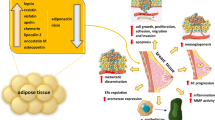
Abstract
Response to chemoradiotherapy (CRT) in patients with locally advanced rectal cancer is highly variable. Identification of CRT non-responders and definite accurate biomarkers of response are unmet needs. In turn, adipokines might impact on colorectal cancer development. We hypothesized that imbalance in leptin and adiponectin modulates stemness potential CRT response in rectal cancer. Pre-CRT serum and tissue samples were collected from a cohort of locally advanced rectal cancer patients (n = 33), submitted to long-course CRT and proctectomy. Adiponectin and leptin were measured by ELISA in serum. In tumour biopsies, mRNA expression of stemness-related genes was evaluated by qRT-PCR and transcription factor STAT3 by immunoblotting. Correlations with clinical data and accuracy of potential CRT response biomarkers were evaluated. Carcinoembryonic antigen (CEA) but not leptin or adiponectin distinguished CRT responders from non-responders (p < 0.05). However, higher leptin and lower adiponectin serum levels were associated with positive extramesorectal nodes and extramural vascular invasion. mRNA expression of stemness factors was inversely correlated with adiponectin but positively correlated with leptin. STAT3 phosphorylation presented similar results. CEA levels together with STAT3 activation and OCT4/KLF4 expression accurately identified rectal cancer patients, CRT non-responders (AUROC 0.80; p < 0.05). Adipokines might impact rectal cancer stemness and patient prognosis. The leptin/STAT3 signalling axis provides the rational for a potential biomarker panel that identifies rectal cancer patients who will not benefit from CRT treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is the third most incident (10.0%) and second most mortal (9.4%) worldwide [55]. Rectal cancer accounts for 35% of all colorectal cancer cases in the European Union [24]. Despite sharing common risk factors and often considered a single entity, colon and rectal cancers present fundamental differences at anatomical and therapeutic levels. While a surgical approach is valid in both cases, neoadjuvant chemoradiotherapy (CRT) is only considered for rectal cancer [58].
Preoperative TNM (T, tumour size; N, lymph node spread; M, metastasis) staging is crucial to identify patients with locally advanced rectal cancer that require neoadjuvant long-course CRT followed by total mesorectal excision [37]. Preoperative CRT offers many advantages, such as tumour downstaging and downsizing, increasing curative resections (R0 resection rates), sphincter-sparing surgery and decreasing local recurrence [34]. Nevertheless, nearly 30% of patients exhibit CRT resistance and are at increased risk of disease progression, unnecessary toxicity and intestinal and genitourinary morbidity [14, 34].
Pathological response to CRT, assessed as tumour regression grade (TRG), can range from complete or partial response to non-response, in patients resistant to CRT [24]. Accurate and early prediction of CRT response would benefit the selection of the best responders for neoadjuvant treatment and conservative non-surgical approaches [23, 26] and the identification of non-responders sparing patients from unbeneficial CRT and favouring surgical resection. The worldwide incidence of rectal cancer is expected to rise from 0.73 million new cases in 2020 to 1.16 million by 2040, representing a 58.5% change [21]. Concurrently, rectal cancer patients that will be subjected to unbeneficial CRT approaches will also increase, since it is lacking an accurate stratification between responders and non-responders based on each individual clinical staging. To date, CRT response cannot be clinically predicted, but molecular features and signatures have been explored, such as miRNA expression, KRAS and TP53 mutations or carcinoembryonic antigen (CEA) levels [4, 8,9,10, 12, 46, 49, 52, 53]. The inclusion of molecular markers in a biomarker algorithm would greatly benefit patient stratification.
In the last decades, obesity has emerged as a global epidemic and is considered a risk factor for both colon and rectal cancers [24, 30]. A growing body of evidence supports the role of obesity in colorectal cancer development, progression, response to therapy and outcome [5, 25, 38]. In fact, 5.2% of all rectal cancers are attributable to obesity alone [22], and the relative risk of rectal cancer development is increased with high body-mass index (BMI), particularly in men [42, 44]. Hence, imbalances in metabolism-related hormones such as adipokines play a role in colorectal cancer development. Two of the most well-studied adipokines in the context of colorectal cancer are adiponectin and leptin, which are produced by adipose tissue and are altered in obesity. Yet, they have antagonistic roles in colonic tumorigenesis. While adiponectin has been suggested to be protective [19], leptin is believed to promote cell growth, motility and invasion [29].
Adipokines may also influence cancer stem cell (CSC) biology and response to therapy. Indeed, 5-fluorouracil therapy is offset by higher leptin levels and accompanied by CSC survival [6]. CSCs display high self-renewal capacities, plasticity, resistance to tumour microenvironment stress factors and quiescence, thus being responsible for cancer relapse, metastization and resistance to radio- and chemotherapy [32, 64]. Moreover, in vitro and in vivo studies have shown that obesity can modulate stem cell responsiveness in carcinogenesis [13]. These facts bring forward a potential role for adipokines, like adiponectin and leptin, to modulate not only cancer development but also prognosis and response to therapy.
Here we hypothesized that adipokine imbalance plays a role in rectal cancer development and stemness potential and, ultimately, modulates response to CRT. We explored the influence of adiponectin and leptin on rectal cancer oncogenic pathways, identifying molecular markers and assessing their potential as prognostic biomarkers of response to CRT.
