Abstract
Among the dietary amines present in foods and beverages, tyramine has been widely studied since its excessive ingestion can cause catecholamine release and hypertensive crisis. However, tyramine exerts other actions than depleting nerve endings: it activates subtypes of trace amine associated receptors (TAARs) and is oxidized by monoamine oxidases (MAO). Although we have recently described that tyramine is antilipolytic in human adipocytes, no clear evidence has been reported about its effects on glucose transport in the same cell model, while tyramine mimics various insulin-like effects in rodent fat cells, such as activation of glucose transport, lipogenesis, and adipogenesis. Our aim was therefore to characterize the effects of tyramine on glucose transport in human adipocytes. The uptake of the non-metabolizable analogue 2-deoxyglucose (2-DG) was explored in adipocytes from human subcutaneous abdominal adipose tissue obtained from women undergoing reconstructive surgery. Human insulin used as reference agent multiplied by three times the basal 2-DG uptake. Tyramine was ineffective from 0.01 to 10 µM and stimulatory at 100 µM-1 mM, without reaching the maximal effect of insulin. This partial insulin-like effect was not improved by vanadium and was impaired by MAO-A and MAO-B inhibitors. Contrarily to benzylamine, mainly oxidized by semicarbazide-sensitive amine oxidase (SSAO), tyramine activation of glucose transport was not inhibited by semicarbazide. Tyramine effect was not dependent on the Gi-coupled receptor activation but was impaired by antioxidants and reproduced by hydrogen peroxide. In all, the oxidation of high doses of tyramine, already reported to inhibit lipolysis in human fat cells, also partially mimic another effect of insulin in these cells, the glucose uptake activation. Thus, other MAO substrates are potentially able to modulate carbohydrate metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the various biogenic amines found in food or generated by intestinal microbiota during digestion [47], tyramine is associated with a deplorable capacity to generate hypertension [39] since it releases catecholamines from nerve terminals [3, 5]. The tyramine intravenous infusion induces in humans a pressor response, which increases by 45 mm Hg the systolic blood pressure with an administered dose as low as 5 nmol/kg [57]. However, the pressor effect of tyramine is largely reduced when it is administered orally, and more especially when tyramine intake is associated with food ingestion [68]. Nevertheless, the pioneering studies of Mueller and Horwitz have shown that no change in blood glucose and in free fatty acids (FFA) was observed during tyramine intravenous infusion, while equipressor doses of adrenaline and noradrenaline raised both circulating glucose and FFA [57]. Although this suggested since decades that tyramine could exhibit properties other than releasing endogenous catecholamines, only scarce observations of the direct effects of tyramine on targets other than nerve endings have been reported so far, and more particularly with regard to the cell type that focuses the interest of the present study—the adipocyte.
In healthy subjects, a local lipolytic response was observed when tyramine was administered into subcutaneous adipose tissue via a microdialysis probe (at initial concentrations of 3.5 mM) [1]. Since such lipolytic effect was not reproduced when tyramine was administered directly in vitro on human isolated adipocytes, it was concluded that the catecholamine releasing property of tyramine was supporting the observed effect [1]. Similarly, the tyramine-induced catecholamine release has been used in humans to demonstrate the adrenergic inhibition of insulin secretion in the pancreas [22]. Then, tyramine has been evidenced to activate some of the trace amine-associated receptors (TAARs), namely the TAAR1 expressed in many cell types in humans [48] and was not more considered as only a catecholamine-releasing agent.
