or unexpected distant metastases [10–13]. Some previous reports have been issued on the relationship between pre-ablation serum Tg level and RxWBS patterns [10, 11].
However, few studies have been conducted to determine how remnant thyroid tissues, visualized by RxWBS, affect serum Tg changes by RAI therapy. Therefore, we investigated the relationship between iodine uptake patterns and serum Tg changes after RAI therapy.
Materials and Methods
Patients
Initially, we investigated all patients with papillary thyroid carcinoma (PTC) treated with high dose (> 1.11 GBq) 131I at our institution between April 2013 and August 2015. Patients that underwent total thyroidectomy (TT) with dissection of cervical lymph nodes and had received initial RAI therapy were included. Patients that met any of the following criteria were excluded: (a) high probability of the remaining regional or distant metastasis based on pre-ablation or post-ablation studies, (b) serum anti-Tg antibody (TgAb) ≥ 60 IU/mL, (c) negative iodine uptake of the anterior neck on RxWBS, or (d) undetectable serum Tg before and after RAI therapy. Finally, 250 patients were enrolled in this study. This retrospective study has been approved by our institutional review board and the need for written informed consent was waived.
Radioactive Iodine Ablation Therapy and Serum Tg Measurement
For RAI therapy, all patients were prepared by thyroid hormone withdrawal for 4 weeks: levothyroxine was withdrawn for 4 weeks, with supplementary tri-iodothyronine for the first 2 weeks of this preparation period. Patients received a low iodine diet (LID) for 2 weeks prior to RAI therapy in accord with institutional practice. On the first day of admission, serum Tg, TgAb, and thyroid-stimulating hormone (TSH) levels were measured before 131I administration. Serum TSH level was 30 μIU/mL or higher prior to 131I administration in all subjects. Dosages of 131I were determined in accord with institutional guidelines and ranged from 3.70 to 6.66 GBq. RxWBS was performed 7 days after RAI therapy and on the same day, serum Tg, TgAb, and TSH levels were remeasured to assess changes after RAI therapy.
Serum Tg levels were measured using a radioimmunoassay kit (Tg-pluS RIA, BRAHMS GmbH, Hennigsdorf, Germany) with a lower detection limit of 0.15 ng/mL. Serum TgAb levels were also measured using a radioimmunoassay kit (anti-Tgn RIA, BRAHMS GmbH, Hennigsdorf, Germany) with a lower detection limit of 20 U/mL. Serum TSH levels were measured using TSH-CTK-3 immunoradiometric assay (DiaSorin SpA, Saluggia, Italy) with a lower detection limit of 0.07 mIU/L.
Study Design
Patients were allocated to one of two groups according to the presence of midline uptake above the thyroidectomy bed on RxWBS, that is, patients without midline uptake above the thyroidectomy bed were allocated to group 1, and those with midline uptake above the thyroidectomy bed were allocated to group 2 (Fig. 1). Patients with ambiguous RxWBS findings that could not be assigned to a group, such as patients with a prominent star artifact on RxWBS, were excluded. Two experienced nuclear medicine physicians reviewed RxWBS images and classified uptake patterns and excluded inappropriate scans. Clinical and pathological variables, including age, gender, tumor and lymph node stages, 131I dosage, and pre- and post-ablation level of serum Tg, TgAb, and TSH, were compared. In addition, Tg ratios (defined as ratio of pre-ablation Tg to post-ablation Tg) were also compared to assess degrees of serum Tg change. Tumors and lymph node metastases were classified using the classification system of the International Union Against Cancer and the 7th edition of the American Joint Committee on Cancer (AJCC) cancer staging manual.