Materials and methods
Patients and sample collection
Tissue and serum samples were collected from 33 patients diagnosed with rectal cancer (stages I–IV, American Joint Committee on Cancer, AJCC) between 2017 and 2019 in the Surgical Department of Hospital Beatriz Ângelo (Loures, Portugal). Patients underwent preoperative staging with thoraco-abdominal-pelvic-computed tomography and pelvic magnetic resonance (MR) or endoanal ultrasound when pelvic MR was not clinically possible. Eligibility criteria consisted of treatment with long-course CRT and proctectomy. The indications for CRT were patients with locally advanced rectal cancer, as per the ESMO guidelines [24], including stage IV patients if they had oligometastatic disease susceptible of resection in a curative intent setting. Patients were excluded if presenting other histological types of rectal malignancy, if not submitted to CRT or proctectomy, if pregnant or if under 18 years old. Patients were subjected to long-course CRT consisting of a total dose of 50.4 Gy of pelvic irradiation, delivered with capecitabine or 5-fluorouracil, and subsequent proctectomy, 10 to 12 weeks later. Tissue samples were collected from all patients, prior to CRT, during pre-therapeutic colonoscopy, and after CRT, from the proctectomy specimens. Samples were directly frozen with CO2 prior to storage at − 80 °C. Serum samples were also collected at the time of pre-treatment staging colonoscopy. For histopathological analysis, formalin-fixed paraffin-embedded tissue sections were routinely stained with haematoxylin–eosin and post-CRT samples graded by TRG according to the College of American Pathologist guidelines (CAP, TNM 7th edition) by two independent and blinded experienced pathologists. Samples were categorized as TRG0 or complete response (no viable tumour cells), TRG1 or moderate score (single cells or small groups of cancer cells), TRG2 or minimal response (residual cancer outgrown by fibrosis) or TRG3 or poor response (minimal or no tumour killing with extensive residual cancer). Subsequently, two groups of patients were defined: pathological responders (TRG0, 1 and 2; n = 18) and pathological non-responders (TRG3; n = 15). Patients had up to 47 months of follow-up. Sample collection was carried out after written and signed consent. The study protocol was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional Human Research Committee and Ethical Committee from Hospital Beatriz Ângelo.
Serum hormone levels
Pre-CRT serum levels of adiponectin and leptin were measured in single determination using specific enzyme-linked immunosorbent assay (ELISA) kits (Mediagnost GmbH, Reutlingen, Germany) according to the manufacturer’s instructions. To assess how adiponectin and leptin levels associated with future response to CRT and clinical outcomes, their pre-CRT serum levels were correlated with TGR status, positive extramesorectal nodes (EMN) and extramural vascular invasion (EMVI) and mRNA expression of stemness Yamanaka factors evaluated in pre-CRT tumoral biopsy tissue.
Total RNA extraction and quantitative real-time PCR
For gene expression analysis, total RNA was isolated from fresh-frozen pre-CRT rectal tumour biopsies using TRIzol® reagent (Invitrogen, Thermo Fisher Scientific, Paisley, UK) and reverse-transcribed to cDNA using NZY First-Strand cDNA Synthesis Kit (NZYTech, Lisbon, Portugal). Quantitative real-time PCR (qRT-PCR) was performed on 384-well QS7 Flex Real-Time PCR System (Applied BioSystems, MA, USA) using SensiFAST SYBR Hi-ROX Kit (Bioline, Meridian Bioscience, OH, USA). Primer sequences are listed in Table S1. Gene expression was quantified using the relative standard curve method and normalized to β-actin levels. All procedures were performed according to the manufacturer’s instructions. Besides correlation with pre-CRT serum adipokines levels, mRNA expression of Yamanaka factors (cMYC, Kruppel-like factor 4 (KFL4), octamer-binding transcription factor 4 (OCT4) and sex-determining region Y-box 2 (SOX2)) was correlated with TRG status and prognostic features (ENM and EMVI).
Total protein isolation and immunoblotting
Total protein extracts were isolated from pre-CRT rectal tumour biopsies. For this purpose, samples were homogenized in lysis buffer containing 10 mM Tris–HCl (pH 7.6), 2.5 mM MgCl2, 0.75 mM KAc, 0.5% Nonidet P-40 (VWR, Pennsylvania, USA), 1 mM dithiothreitol (DTT) and 1 × Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific) for at least 30 min. Protein lysates were sonicated and then centrifuged at 10,000 × g, for 10 min at 4 °C. Protein concentrations were determined using Bio-Rad Protein Assay reagent (Bio-Rad, CA, USA), according to the manufacturer’s instructions. Total protein extracts were stored at − 80 °C. To evaluate steady-state protein expression, immunoblot analysis was performed. Briefly, 40 µg of total protein was denatured and separated on 8% sodium dodecyl sulphate polyacrylamide electrophoresis gels (SDS-PAGE) and transferred onto nitrocellulose membranes (RTA Transfer Kit, Bio-Rad) using Trans-Blot Turbo Transfer System (Bio-Rad). After blocking with 5% (w/v) no-fat dry milk in PBS-tween-20 (0.5% v/v), blots were incubated overnight at 4 °C with primary mouse antibody reactive to signal transducer and activator of transcription 3 (STAT3) (124H6; 1:1000; #9139 T; Cell Signaling Technology Inc., MA, USA) or primary rabbit antibody reactive to phosphorylated STAT3 (p-STAT3) (Tyr705; 1:2000; #9145 T; Cell Signaling Technology Inc.). Next, membranes were incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (all 1:5000; Bio-Rad) for 2 h at room temperature. Lastly, proteins of interest were detected by chemiluminescence using Immobilon Western Chemiluminescent HRP Substrate (Millipore, MA, USA) or SuperSignalTM West Femto Maximum Sensitivity Substrate (Thermo Fischer Scientific) and acquired with a ChemiDoc XRS-imaging system (Bio-Rad). β-actin (1:40,000; #A5441; Sigma-Aldrich) was used as loading control. The relative density of protein bands was analysed with Image Lab version 5.0 densitometric analysis software (Bio-Rad).