More recently, we have reported that, in the 0.1–1 mM range, tyramine acutely inhibits lipolytic activation of human adipocytes either by β-adrenergic agonists, isobutylmethylxanthine (IBMX), or atrial natriuretic peptide [12]. This lipolysis inhibition was not mediated by Gi-coupled receptor activation but was dependent on tyramine oxidation by fat cells and the subsequent generation of hydrogen peroxide, a reactive oxygen species (ROS) known for its antilipolytic effects [43, 58]. Hydrogen peroxide is, together with ammonia and aldehydes, an end-product of the oxidation of endogenous or exogenous amines by amine oxidases. The amine oxidases expressed in adipocytes belong to two distinct families [7]. The first one has FAD as cofactor and is mainly represented by the monoamine oxidases A and B (MAO-A, MAO-B), having catecholamines, serotonin, and tyramine among their numerous substrates [72]. The other family encompasses amine oxidases requiring copper as coenzyme and having an almost larger panel of substrates, including aliphatic amines and non-aromatic primary amines [45]. This latter family known as copper-containing amine oxidases (AOCs) has its major member either named primary amine oxidase, vascular adhesion protein-1, or semicarbazide-sensitive amine oxidase (AOC3/PrAO/VAP-1/SSAO). This member, hereafter called SSAO, is one of the most abundant membrane proteins in differentiated adipocytes [52, 56, 65]. Tyramine is a MAO and SSAO substrate in animal models, while it is not considered as a good substrate for human AOC3 [31].
Tyramine belongs to the MAO and SSAO substrates reported to mimic several in vitro insulin actions in rodent adipocytes, such as inhibition of lipolysis and stimulation of glucose uptake [55, 71]. Tyramine has also been reported to facilitate adipocyte differentiation of murine preadipocytes [11, 20] and to exhibit antihyperglycemic properties in diabetic rodents, either when tested as a pharmacologic agent [69, 70] or as a phytochemical agent extracted from medicinal plants [2, 42]. However, while the observation of an insulin-like effect of tyramine on glucose uptake has been reported for the first time in rat adipocytes more than two decades ago [49], this effect has never been documented in human fat cells, at least to our knowledge. The aim of the present study was therefore to characterize the direct effects of tyramine on hexose transport in human adipocytes. This aim might appear of limited interest when considering that other MAO and/or SSAO substrates such as benzylamine and methylamine have already been reported to activate hexose uptake and inhibit lipolysis in both rodent and human fat cells [14, 30, 54, 67]. However, such extrapolation remains necessary since numerous interspecies differences exist between the responses of human adipocytes and those of rodent ones [38]. Moreover, interspecific differences also exist regarding the substrate selectivity of MAOs and AOCs [35, 46]. Other arguments argued for a detailed verification of direct effects of tyramine on glucose consumption in human fat cells. First, the stimulation of glucose uptake by benzylamine or methylamine is substantially potentiated by vanadium in rodent adipocytes [49, 74] but not in human ones [54]. Second, the usefulness of tyramine-free diets in clinical nutrition established to avoid the sadly known “cheese effect” in patients treated by MAO inhibitors [18] has been repeatedly questioned [23, 60, 64]. Last, the overconsumption of dietary tyramine in mice has not revealed dramatically noxious effects on the cardiovascular system, while it tended to facilitate glucose handling [15].
Thus, the following results explore the direct influence of tyramine on glucose transport into human adipocytes. They will show a modest but indisputable activation of hexose uptake in response to high doses of tyramine, which is predominantly dependent on its degradation by MAOs while mostly independent from Gi-coupled TAARs.
Materials and methods
Adipose tissue sampling from human subjects
For studies on glucose transport in freshly isolated adipocytes, subcutaneous abdominal adipose tissue samples were obtained from a total of 59 women undergoing plastic surgery at the Rangueil hospital, Toulouse (France). Their mean age was 43 (range: 25–70 year) and their mean body mass index (BMI) was 25.9 ± 0.4 kg/m2. The study was validated by the local ethics committee for the protection of individuals (Comité de Protection des Personnes Sud Ouest et Outre Mer II) under the reference: DC-2014–2039. After the surgical removal, pieces of subcutaneous abdominal human adipose depot were transported to the laboratory in less than half an hour. As the pharmacological analysis of the mechanisms of action of tyramine progressed, successive series of experiments were formed on subgroups ranging in size from 5 to 20 individuals, as noted elsewhere.
Animals
Normoglycemic Wistar rats of both sexes (a total of 23 animals of 200–250 g body weight) were purchased from Charles River (L’Arbresle, France) for an interspecies comparative approach. Rat adipose depots from subcutaneous and visceral locations were removed and pooled as described previously [49].