Classification of the 250 study subjects by uptake pattern on post-ablation whole body scan. a If iodine uptake was observed only in the thyroidectomy bed, patients were assigned to group 1 (negative midline uptake). b On the other hand, if localized iodine uptake was observed in the central area above the thyroidectomy bed (arrowed), patients were assigned to group 2 (positive midline uptake)
Statistical Analysis
Univariate analysis was performed prior to multivariate analysis using chi-square for categorical variables and Student’s t-test for continuous variables, and this was followed by multivariate analysis. Initially multiple linear regression analysis was performed to identify multiple collinearity among clinical variables and this was followed by multiple logistic regression analysis to identify variables that showed significant intergroup differences. Multiple logistic regression analysis included all variables with a P value less than 0.25 by univariate analysis. Results are presented as means ± standard deviations. P value less than 0.05 was considered statistically significant, and the analysis was performed using SPSS version 21.0 for Windows® (IBM Corp., Armonk, NY, USA).
Results
Patient Characteristics
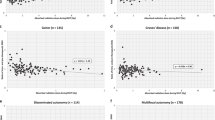
A total of 250 patients were enrolled; patient characteristics are summarized in Table 1. The frequency of the micropapillary carcinoma was more than 50 %. Solitary tumors were more common than multifocal tumors (53.2 vs. 46.8 %), and unilateral tumors were more common than bilateral tumors (67.2 vs. 32.8 %). Fewer patients had extrathyroidal extension (43.2 vs. 56.8 %). Regarding tumor (T) and lymph node (N) staging, T1 and N1a diseases were the most common (55.6 and 74.8 %, respectively). The mean time from surgery to RAI therapy was 101.97 days. Doses of 3.70 GBq 131I were administered to 138 patients, 5.55 GBq to 18 patients, and 6.66 GBq to 94 patients. Patients with midline uptake on RxWBS were more common than patients without midline uptake (59.6 vs. 40.4 %).
Comparison of Pre-ablation Characteristics in the Two Groups
Table 2 shows the results of univariate and multivariate analysis of clinical factors according to RxWBS pattern. One hundred and one patients were included in group 1 (negative midline uptake), and 149 patients in group 2 (positive midline uptake). No significant difference was found between the two groups, with respect to age, gender, T, N staging, tumor multiplicity or bilaterality, tumor size, time from operation to RAI therapy or dose of 131I. Pre-ablation laboratory values on the day of RAI therapy, including Tg, TgAb, and TSH, were not significantly different.
Comparison of Post-ablation Characteristics
In all 250 study subjects, no significant pre- to post-ablation change was observed in serum TgAb (25.86 ± 10.44 U/mL vs. 25.86 ± 11.49 U/mL), while serum TSH decreased from 79.73 ± 29.94 μIU/mL to 38.07 ± 30.92 μIU/mL. And no significant difference was found in the serum level of post-ablation TgAb and TSH between the two groups, in common with the serum level of the pre-ablation TgAb and TSH (Table 2).
Serum Tg increased from 1.86 ± 2.28 ng/mL to 23.62 ± 44.34 ng/mL after RAI therapy, in all 250 study subjects. In group 1, serum Tg level increased about 5-fold after RAI therapy (1.71 ± 2.38 ng/mL to 8.12 ± 11.05 ng/mL), whereas in group 2, it increased by more than 17-fold after RAI therapy (1.98 ± 2.20 ng/mL to 34.12 ± 54.31 ng/mL) (Fig. 2). Although pre-ablation serum Tg level was not significantly different between the two groups, post-ablation serum Tg level was significantly higher in group 2 (8.12 ± 11.05 ng/mL vs. 34.12 ± 54.31 ng/mL, P < 0.001). In addition, Tg ratios were significantly different between the two groups (7.81 ± 8.98 vs. 20.01 ± 19.84 (group 1 vs. group 2, respectively; P < 0.001) (Fig. 3). To exclude the effect of administered RAI on the serum Tg assay, we randomly selected 50 post-ablation serum samples for Tg assay and rechecked Tg values 1 to 2 months after initial assays, and found no significant difference (5.50 ± 13.74 ng/mL vs. 5.74 ± 14.47 ng/mL, P = 0.172 by paired T-test). This result is consistent with that of a recent study [14].