Statistical analysis
All data and statistical analysis were carried out with GraphPad Prism 8.4.2 software (California, USA) and IBM SPSS Statistics version 27 (IBM, NY, USA). Normality of value distribution was determined using the Shapiro–Wilk test. Differences in frequencies between responders and non-responders were evaluated using the Qui-square test. Differences in median values (expressed as minimum–maximum values) between responders and non-responders were evaluated with the unpaired t-test or Mann–Whitney U test, according to the normality of value distribution. Two-tailed Spearman correlations were used to correlate hormone serum levels and mRNA expression. Uni-parameter receiver operating characteristic (ROC) curve analysis was used to assess discriminatory power between TRG0 2 and TRG3. Variables with area under the ROC curve (AUROC) superior to 0.6 were selected for binomial logistic regression analysis, and multi-parameter ROC curves were established for relevant parameter combinations. AUROC value superior to 0.8 was considered very good. Cutoff values were retrieved from the Youden Index. Associated sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were estimated using www.medcalc.org online calculator. A p-value inferior to 0.05 was considered significant. GraphPad Prism was used for unpaired t-test, Mann–Whitney U test, Spearman correlation, AUROC analysis and graph production. SPSS was used for descriptive statistics, Qui-square test and binomial logistic regression.
Results
Patient clinical parameters
Clinical and demographic features of the 33 rectal cancer patients according to their TRG status are summarized in Table 1. Pre-CRT CEA levels were significantly higher in pathological non-responders (TRG3) than in responders (TRG0-2) (p < 0.05).
Opposing role of adiponectin and leptin serum levels in clinical prognosis
Although not significant, pre-CRT adiponectin serum values were inversely associated with BMI (Spearman r = − 0.21, p = 0.24) (Fig. 1A). There were no significant differences in pre-CRT median serum levels of adiponectin between TRG0-2 (8.78 (4.56–13.96) µg/mL) and TRG3 (7.88 (2.26–21.79) µg/mL) patients (p = 0.64; Fig. 1B and Fig. S1). However, there was a non-significant increase in adiponectin median levels in patients with negative EMN (9.13 (2.50–21.79) µg/mL) versus positive EMN (6.81 (2.26–20.34) µg/mL) (p = 0.11; Fig. 1C and Fig. S1) and in patients without EMVI (8.77 (2.50–21.79) µg/mL) versus those with EMVI (7.36 (2.26–15.13) µg/mL) (p = 0.59; Fig. 1D and Fig. S1). Also, higher pre-CRT serum adiponectin levels were associated with lower mRNA expression of stemness Yamanaka factors OCT4 (p < 0.05), KFL4 and cMYC (both p = 0.08) in tumour tissue (Fig. 1E).
Higher levels of pre-CRT serum adiponectin correlate with negative EMN, absence of EMVI and reduced stemness potential in pre-CRT tumour biopsies. A Correlation of adiponectin serum levels with BMI. Adiponectin serum levels in B pathological responders (TRG0-2; n = 18) and pathological non-responders (TRG3; n = 15); rectal cancer patients with and without C positive EMN (no EMN, n = 22; EMN, n = 11) and D EMVI (no EMVI, n = 21; EMVI, n = 12). E Correlation of adiponectin serum levels with mRNA expression of Yamanaka factors in pre-CRT tumour tissue biopsies (cMYC, OCT4 and KLF4, n = 33; SOX2, n = 32). Data are expressed as median (minimum–maximum) values. BMI, body mass index; EMN, extramesorectal nodes; EMVI, extramural vascular invasion; KFL4, Kruppel-like factor 4; OCT4, octamer-binding transcription factor 4; SOX2, sex determining region Y-box 2; TRG, tumour regression rate
Conversely, pre-CRT leptin serum levels were positively correlated with BMI (Spearman r = 0.55, p < 0.001) (Fig. 2A). In addition, although median pre-CRT serum levels of leptin did not significantly change between TRG0-2 (4.37 (0.42–38.76) ng/mL) and TRG3 patients (2.92 (0.75–24.34) ng/mL) (p = 0.99; Fig. 2B and Fig. S1), a non-significant increase in serum leptin levels was observed in patients with positive EMN (11.02 (0.42–38.76) ng/mL) versus negative EMN (2.89 (0.44–24.34) ng/mL) (p = 0.06; Fig. 2C and Fig. S1) and again in patients with EMVI (5.80 (0.75–38.76) ng/mL) versus those without EMVI (2.86 (0.42–24.34) ng/mL) (p = 0.23; Fig. 2D and Fig. S1). Additionally, a significant positive correlation was found between serum leptin and OCT4 mRNA levels in tumour tissue (p < 0.05; Fig. 2E).