Adipocyte preparations and lipogenic activities
Pieces of adipose tissue (AT) were minced with scissors and digested by collagenase for adipocyte preparation as previously described [40]. The medium used for the collagenase digestion at 37 °C under agitation was Krebs–Ringer containing 15 mM sodium bicarbonate, 10 mM HEPES, 3.5% bovine serum albumin (pH 7.4) and was lacking of glucose as described in [24]. After digestion, the buoyant adipocytes were separated from the stromavascular fraction by filtration through a 250-µm nylon mesh-screen and two washes in the same buffer without collagenase. For both species, fat cell suspensions were prepared, diluted and handled at 37 °C within 3 h from the AT removal. Tetrazolium-based cell viability tests with tyramine were already performed on human mature adipocytes in a concentration range of 10−5–10−3 M [12], as it was the case for insulin and other pharmacologic agents studied here, and did not reveal immediate toxic effect [40].
The incorporation of D-[3H]-glucose into cell lipids was measured on 90 min according to [27], with a simple assay method based on the extraction and counting of the neosynthesized lipids by a liquid scintillation cocktail that is not miscible with aqueous solutions, as originally described by Moody et al. [53].
Assay of 2-deoxyglucose uptake in adipocytes
The only source of glucose for the fat cell preparations during glucose transport assays was the non-metabolizable analogue [3H]-2-DG at a final concentration of 0.1 mM (approximately 1,300,000 dpm/vial), as described previously [26, 54]. However, 2 mmol/L pyruvate was present in the medium throughout the experiments for energy supply (during the 45-min incubation with studied agents, and the subsequent 10-min 2-DG uptake assay), as previously stated [26, 54]. Human fat cells were incubated in 400 µL of medium, then [3H]-2-DG was added as 100 µL portions and hexose uptake assays were stopped 10 min later with 100 µL of 100 µM cytochalasin B. Then, 200 µL of cell suspension were immediately transferred to plastic centrifugation microtubes prefilled with dinonyl-phthalate (density 0.98 g/mL) before a 40 s spin, to separate the undamaged buoying adipocytes from the medium. The upper part of the tubes, containing [3H]-2-DG internalized in fat cells above the silicon layer, was then counted in scintillation vials. No radioactivity was detected in the silicone layer, in agreement with the hydrophilic nature of [3H]-2-DG. The radiolabeled hexose separated in the upper part but not really internalized in the cells was determined with adipocytes immediately blocked by cytochalasin B at time 0 upon [3H]-2-DG addition. This non-specific portion was subtracted from all assays, and averaged only 1–5% of the radioactivity found in the upper phase, as reported in [54]. For each individual, the tested suspension of undamaged cells contained approximately 20 mg lipids/400 µL, determined as previously described [49].
Amine oxidase activity
Oxidase activity was measured by two methods, as already described [41]: by a radiochemical assay using [14C]-tyramine (from Sigma-Aldrich, 500,000 dpm/point) and extraction/counting of the radioactive aldehydic products of oxidation in toluene/ethyle acetate and by a fluorometric assay using Amplex Red (from Fluoroprobes) as a fluorescent probe for a peroxidase-based detection of the released hydrogen peroxide. In both cases, thawed adipose tissue samples were homogenized in 200 mM phosphate buffer (pH 7.4) containing antiprotease cocktail just prior the determination of their amine oxidase activity on 30 min, as previously reported [13].
Chemicals
p-Tyramine hydrochloride, benzylamine hydrochloride, sodium orthovanadate, clorgyline, selegiline, pargyline, semicarbazide, human insulin, collagenase, as well as most of the reagents were from Sigma-Aldrich-Merck. Ro 41–1049 (N-(2-Aminoethyl)-5-(3-fluorophenyl)-4-thiazolecarboxamide) and Ro 16–6491 (N-(2-Aminoethyl)-4-chlorobenzamide) were from Roche (Neuilly/Seine, France). BTT 2052 was a generous gift from D. Smith and M. Salmi (Biotie Therapies, Turku, Finland).
Statistical analyses
All statistical analyses were performed using GraphPad Prism 5.0 for Windows (GraphPad Software Inc., San Diego, CA). One-way ANOVA followed by Dunnett post-hoc tests and Student’s t-tests were performed to determine differences between the oxidation treatments. NS means non-significant difference. All values in figures and tables are presented as mean ± SEM.