Change of pre- and post-ablation serum thyroglobulin (Tg) level in each group. a In group 1, serum Tg level increased about 5-fold after RAI therapy (1.71 ± 2.38 ng/mL to 8.12 ± 11.05 ng/mL). b On the other hand, it increased by more than 17-fold after RAI therapy in group 2 (1.98 ± 2.20 ng/mL to 34.12 ± 54.31 ng/mL)
No significant collinearity was found among clinical variables by multiple linear regression analysis, even among pre-ablation Tg, post-ablation Tg, and Tg ratio (variance inflation factors were 1.397, 1.849, and 1.588, respectively). Multiple logistic regression analysis was performed using all variables with a P value less than 0.25 by univariate analysis. Post-ablation Tg (OR = 1.060, 95 % CI = 1.028–1.092, P < 0.001) and Tg ratio (OR = 1.059, 95 % CI = 1.023–1.096, P = 0.001) continued to maintain significance and no other variable was found to be significantly different in the two study groups.
Figure 4 shows representative cases. In the patient without midline uptake, no change in serum Tg was observed after RAI therapy (both 0.3 ng/mL). However, in the patient with midline uptake, post-ablation serum Tg (18.1 ng/ml) and Tg ratio (45.25) were considerably higher than those of the patient without midline uptake, although pre-ablation serum Tg level was almost the same (0.4 ng/ml).
Post-ablation whole body scans of one patient from each group. No significant difference was evident with respect to pathologic staging (T1 N1a Mx) or administered 131I dose (3.70 GBq). a In the patient without midline uptake, no significant pre- to post-ablation change in serum thyroglobulin (Tg) level was observed (0.3 ng/mL and Tg ratio 1.00). b However, in the patient with midline uptake, although pre-ablation Tg was almost the same (0.4 ng/mL) as in the patient without midline uptake, post-ablation serum Tg was significantly elevated to 18.1 ng/mL and Tg ratio was 45.25
Discussion
Several studies have addressed alterations in biochemical markers after external irradiation of the thyroid [15–18]. Nishiyama et al. [15] reported that serum Tg and thyroid hormone levels were elevated due to cellular damage after incidental external irradiation to the thyroid. Increased cellular membrane permeability and apoptosis after radiation exposure have been suggested to be the most important mechanisms responsible for those phenomena [16, 17]. In view of those reports, it appears that serum Tg elevation is related to cellular damage after RAI therapy, and that post-ablation serum Tg level reflects the magnitude of the cellular damage caused by RAI therapy. Notably, the stimulating effect of TSH could be ruled out as a cause of serum Tg elevation because post-ablation serum TSH levels were significantly lower than pre-ablation serum TSH levels (79.73 ± 29.94 μIU/ml vs. 38.07 ± 30.92 μIU/ml, P < 0.001).
Bernier et al. [8] investigated the prognostic value of an increase in serum Tg in patients with DTC. Tg ratios were calculated by dividing post-ablation Tg (measured 5 days after RAI therapy) by pre-ablation Tg and compared with pre-ablation serum Tg levels. A higher Tg ratio (>20) was related to ablation success despite a higher pre-ablation Tg level (≥5 ng/ml). Kim et al. [7] also compared pre-ablation stimulated Tg levels with Tg ratios and the result was similar with that of Bernier et al. [8].
Presumably, post-ablation Tg level and Tg ratio were found to be potential prognostic factors because they conceptually reflect therapeutic responsiveness to RAI, whereas pre-ablation Tg does not. However, previous studies failed to demonstrate that Tg ratio had a greater clinical impact than pre-ablation stimulated Tg. There could be several reasons why the prognostic ability of Tg ratio is limited and it is crucial to evaluate which factors affect post-ablation Tg level or Tg ratio, other than cellular damage.