Higher levels of pre-CRT serum leptin correlate with positive EMN, EMVI and increased stemness potential in pre-CRT tumour biopsies. A Correlation of leptin serum levels with BMI. Leptin serum levels in B pathological responders (TRG0-2; n = 18) and pathological non-responders (TRG3; n = 15); rectal cancer patients with and without C positive EMN (no EMN, n = 22; EMN, n = 11) and D EMVI (no EMVI, n = 21; EMVI, n = 12). E Correlation of leptin serum levels with mRNA expression of Yamanaka factors in pre-CRT tumour tissue biopsies (all n = 33). Data are expressed as median (minimum–maximum) values. BMI, body mass index; EMN, extramesorectal nodes; EMVI, extramural vascular invasion; KFL4, Kruppel-like factor 4; OCT4, octamer-binding transcription factor 4; SOX2, sex determining region Y-box 2; TRG, tumour regression rate
Increased expression of stemness-related genes and STAT3 activation associate with a poor prognosis
Although not significant, mRNA expression of the Yamanaka factors, cMYC, KLF4, OCT4 and SOX2, were increase in TRG3 patients (Fig. 3A), patients with positive EMN (Fig. 3B) and patients with EMVI (Fig. 3C). STAT3 phosphorylation was slightly increased in TRG3 patients (Fig. 4A), with positive EMN (Fig. 4B) and EMVI (Fig. 4C), although not significant. Additionally, although no correlations were found neither with leptin serum levels nor with patient survival (data not shown), STAT3 activation increased with mRNA expression of Yamanaka factors (Fig. 4D). Thus, STAT3 phosphorylation may contribute to worse prognosis and poor response to CRT.
Increased expression of Yamanaka factors in pre-CRT tumour biopsy tissues associates with poor prognostic factors and lower patient survival. mRNA expression of Yamanaka factors cMYC, KLF4, OCT4 and SOX2 in rectal cancer patients according to A TRG (TRG0-2, n = 15–18; TRG3, n = 12–14); B presence of positive EMN (no EMN, n = 18–21; EMN, n = 10–11) and C presence of EMVI (no EMVI, n = 18–21; EMVI, n = 10–12). mRNA expression data are expressed as mean ± standard error of the mean (SEM) of individual fold change to median. EMN, extramesorectal nodes; EMVI, extramural vascular invasion; KFL4, Kruppel-like factor 4; OCT4, octamer-binding transcription factor 4; SOX2, sex determining region Y-box 2; TRG, tumour regression rate
Increased STAT3 activation in pre-CRT tumour biopsy tissues associates with poor prognostic features and increased mRNA expression of Yamanaka factors. A Immunoblotting and densitometry of p-STAT3 and STAT3, in pre-CRT tumour biopsy tissues according to TRG (TRG0-2, n = 18; TRG3, n = 14). STAT3 activation according to presence of B positive EMN (no EMN, n = 22; EMN, n = 10) and C EMVI (no EMVI, n = 21; EMVI, N = 11). D Correlation between STAT3 activation and mRNA expression of Yamanaka factors in pre-CRT tumour biopsy tissues. Blots of p-STAT3 were normalized to total STAT3. Data are expressed as mean ± SEM. EMN, extramesorectal ganglia; EMVI, extramural vascular invasion; KFL4, Kruppel-like factor 4; OCT4, octamer-binding transcription factor 4; SOX2, sex determining region Y-box 2; STAT3, signal transducer and activator of transcription 3; TRG, tumour regression rate
STAT3 activation, OCT4 and KFL4 in tumour tissue are biomarkers for CRT response
Since STAT3 activation and OCT4/KFL4 mRNA expression protruded in the TRG0-2 versus TRG3 comparison, their performance as biomarkers for the identification of pathological non-responders was evaluated and compared to that of pre-CRT CEA serum levels, the only serological parameter with significant differences between responders and non-responders. ROC curve analysis was carried out (Table 2), and AUROC values were assessed for each parameter individually (Fig. 5). In the identification of pathological non-responders, pre-CRT CEA serum levels presented an AUROC value of 0.78 (p = 0.02), with an overall accuracy of 80% and positive predictive value (PPV) of 70%. STAT3 activation (p = 0.14), OCT4 (p = 0.34) and KLF4 (p = 0.18) expression levels presented average AUROC values between 0.62 and 0.68. Surprisingly, STAT3 activation levels presented specificity and PPV values of 100% in the identification of non-responders to CRT (Table 2). Still, none of these parameters could produce a satisfactory distinction between pathological responders and non-responders. However, when all parameters were combined in a four-biomarker panel, the AUROC value increased to 0.8 (p = 0.01), with an overall accuracy of 80% and very high specificity and PPV values of 93.8% and 83.3%, respectively, for the identification of non-responders (Table 2).
ROC curves for pre-CRT CEA serum levels (A), STAT3 activation (B) and mRNA expression of OCT4 (C) and KLF4 (D) as prognostic biomarkers for the identification of pathological non-responders. p-STAT3/STAT3 was evaluated by immunoblotting and OCT4 and KFL4 mRNA expression evaluated by qRT-PCR in tissues from pre-CRT tumour biopsies. AUROC, area under the ROC curve; CEA, carcinoembryonic antigen; CI, confidence interval; KLF4, Kruppel-like factor 4; OCT4, octamer-binding transcription factor 4; STAT3, signal transducer and activator of transcription 3
Discussion
In this study, we explored the role of adipokines on rectal cancer stemness, impact on clinical outcome and predictive response to CRT. In that regard, we analysed pre-CRT serum and biopsy tissue samples from a small cohort of rectal cancer patients. Correlations between adipokines, mRNA expression of stemness factors, prognosis factors and response to CRT were explored. This patient cohort includes 16 non-obese rectal cancer patients, 13 overweight, three obese and one severely obese, averaging a median BMI of 25. Nevertheless, the opposed roles of adiponectin and leptin were still observed. Indeed, our results showed that lower levels of serum adiponectin tended to associate with both the presence of EMVI, a prognostic factor for distance relapse and the presence of positive EMN, an indicator of advanced disease stage and prognostic factor for metastization. In agreement, both in vivo and in vitro studies have suggested a protective role of adiponectin [17, 19, 31, 39], and several clinical studies suggest that decreased levels of circulating adiponectin may be an increased risk factor for colorectal cancer development [11, 36, 45].