Results
Stimulation of glucose transport into human adipocytes by insulin and tyramine: influence of vanadate
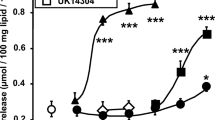
It was first studied whether tyramine exhibits dose-dependent effects on glucose transport in human adipocytes. The stimulatory agent of reference was bovine insulin. Also included was the study of the influence of vanadate since this agent acts synergistically with tyramine on the stimulation of hexose uptake in rat fat cells. As expected, insulin induced a classical sigmoidal dose-dependent stimulation of 2-DG uptake, with a maximum reached at 100 nM. By contrast, tyramine was stimulatory only at 0.1 and 1 mM (Fig. 1). Moreover, the maximal effect of insulin increased more than three times the level of basal transport while the effect of 1 mM tyramine did not exceed doubling. The presence of 10 µM sodium orthovanadate did not enhance the basal, insulin-stimulated, or tyramine-stimulated 2-DG uptake (Fig. 1).
Dose-dependent activation of glucose transport by insulin and tyramine in human adipocytes. The uptake of 2-deoxyglucose (2-DG) was assessed on 10 min after 45-min incubation of human subcutaneous adipocytes without (basal) or with increasing concentrations of insulin (circles) or tyramine (squares) in the absence (black symbols) or the presence of 0.1 mM sodium orthovanadate (open symbols). Each point is the mean ± SEM of 12–14 determinations. 2-DG uptake was expressed as nmol transported/100 mg cell lipids/10 min. The mean amount of cell lipids per assay was 22.2 ± 2.4 mg (n = 14 individuals). No significant influence of the factor vanadate was evidenced by ANOVA followed by Dunnett test. Different from corresponding basal at: *p < 0.05; **p < 0.01; ***p < 0.001
These first observations indicated that the synergism between vanadium and tyramine, already documented in rodent adipocytes [49], does not apply for humans. Although, these experiments were performed on a relatively large number of individuals (n = 14 with a mean age of 42 ± 3 years) it was further studied on other human adipocyte preparations whether different experimental conditions allowed the detection of a synergism between vanadate and tyramine.
Figure 2 shows that, regarding the stimulation of hexose uptake, there was no notable influence of larger doses of vanadate or of longer pre-incubation time with the transition metal on a putative synergism with tyramine. Vanadate did not alter significantly basal, insulin- or tyramine-stimulated 2-DG uptake when tested at 0.2 and 1 mM for 45 min (Fig. 2A). Even when the incubation with 0.1 mM vanadate lasted 3 h, there was no any synergism between vanadium and tyramine on glucose transport activity (Fig. 2B). Again, in these additional subjects, the effect of 1 mM tyramine on glucose transport was modest but significant, since it reproduced 25 to 35% of the maximal stimulation induced by 100 nM insulin.
Lack of influence of larger vanadate doses or longer incubation time on the tyramine-induced stimulation of hexose uptake in human adipocytes. A Increasing doses of vanadate. 2-DG uptake was measured after 45-min incubation of human subcutaneous adipocytes in the absence (control, closed circles) or the presence of sodium orthovanadate at 0.2 mM (open triangles) or 1 mM (open squares). Tested conditions were: 100 nM insulin, basal, and 0.1–1 mM tyramine. Each point is the mean ± SEM of 10 determinations and in several occurrences, error bar lies within the caption. B Increasing incubation time. After 3-h incubation without (control, closed symbols) or with 0.1 mM vanadate (open symbols), 2-DG uptake was determined in response to the indicated doses of insulin (circles) or tyramine (squares). Each point is the mean ± SEM of 6 determinations. No significant influence of exposure to vanadate could be detected. Different from the basal uptake at: *p < 0.05; **p < 0.01; ***p < 0.001
Comparison of the short-term insulin-mimicking effects of vanadate, hydrogen peroxide, and tyramine in rat and human adipocytes
At this stage, it was mandatory to verify whether the synergism originally described in rat adipocytes occurred not only between vanadium and tyramine [49] but also between vanadium and hydrogen peroxide, the combination of which led to the discovery of the potent insulin-mimicking actions of peroxovanadate [44]. In this comparative verification step, rat adipocytes were much responsive to insulin activation of hexose transport than human fat cells: baseline uptake was multiplied by 8 times with insulin in rats and only by 2–3 times in humans (Fig. 3). Nevertheless, the combination of vanadate and hydrogen peroxide elicited an uptake higher than that elicited by each agent separately, and their synergism reached 70–85% of the maximal insulin effect in both rat and human adipocytes (Fig. 3). By contrast, it was evident that the synergism occurring between vanadate and tyramine was limited to rat adipocytes only (Fig. 3). Thus, it was apparently not an interspecific difference in the insulin-like properties of peroxovanadate (chemically generated after vanadate and hydrogen peroxide combination) that explained the differences between the two species.