Jung et al. [19] reported that ablation failure was related to a high pre-ablation serum Tg level and positive neck uptake on a Tc-99m pertechnetate salivary scan, which suggests a large amount of remaining thyroid tissue after surgery. Interestingly, all cases showing positive uptake on a salivary scan were localized in the central area above the thyroidectomy bed. Lee et al. [20] concluded midline uptake above the thyroidectomy bed (visualized by RxWBS) should be considered as a thyroglossal duct remnant (TGDR); furthermore, midline uptakes were still observed on follow-up diagnostic iodine scan in 15 % of patients with midline uptake on RxWBS.
Taken together, positive midline uptake on RxWBS could suggest a large amount of remnant thyroid tissues, such as TGDR [20, 21], and post-ablation serum Tg might be affected by midline uptake regarded as TGDR. However, remnant burden alone is not sufficient to explain serum Tg elevation in patients with midline uptake. First of all, there was no significant difference of baseline serum Tg between the two groups. Some studies suggest that pre-ablation Tg is related with remnant burden [22], although serum Tg is not actively released into blood stream in proportion to cellular burden and has limitation in estimation of small remnant burden. In addition, there is no known relationship between iodine uptake and remnant burden, although similar iodine uptake intensity suggested similar iodine accumulation [23]. According to Fig. 4, both of the patients have uptake of 131I on RxWBS with similar intensity but the location of uptake is different: patients on the left side with uptake in the thyroidectomy bed and patients on the right side with uptakes in the thyroidectomy bed and midline above thyroidectomy bed. Although intensity of 131I uptake on RxWBS and baseline serum Tg were similar, patients with midline uptake demonstrated higher Tg ratio. Lastly, our study showed wide ranges of post-ablation serum Tg levels (0.2–378.00 ng/mL) and of Tg ratios (1.00–155.00) even in patients with midline uptake on RxWBS. This wide range of distribution might be a result of individual differences in the amount of remnant thyroid tissue or an unknown characteristic of TGDR. However, at least, it is presumable that serum Tg elevation after RAI therapy might be affected not only by remnant burden but also by other factors such as RAI sensitivities of remnant tissues or TGDR. Accordingly, this study provides new insight for the meaning of Tg elevation after RAI therapy, because serum Tg levels could be elevated differentially according to RxWBS uptake patterns. Further investigations are needed to elucidate other possible mechanisms.
It could be somewhat controversial to regard all midline uptakes in group 2 as TGDR, because pathologic and radiologic investigations were not performed in all patients. Actually, midline uptake pattern is a somewhat unique imaging finding, compared with other remnant uptake in thyroidectomy bed and that might be the reason why previous studies have considered those uptakes as TGDR without pathological diagnosis. On the other hand, prevalence of midline uptake regarded as TGDR in our study is much higher than that in other studies [24–28]. Average prevalence is approximately 7 % [24–26] but reported prevalence has quite a wide range; up to 41.3 % [27]. And TGDR indicates quite a wide range of pathologic entities, from scanty ectopic thyroid tissue along the suspected route of the thyroglossal tract, to ectopic thyroid tissue with complete thyroglossal tract [28]. Iodine scan is usually very sensitive to detect small remnant tissues or TGDR, which could affect the prevalence of midline uptake regarded as TGDR. In addition, cases included in this study may not reflect all of the population because we excluded some cases based on exclusion criteria.
This study has several limitations. First, we could not explore the clinical implications of the relationship between midline uptake and an elevated serum Tg level, due to insufficient follow-up duration. Instead, we tried to focus on the meaning of a serum Tg level change after RAI therapy by considering RxWBS uptake patterns. Second, RxWBS uptake patterns were determined mainly by using planar images because single photon emission computerized tomography/computerized tomography was performed in some of the patients. Moreover, pathologic investigations on the nature of midline uptake were not performed in those patients. Although two experienced nuclear medicine physicians reviewed RxWBS images and classified uptake patterns, further investigations are necessary to characterize midline uptake.