In turn, patients with positive EMN and EMVI presented slightly increased leptin serum levels, which correlated with mRNA expression of Yamanaka factors. In fact, several studies have demonstrated an oncogenic role of leptin, by promoting cancer cell growth, motility and invasion [2, 3, 29]. It has been shown a gradual increase in leptin levels during the normal mucosa–adenoma–adenocarcinoma sequence [48], and both leptin and leptin receptor were increased in colorectal tumour tissues when compared to adjacent normal tissues [1]. Still, our results showed that neither adiponectin nor leptin serum levels associated with CRT response. Nonetheless, the observed association between these adipokines and stemness-associated genes prompted us to explore further links that could influence response to CRT.
Several reports have shown that leptin can activate signalling pathways linked to colorectal CSCs and critical for the maintenance of stemness traits [15, 43, 50, 60]. The involvement of leptin in the JAK/STAT3 axis in colorectal cancer development is of particular interest. Upon leptin binding to its cellular membrane receptor, JAK-2 is phosphorylated and prompts STAT3 phosphorylation and translocation into the nucleus, where it regulates gene expression by binding to promoter regions of target genes [18, 41, 56]. In particular, STAT3 activation has been described to induce the expression of the stemness factors OCT4 and SOX2, which in turn would induce the expression of leptin receptor (Ob-R) [16]. Moreover, increased phosphorylation of Ob-R, JAK-2 and STAT3 was observed in both colorectal adenoma and carcinoma tissues when compared to normal colorectal tissues [57].
Here, we have observed that despite no significant changes in serum leptin levels, STAT3 activation and mRNA expression of OCT4 and KLF4 were slightly increased in non-responders (TRG3) and in patients with EMVI and with positive EMN. Previous reports showed that increased OCT4 expression in rectal cancer tumour tissues was associated with more severe disease stages and with poor disease-free survival [51, 62]. Similarly, a clinical study has demonstrated that KLF4 and B lymphoma Mo-MLV insertion region 1 homolog (BMI1) levels were positively correlated in rectal cancer and associated with poor therapeutical response. Further in vitro studies showed that BMI1 deficiency enhanced radiosensitivity, which could be overcome by KLF4 overexpression [27].
Contrary to our observations, a retrospective study where the prognostic value of phosphorylated STAT3 in rectal cancer patients was evaluated did not find any correlations with clinicopathological features and proposed an improved overall survival for patients with p-STAT3 positive tumours. Nevertheless, albeit having a fairly large patient cohort, this study was based on immunohistochemistry of formalin-fixed paraffin-embedded samples, and their analysis was limited to the 37.5% of p-STAT3-positive samples [40].
Here, we observed that STAT3 activation by immunoblotting was increased in non-responders (TRG3). More recent in vitro and in vivo studies in colorectal cancer models have shown that silencing or de-phosphorylation of STAT3 could suppress cell proliferation and tumour growth [33, 35, 63]. Moreover, it has been demonstrated that STAT3 protein expression positively correlated with CRT resistance in vitro and that STAT3 inhibition in vivo sensitized mice to CRT and delayed tumour regrowth [54]. Hence, STAT3 activation and mRNA expression levels of KLF4 and OCT4 seemed plausible biomarker candidates to stratify CRT response. We found that those markers presented moderated AUROC values between 0.62 and 0.68, and overall accuracies in identifying non-responders ranged between 68 and 80% and would benefit from improved sensitivity.
To date, there are no truly validated biomarkers of response to CRT. CEA, as the most widely used tumour marker in colorectal cancer, might be a potential candidate. Here, pre-CRT serum levels alone allowed the distinction between responders and non-responders with an AUROC of 0.78. However, studies have demonstrated that serum CEA is not sensitive enough to be used alone as a biomarker, and its potential has been explored in association with tumour size ratio or by monitorization of its clearance pattern [7, 20, 28]. Moreover, CEA can be affected by smoking status and is more efficient as an adjunct for the assessment of complete response rather than identifying non-responders [28, 59]. Hence, we combined CEA levels with STAT3 activation and OCT4 and KFL4 expression levels as a multi-parameter biomarker panel. This strategy allowed to increase the AUROC value to 0.8, with excellent specificity and PPV values of 93.8% and 83.3%.
Other approaches in the literature have identified vascular endothelial growth factor (VEGF) expression or miRNA signatures with AUROC values proximal to 0.6, however focusing on the identification of complete responders [8, 9, 47, 61], while a 13-gene signature with 76% accuracy was proposed for the identification of non-responders [10]. Besides focusing more on the identification of complete responders (TRG0) rather than non-responders (TRG3), most of these studies encompass patients with heterogeneous treatment schedules. Importantly, our study only included patients that followed the same therapeutic approach and scheduling and benefited from access to fresh frozen biopsy samples. However, some limitations are still recognized and shared with other studies, such as the small cohort size from a single hospital centre and the non-randomized retrospective study design in need of prospective validation, with the inclusion of obese patients, whose adipokine levels might be dramatically deregulated. Mechanistic studies of the leptin/STAT3 signalling may clarify its influence on response to CRT, and the role of tissue leptin and its receptor Ob-R still deserve further investigation.