Comparison of the influence of vanadate on glucose transport by adipocytes in rat and in humans. Rat or human adipocytes were incubated for 45 min in the absence (basal) or the presence of 0.1 µM bovine insulin, or with 1 mM hydrogen peroxide (H2O2), or 1 mM tyramine; under control conditions without (colored bars) or with sodium orthovanadate (+ vanadate 0.1 mM, open bars). Thereafter, 2-DG uptake was measured over a 5-min period for rat (A) or a 10-min period for human adipocytes (B). 2-DG uptake was expressed as nmol transported/100 mg cell lipids/ 5 or 10 min, respectively. Results are mean values ± SEM of the number of experiments indicated in parentheses. When referring to basal, the tested factors activated significantly 2-DG uptake at: °°p < 0.01; °°°p < 0.001. The combination with vanadate was significantly different from corresponding control at: **p < 0.01; ***p < 0.001 (ANOVA followed by Dunnett test)
It was also compared whether glucose metabolism was activated by insulin and tyramine, downstream the activation of transmembrane uptake. To this aim, the incorporation of tritiated glucose into cell lipids was investigated. This comparative approach definitely confirmed that the human adipocytes are less metabolically active than the rat ones. The activator effect of insulin was easily evidenced in rat adipocytes since it is increasing by approximately eight times the basal lipogenic activity. A lipogenic effect was also evidenced in rat for the combination of vanadium with either hydrogen peroxide or tyramine (Fig. 4A). However, human adipocytes were much less sensitive to insulin as no significant activation of lipogenic activity could be detected: the maximal response was lower than twice as much as baseline (Fig. 4B). Vanadium, hydrogen peroxide, and tyramine were also unable to clearly activate the weak glucose-dependent lipogenic activity of human adipocytes. For all these agents, a tendency to reproduce the responses observed in rat adipocytes was found in a blunted manner only.
Influence of tyramine and vanadate on the glucose incorporation into lipids of rat and human adipocytes. Fat cells were incubated for 90 min without (basal) or with the indicated final concentrations of insulin, hydrogen peroxide (H2O2), or tyramine, in the absence (control, colored bars) or in the presence of sodium orthovanadate (+ 0.1 mM vanadate, open bars). Glucose incorporation into lipids was expressed as fold increase over basal lipogenesis, which was equivalent to 27.0 ± 5.4 and 4.8 ± 2.4 mol of glucose incorporated/100 mg lipids/90 min in rat (A, red and white columns) and human adipocytes (B, blue and white columns), respectively. Mean ± SEM of the number of cases indicated in parentheses. With reference to the basal, the tested factors activated significantly glucose-dependent lipogenesis at: °°p < 0.01; °°°p < 0.001. The combination with vanadate was significantly different from corresponding control at: ***p < 0.001 (ANOVA followed by Dunnett test)
Since glucose incorporation into lipids of human adipocytes was not readily activated by insulin or tyramine in human adipocytes, it was investigated whether the initial effect of tyramine on hexose entry could be mediated by Gi-coupled trace amine receptors (TAARs) since some of them are expressed in adipose tissue and some of them can be activated by tyramine [21].