Conclusion
Serum Tg levels after RAI therapy were found to be significantly elevated in patients with midline uptake on RxWBS. Therefore, midline uptake is believed to be a major cause of serum Tg elevation after RAI therapy. Our results may offer a means to further understand how RAI affects remnant thyroid tissue, and suggests serum Tg elevation might be a precise surrogate marker of early therapeutic responsiveness to RAI by considering uptake patterns on RxWBS. Further investigations are needed to elucidate the implications (e.g., ablation success) of the relationship between midline uptake and an elevated serum Tg level.
References
Park HJ, Jeong GC, Kwon SY, et al. Stimulated serum thyroglobulin level at the time of first dose of radioactive iodine therapy is the most predictive factor for therapeutic failure in patients with papillary thyroid carcinoma. Nucl Med Mol Imaging. 2014;48:255–61.
Lee JI, Chung YJ, Cho BY, et al. Postoperative-stimulated serum thyroglobulin measured at the time of 131I ablation is useful for the prediction of disease status in patients with differentiated thyroid carcinoma. Surgery. 2013;153:828–35.
Webb RC, Howard RS, Stojadinovic A, et al. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a meta-analysis involving 3947 patients. J Clin Endocrinol Metab. 2012;97:2754–63.
Kendler DB, Vaisman F, Corbo R, Martins R, Vaisman M. Pre-ablation stimulated thyroglobulin is a good predictor of successful ablation in patients with differentiated thyroid cancer. Clin Nucl Med. 2012;37:545–9.
Vaisman A, Orlov S, Yip J, et al. Application of post-surgical stimulated thyroglobulin for radioiodine remnant ablation selection in low-risk papillary thyroid carcinoma. Head Neck. 2010;32:689–98.
Sawka AM, Orlov S, Gelberg J, et al. Prognostic value of postsurgical stimulated thyroglobulin levels after initial radioactive iodine therapy in well-differentiated thyroid carcinoma. Head Neck. 2008;30:693–700.
Kim YI, Im HJ, Paeng JC, et al. Serum thyroglobulin level after radioiodine therapy (Day 3) to predict successful ablation of thyroid remnant in postoperative thyroid cancer. Ann Nucl Med. 2015;29:184–9.
Bernier MO, Morel O, Rodien P, et al. Prognostic value of an increase in the serum thyroglobulin level at the time of the first ablative radioiodine treatment in patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2005;32:1418–21.
Muratet JP, Giraud P, Daver A, et al. Predicting the efficacy of first iodine-131 treatment in differentiated thyroid carcinoma. J Nucl Med. 1997;38:1362–8.
Kim K, Kim SJ, Kim IJ, et al. Clinical significance of diffuse hepatic visualization and thyroid bed uptake on post-ablative iodine-131 whole body scan in differentiated thyroid cancer. Onkologie. 2012;35:82–6.
Lee HJ, Rha SY, Jo YS, et al. Predictive value of the pre-ablation serum thyroglobulin level after thyroidectomy is combined with post-ablation 131I whole body scintigraphy for successful ablation in patients with differentiated thyroid carcinoma. Am J Clin Oncol. 2007;30:63–8.
Hindie E, Zanotti-Fregonara P, Keller I, et al. Bone metastases of differentiated thyroid cancer: impact of early 131I-based detection on outcome. Endocr Relat Cancer. 2007;14:799–807.
Pelizzo MR, Boschin IM, Toniato A, et al. Papillary thyroid microcarcinoma (PTMC): prognostic factors, management and outcome in 403 patients. Eur J Surg Oncol. 2006;32:1144–8.
Park S, Bang JI, Lee HY, Kim SE. Does (131)I radioactivity interfere with thyroglobulin measurement in patients undergoing radioactive iodine therapy with recombinant human TSH? Nucl Med Mol Imaging. 2015;49:122–6.
Nishiyama K, Kozuka T, Higashihara T, Miyauchi K, Okagawa K. Acute radiation thyroiditis. Int J Radiat Oncol Biol Phys. 1996;36:1221–4.