Conclusion
We highlight the role of adipokines on rectal cancer patient prognosis. By exploring the leptin/STAT3 axis and its association with stemness potential in rectal cancer patients, we showed that CEA levels together with STAT3 activation and OCT4 and KLF4 mRNA expression levels can be valuable predictors of CRT response and potentially identify non-responders, sparing them from unbeneficial treatment and associated side effects.
Data availability
The data that support the current study are available within the article, supplementary materials and from the corresponding author upon reasonable request.
References
Al-Shibli SM, Harun N, Ashour AE et al (2019) Expression of leptin and leptin receptors in colorectal cancer—an immunohistochemical study. PeerJ 7:e7624. https://doi.org/10.7717/peerj.7624
Amemori S, Ootani A, Aoki S, et al (2007) Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am J Physiol Hear Circ Physiol 292. https://doi.org/10.1152/ajpgi.00145.2006
Aparicio T, Kotelevets L, Tsocas A et al (2005) Leptin stimulates the proliferation of human colon cancer cells in vitro but does not promote the growth of colon cancer xenografts in nude mice or intestinal tumorigenesis in ApcMin/+ mice. Gut 54:1136–1145. https://doi.org/10.1136/gut.2004.060533
Azizian A, Gruber J, Ghadimi BM, Gaedcke J (2016) MicroRNA in rectal cancer. World J Gastrointest Oncol 8:416–426. https://doi.org/10.4251/wjgo.v8.i5.416
Bardou M, Barkun AN, Martel M (2013) Obesity and colorectal cancer Gut 62:933–947. https://doi.org/10.1136/gutjnl-2013-304701
Bartucci M, Svensson S, Ricci-Vitiani L et al (2010) Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocr Relat Cancer 17:823–833. https://doi.org/10.1677/ERC-10-0083
Cai D, Huang Z, Yu H et al (2019) Prognostic value of preoperative carcinoembryonic antigen/tumor size in rectal cancer. World J Gastroenterol 25:4945–4958. https://doi.org/10.3748/wjg.v25.i33.4945
Campayo M, Navarro A, JC B et al (2018) Predictive response signature for preoperative chemoradiotherapy in rectal cancer. Inl J Colorectal Dis 13:e0206542. https://doi.org/10.1371/journal.Pone.0206542
Caramés C, Cristóbal I, Moreno V et al (2015) MicroRNA-21 predicts response to preoperative chemoradiotherapy in locally advanced rectal cancer. Int J Colorectal Dis 30:899–906. https://doi.org/10.1007/s00384-015-2231-9
Casado E, García VM, Sánchez JJ et al (2011) A combined strategy of SAGE and quantitative PCR provides a 13-gene signature that predicts preoperative chemoradiotherapy response and outcome in rectal cancer. Clin Cancer Res 17:4145–4154. https://doi.org/10.1158/1078-0432.CCR-10-2257
Chong DQ, Mehta RS, Song M et al (2015) Prediagnostic plasma adiponectin and survival among patients with colorectal cancer. Cancer Prev Res 8:1138–1145. https://doi.org/10.1158/1940-6207.CAPR-15-0175
Chow OS, Kuk D, Keskin M et al (2016) KRAS and combined KRAS/TP53 mutations in locally advanced rectal cancer are independently associated with decreased response to neoadjuvant therapy. Ann Surg Oncol 23:2548–2555. https://doi.org/10.1245/s10434-016-5205-4
DeClercq V, McMurray DN, Chapkin RS (2015) Obesity promotes colonic stem cell expansion during cancer initiation. Cancer Lett 369:336–343. https://doi.org/10.1016/j.canlet.2015.10.001
Dossa F, Chesney TR, Acuna SA, Baxter NN (2017) A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2:501–513. https://doi.org/10.1016/S2468-1253(17)30074-2
Endo H, Hosono K, Uchiyama T et al (2011) Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut 60:1363–1371. https://doi.org/10.1136/gut.2010.235754
Feldman DE, Chen C, Punj V et al (2012) Pluripotency factor-mediated expression of the leptin receptor (OB-R) links obesity to oncogenesis through tumor-initiating stem cells. Proc Natl Acad Sci U S A 109:829–834. https://doi.org/10.1073/pnas.1114438109
Fenton JI, Birmingham JM (2010) Adipokine regulation of colon cancer: adiponectin attenuates interleukin-6-induced colon carcinoma cell proliferation via STAT-3. Mol Carcinog 49:700–709. https://doi.org/10.1002/mc.20644
Frühbeck G (2006) Intracellular signalling pathways activated by leptin. Biochem J 393:7–20. https://doi.org/10.1042/BJ20051578
Fujisawa T, Endo H, Tomimoto A et al (2008) Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut 57:1531–1538. https://doi.org/10.1136/gut.2008.159293
Gago T, Caldeira P, Cunha AC et al (2021) Can we optimize CEA as a response marker in rectal cancer? Rev Esp Enfermedades Dig 113:423–428. https://doi.org/10.17235/reed.2020.7321/2020
Global Cancer Observatory: Cancer Tomorrow (2022) Lyon, Fr. Int. Agency Res. Cancer. Available from https://gco.iarc.fr/tomorrow/. Accessed Jan 2022
Global Cancer Observatory: Causes (2022) Lyon, Fr. Int. Agency Res. Cancer. Available from https://gco.iarc.fr/causes/obesity/. Accessed Jan 2022