Tyramine activation of glucose uptake after blockade of Gi protein coupling by pertussis toxin
Although the involvement of Gi proteins in insulin action is most involved in the generation of reactive oxygen species [34] than in activation of glucose transporters [25], the description in transfected cells of a Gi-dependent stimulation of glucose transporter translocation by adrenaline [73] prompted us to study whether Gi blockade by Pertussis toxin treatment (PTX, 100 ng/mL during 2 h at 37 °C) could alter or not the tyramine insulin-like effects. In fact, the stimulation of hexose uptake by insulin or by tyramine was not modified by PTX-induced blockade of Gi-coupled signal transduction (Table 1). The PTX experiments confirmed that the effect of tyramine was not potentiated by vanadium in human fat cells, while the combination of hydrogen peroxide and vanadate exhibited a synergistic activation of hexose uptake that was almost equivalent to that obtained with insulin 10 nM, regardless of the treatment (Table 1). Additionally, the tendency of isobutylmethylxanthine (IBMX) to inhibit glucose transport remained unaltered after PTX treatment, since it is predominantly mediated by a direct interaction with glucose transporters rather than with Gi-coupled receptors [66].
Impairment of tyramine stimulation of hexose uptake by monoamine oxidase inhibitors
It was then investigated whether tyramine activation of 2-DG uptake was impaired by the selective MAO-A inhibitor clorgyline or by the selective MAO-B inhibitor selegiline. The basal 2-DG uptake of human adipocytes was not altered by increasing doses of both inhibitors (Fig. 5A). An inhibition of 1 mM tyramine-induced 2-DG uptake was obtained with 1 mM of each inhibitor. Moreover, the inhibitory potency of clorgyline tended to be higher than that of selegeline, especially in the 0.01–0.1 mM range. However, 1 mM selegiline also produced a modest but significant inhibition of insulin-stimulated 2-DG uptake (Fig. 5A). As the inhibition of tyramine action was not total with either MAO-A or MAO-B inhibitor, other selective inhibitors were studied. The MAO-A inhibitor Ro 41–1049 and the MAO-B inhibitor Ro 16–6491 were devoid of unselective action since they did not alter significantly basal or insulin-stimulated 2-DG uptake (Fig. 5B). In the 0.1–1 mM range, these molecules exhibited a similar magnitude of inhibition on tyramine-induced 2-DG uptake (Fig. 5B). All these results strongly suggested that MAO oxidation of tyramine was involved in its activation of hexose uptake, but they were not supporting that tyramine effect was solely depending on either MAO-A or MAO-B.
Inhibition of tyramine-induced stimulation of hexose uptake in human adipocytes by selective inhibitors of MAO-A and MAO-B. A Inhibition by clorgyline and selegiline. The MAO-A inhibitor clorgyline (black symbols and lines) and the MAO-B inhibitor selegiline (open symbols, dotted lines) were tested at doses increasing from 10 µM to 1 mM on the basal 2-DG uptake (circles), and against 1-mM tyramine-induced (triangles) or 100-nM insulin-induced (squares) stimulation. 2-DG uptake assay was immediately performed on 10 min after 45-min incubation with the indicated agents and expressed as the percentage of stimulation by insulin (set at 100%), with baseline set at 0%. Each point is the mean ± SEM of 10 to 15 adipocyte preparations, in which tyramine effect reached 34.8 ± 4.1% in control condition, without inhibitor. Different from respective control at: *p < 0.05. B Inhibition by the MAO-A inhibitor Ro 41–1049 (black symbols and lines) and the MAO-B inhibitor Ro 16–6491 (open symbols, dotted lines). The inhibitors were tested at doses increasing from 10 µM to 1 mM on basal (circles), and against 1 mM tyramine-induced (triangles) or 100-nM insulin-induced (squares) 2-DG uptake. Mean ± SEM of 10 to 17 determinations, in which tyramine effect reached 27.0 ± 2.7% of insulin effect, without inhibitor. Different from respective control at: **p < 0.01
So, we included pargyline, a less selective MAO-A/MAO-B inhibitor in the inhibition experiments taking into account that MAO-A and MAO-B coexist in human adipocytes [61] and that tyramine is a substrate for both forms [8]. Since tyramine is a recognized SSAO substrate in rat [49], a SSAO inhibitor was also tested, alone and in combination with pargyline. The effect of 1 mM tyramine on 2-DG uptake was inhibited by 1 mM pargyline, and practically not by semicarbazide, while a stronger inhibition was found when both inhibitors were combined (Fig. 6). A mirrored situation was observed for 0.1 mM benzylamine, which was sensitive to semicarbazide alone and further blocked by the presence of both inhibitors (Fig. 6). This latter verification was dealing with the fact that benzylamine is a SSAO and a MAO-B substrate [19]. Although these inhibitors required millimolar dose to abolish the actions of amines, it can be noted that they did not hamper basal or insulin-stimulated glucose transport (Fig. 6).