Cramp WA, Yatvin MB, Harms-Ringdahl M. Recent developments in the radiobiology of cellular membranes. Acta Oncol. 1994;33:945–52.
Ramakrishnan N, McClain DE, Catravas GN. Membranes as sensitive targets in thymocyte apoptosis. Int J Radiat Biol. 1993;63:693–701.
Schlumberger M, Sebagh M, De Vathaire F, et al. Thyroid iodine content and serum thyroglobulin level following external irradiation to the neck for Hodgkin’s disease. J Endocrinol Invest. 1990;13:197–203.
Jung JS, Lee SM, Kim SJ, Choi J, Han SW. Prediction of the success of thyroid remnant ablation using pre-ablative 99mTc pertechnetate scintigraphy and post-ablative dual 131I scintigraphy. Nucl Med Commun. 2015;36:38–44.
Lee M, Lee YK, Jeon TJ, et al. Frequent visualization of thyroglossal duct remnant on post-ablation 131I-SPECT/CT and its clinical implications. Clin Radiol. 2015;70:638–43.
Lee SW, Lee J, Lee HJ, et al. Enhanced scintigraphic visualization of thyroglossal duct remnant during hypothyroidism after total thyroidectomy: prevalence and clinical implication in patients with differentiated thyroid cancer. Thyroid. 2007;17:341–6.
Bachelot A, Cailleux AF, Klain M, et al. Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid. 2002;12:707–11.
Brandt MP, Kloos RT, Shen DH, et al. Micro-single-photon emission computed tomography image acquisition and quantification of sodium-iodide symporter-mediated radionuclide accumulation in mouse thyroid and salivary glands. Thyroid. 2012;22:617–24.
de Jong RJ B, Rongen RJ, Lameris JS, Knegt P, Verwoerd CD. Ultrasound characteristics of thyroglossal duct anomalies. ORL J Otorhinolaryngol Relat Spec. 1993;55:299–302.
Ewing CA, Kornblut A, Greeley C, Manz H. Presentations of thyroglossal duct cysts in adults. Eur Arch Otorhinolaryngol. 1999;256:136–8.
Hilger AW, Thompson SD, Smallman LA, Watkinson JC. Papillary carcinoma arising in a thyroglossal duct cyst: a case report and literature review. J Laryngol Otol. 1995;109:1124–7.
Sprinzl GM, Koebke J, Wimmers-Klick J, Eckel HE, Thumfart WF. Morphology of the human thyroglossal tract: a histologic and macroscopic study in infants and children. Ann Otol Rhinol Laryngol. 2000;109:1135–9.
Kurt A, Ortug C, Aydar Y, Ortug G. An incidence study on thyroglossal duct cysts in adults. Saudi Med J. 2007;28:593–7.
Acknowledgments
This work was supported by a grant from the Chonnam National University Hwasun Hospital Institute for Biomedical Science (Grant No. HCRI15001-1).
The authors thank Subin Jeon for assistance in the calculation and analysis of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Geum-Cheol Jeong, Minchul Song, Hee Jeong Park, Jung-Joon Min, Hee-Seung Bom, Ki Seong Park, Sang-Geon Cho, Sae-Ryung Kang, Jahae Kim, Ho-Chun Song and Seong Young Kwon have no conflict of interest to declare.
Ethics Statement
The protocol of this study was approved by the ethics committee in our hospital, and the study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Ethics committee waived the need to obtain informed consent.
The manuscript has not been published before or is not under consideration for publication anywhere else and has been approved by all co-authors.
Rights and permissions
About this article
Cite this article
Jeong, GC., Song, M., Park, H.J. et al. Iodine Uptake Patterns on Post-ablation Whole Body Scans are Related to Elevated Serum Thyroglobulin Levels After Radioactive Iodine Therapy in Patients with Papillary Thyroid Carcinoma. Nucl Med Mol Imaging 50, 329–336 (2016). https://doi.org/10.1007/s13139-016-0421-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-016-0421-1