Glynne-Jones R, Hughes R (2012) Critical appraisal of the “wait and see” approach in rectal cancer for clinical complete responders after chemoradiation. Br J Surg 99:897–909. https://doi.org/10.1002/bjs.8732
Glynne-Jones R, Wyrwicz L, Tiret E et al (2017) Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv22–iv40. https://doi.org/10.1093/annonc/mdx224
Gribovskaja-Rupp I, Kosinski L, Ludwig KA (2011) Obesity and colorectal cancer. Clin Colon Rectal Surg 24:229–243. https://doi.org/10.1055/s-0031-1295686
Habr-Gama A, São Julião GP, Vailati BB et al (2017) Management of the complete clinical response. Clin Colon Rectal Surg 30:387–394. https://doi.org/10.1055/s-0037-1606116
Hsu YC, Luo CW, Huang WL et al (2020) BMI1-KLF4 axis deficiency improves responses to neoadjuvant concurrent chemoradiotherapy in patients with rectal cancer. Radiother Oncol 149:249–258. https://doi.org/10.1016/j.radonc.2020.06.023
Hu H, Zhang J, Cai Y et al (2018) CEA clearance pattern as a predictor for pathologic complete response after neoadjuvant chemoradiation for rectal cancer: results of the FOWARC trial. J Clin Oncol 18:1145. https://doi.org/10.1186/s12885-018-4997-y
Jaffe T, Schwartz B (2008) Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. Int J Cancer 123:2543–2556. https://doi.org/10.1002/ijc.23821
Jochem C, Leitzmann M (2016) Obesity and colorectal cancer. Recent results Cancer Res 208:17–41. https://doi.org/10.1007/978-3-319-42542-9_2
Kim AY, Lee YS, Kim KH et al (2010) Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol Endocrinol 24:1441–1452. https://doi.org/10.1210/me.2009-0498
Kozovska Z, Gabrisova V, Kucerova L (2014) Colon cancer: cancer stem cells markers, drug resistance and treatment. Biomed Pharmacother 68:911–916. https://doi.org/10.1016/j.biopha.2014.10.019
Li J, Liu YY, Yang XF et al (2018) Effects and mechanism of STAT3 silencing on the growth and apoptosis of colorectal cancer cells. Oncol Lett 16:5575–5582. https://doi.org/10.3892/ol.2018.9368
Li Y, Wang J, Ma X et al (2016) A review of neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Int J Biol Sci 12:1022–1031. https://doi.org/10.7150/ijbs.15438
Lin L, Liu A, Peng Z et al (2011) STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res 71:7226–7237. https://doi.org/10.1158/0008-5472.CAN-10-4660
Lu W, Huang Z, Li N, Liu H (2018) Low circulating total adiponectin, especially its non-high-molecular weight fraction, represents a promising risk factor for colorectal cancer: a meta-analysis. Onco Targets Ther 11:2519–2531. https://doi.org/10.2147/OTT.S157255
McCourt M, Armitage J, Monson JRT (2009) Rectal cancer. Surgeon 7:162–169. https://doi.org/10.1016/S1479-666X(09)80040-1
Moghaddam AA, Woodward M, Huxley R (2007) Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 16:2533–2547. https://doi.org/10.1158/1055-9965.EPI-07-0708
Moon HS, Liu X, Nagel JM et al (2013) Salutary effects of adiponectin on colon cancer: in vivo and in vitro studies in mice. Gut 62:561–570. https://doi.org/10.1136/gutjnl-2012-302092
Monnien F, Zaki H, Borg C et al (2010) Prognostic value of phosphorylated STAT3 in advanced rectal cancer: a study from 104 French patients included in the EORTC 22921 trial. J Clin Pathol 63:873–878. https://doi.org/10.1136/jcp.2010.076414
Mullen M, Gonzalez-Perez RR (2016) Leptin-induced JAK/STAT signaling and cancer growth. Vaccines 4. https://doi.org/10.3390/vaccines4030026
Murphy N, Jenab M, Gunter MJ (2018) Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat Rev Gastroenterol Hepatol 15:659–670. https://doi.org/10.1038/s41575-018-0038-1
Ogunwobi OO, Beales ILP (2007) The anti-apoptotic and growth stimulatory actions of leptin in human colon cancer cells involves activation of JNK mitogen activated protein kinase, JAK2 and PI3 kinase/Akt. Int J Colorectal Dis 22:401–409. https://doi.org/10.1007/s00384-006-0181-y
O’Sullivan J, Lysaght J, Donohoe CL, Reynolds JV (2018) Obesity and gastrointestinal cancer: the interrelationship of adipose and tumour microenvironments. Nat Rev Gastroenterol Hepatol 15:699–714. https://doi.org/10.1038/s41575-018-0069-7
Otake S, Takeda H, Fujishima S et al (2010) Decreased levels of plasma adiponectin associated with increased risk of colorectal cancer. World J Gastroenterol 16:1252–1257. https://doi.org/10.3748/wjg.v16.i10.1252
Ourô S, Mourato C, Ferreira MP et al (2020) Evaluation of tissue and circulating mir-21 as potential biomarker of response to chemoradiotherapy in rectal cancer. Pharmaceuticals 13:1–14. https://doi.org/10.3390/ph13090246
Ourô S, Mourato C, Velho S et al (2020) Potential of miR-21 to predict incomplete response to chemoradiotherapy in rectal adenocarcinoma. Front Oncol 10:577653. https://doi.org/10.3389/fonc.2020.577653
Paik SS, Jang SM, Jang KS et al (2009) Leptin expression correlates with favorable clinicopathologic phenotype and better prognosis in colorectal adenocarcinoma. Ann Surg Oncol 16:297–303. https://doi.org/10.1245/s10434-008-0221-7
Probst CP, Becerra AZ, Aquina CT et al (2016) Watch and wait?—Elevated pretreatment CEA is associated with decreased pathological complete response in rectal cancer. J Gastrointest Surg 20:43–52. https://doi.org/10.1007/s11605-015-2987-9
Rouet-Benzineb P, Aparicio T, Guilmeau S et al (2004) Leptin counteracts sodium butyrate-induced apoptosis in human colon cancer HT-29 cells via NF-κB signaling. J Biol Chem 279:16495–16502. https://doi.org/10.1074/jbc.M312999200
Saigusa S, Tanaka K, Toiyama Y et al (2009) Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol 16:3488–3498. https://doi.org/10.1245/s10434-009-0617-z
Smith FM, Reynolds JV, Miller N et al (2006) Pathological and molecular predictors of the response of rectal cancer to neoadjuvant radiochemotherapy. Eur J Surg Oncol 32:55–64. https://doi.org/10.1016/j.ejso.2005.09.010
Smolskas E, Mikulskytė G, Sileika E et al (2022) Tissue-based markers as a tool to assess response to neoadjuvant radiotherapy in rectal cancer—systematic review. Int J Mol Sci 23:6040. https://doi.org/10.3390/ijms23116040
Spitzner M, Roesler B, Bielfeld C et al (2014) STAT3 inhibition sensitizes colorectal cancer to chemoradiotherapy in vitro and in vivo. Int J Cancer 134:997–1007. https://doi.org/10.1002/ijc.28429
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Tarasiuk A, Mosińska P, Fichna J (2018) The mechanisms linking obesity to colon cancer: an overview. Obes Res Clin Pract 12:251–259. https://doi.org/10.1016/j.orcp.2018.01.005
Uchiyama T, Takahashi H, Endo H et al (2011) Role of the long form leptin receptor and of the STAT3 signaling pathway in colorectal cancer progression. Int J Oncol 39:935–940. https://doi.org/10.3892/ijo.2011.1105
Vecchione L, Stintzing S, Pentheroudakis G et al (2020) ESMO management and treatment adapted recommendations in the COVID-19 era: colorectal cancer. ESMO Open 5:e000826. https://doi.org/10.1136/esmoopen-2020-000826
Wallin U, Rothenberger D, Lowry A et al (2013) CEA – a predictor for pathologic complete response after neoadjuvant therapy for rectal cancer. Dis Colon Rectum 56:859–868. https://doi.org/10.1097/DCR.0b013e31828e5a72
Wang D, Chen J, Chen H et al (2012) Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J Biosci 37:91–101. https://doi.org/10.1007/s12038-011-9172-4
Yu J, Lee SH, Jeung TS, Chang HK (2019) Expression of vascular endothelial growth factor as a predictor of complete response for preoperative chemoradiotherapy in rectal cancer. Medicine (Baltimore) 98:e16190. https://doi.org/10.1097/MD.0000000000016190
You L, Guo X, Huang Y (2018) Correlation of cancer stem-cell markers OCT4, SOX2, and NANOG with clinicopathological features and prognosis in operative patients with rectal cancer. Yonsei Med J 59:35–42. https://doi.org/10.3349/ymj.2018.59.1.35
Zhang BD, Li YR, Ding LD et al (2019) Loss of PTPN4 activates STAT3 to promote the tumor growth in rectal cancer. Cancer Sci 110:2258–2272. https://doi.org/10.1111/cas.14031
Zhang G, Li C, Liu Z et al (2016) Cancer stem cell targets – a review. Eur Rev Med Pharmacol Sci 20:2045–51
Acknowledgements
The authors would like to acknowledge the Mediagnost GmbH (Reutlingen, Germany) for providing easy access to the ELISA kits used in this work.
Funding
This work was supported by the Fundação para a Ciência e Tecnologia (PTDC/MED-FAR/3492/2021 and PD/BD/135467/2017) and by European Horizon 2020 (H2020-MSCA-RISE-2016–734719).
Author information
Authors and Affiliations
Contributions
VM performed experimental work and statistical analysis and wrote the manuscript. SO collected patient samples, curated clinical data and revised the manuscript. MBA contributed to manuscript writing. CMPR was responsible for funding and revised the manuscript. All authors contributed to the experimental design, revision and approval of the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the institution’s Ethical Committee (Comissão de Ética para a Saúde do Hospital Beatriz Ângelo, Project Identification Number 0240) on 13 March 2017. The study was registered in the Portuguese Data Protection Agency (Comissão Nacional de Protecção de Dados) on 27 January 2017.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• To date there are no validated biomarkers of CRT response in rectal cancer.
• Adipokines modulate cancer stemness impacting on patient prognosis and therapy response.
• Leptin/STAT3 signalling axis plays a role in CRT resistance.
• Combination of STAT3, KLF4, OCT4 and CEA allows accurate identification of CRT non-responders.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marques, V., Ourô, S., Afonso, M.B. et al. Modulation of rectal cancer stemness, patient outcome and therapy response by adipokines. J Physiol Biochem 79, 261–272 (2023). https://doi.org/10.1007/s13105-022-00936-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-022-00936-y