A comparison of the sensitivity of human adipocytes to pargyline and semicarbazide of insulin, benzylamine, and tyramine effects on hexose uptake. Human adipocytes were incubated for 45 min in the presence of the indicated activators of 2-DG uptake (insulin, benzylamine, and tyramine) without (control, open columns) or with 1 mM pargyline (red columns), 1 mM semicarbazide (blue columns), or a combination of both (purple columns). Each column is the mean ± SEM of 10 to 20 determinations. Different from respective control by ANOVA at: *p < 0.05; **p < 0.01; ***p < 0.001
Then, BTT 2052, a SSAO blocker of recent generation [50], was studied. It impaired tyramine action, but mostly at 1 mM (2-DG uptake was 24 ± 2.9% with 1 mM tyramine and 8.6 ± 1.7% with tyramine + BTT 2052, when expressed as percentage of insulin effect; n = 4; p < 0.01). However for the lower dose of 100 µM, at which BTT 2052 was demonstrated to be highly SSAO selective [50], the trend to impair tyramine-stimulated glucose uptake was not significant (19.5 ± 3.0%; NS, not shown).
Taken together, these results indicated that the stimulatory effect of tyramine on hexose uptake was rather complex, depending on its oxidation by both MAO-A and MAO-B, and by SSAO to a lesser extend.
Similar sensitivity of tyramine oxidation and of stimulation of hexose uptake to amine oxidase inhibitors
It was observed that the inhibition of tyramine oxidation by human adipose tissue exhibited similarities with the inhibition of its insulin-like action on glucose uptake (Fig. 7). Whatever the method used for measuring tyramine oxidation (either by determining the radioactive adehydic products separated by organic solvent extraction after the oxidation of [14C]-tyramine [13], or by quantifying the hydrogen peroxide release owing to an Amplex red/horseraddish peroxidase fluorometric detection), the major part of this oxidation was sensitive to pargyline, and a minor part was inhibited by semicarbazide. As for tyramine-induced glucose uptake, the largest impairment of tyramine oxidation was obtained when both MAO and SSAO inhibitors were present, regardless of the method.
A comparison of the sensitivity to pargyline and semicarbazide of tyramine oxidation by human adipose tissue homogenates. Oxidation of 1 mM tyramine was performed at 37 °C on 30-min incubation without (control, open columns), or with 1 mM of pargyline, semicarbazide, or pargyline + semicarbazide (parg + semi, purple columns). Left: aldehydic oxidation products of radiolabeled [14C]-tyramine were extracted and counted. Right: hydrogen peroxide was determined by Amplex Red-based fluorescence. Data are expressed as percentage of respective product released under control condition. Mean ± SEM of 3 to 5 homogenates. Different from control by t-test at: ***p < 0.001
Impairment of tyramine stimulation of hexose uptake by antioxidants and PI3K inhibitor
Mandelic acid, which is the parent molecule of many metabolites of catecholamines and tyramine degradation, was inactive from 10−6 to 10−3 M on 2-DG uptake (at 1 mM, it produced 2.3 ± 4.7% of the maximal insulin activation; n = 8; NS). This was also the case of ammonium chloride (0.3 ± 6.1% of insulin max effect, n = 5; NS). These negative results reinforced the hypothesis that hydrogen peroxide mediates the tyramine action of 2-DG uptake. This was further supported when considering that the antioxidants, ascorbic acid, glutathione, and N-acetyl cysteine impaired the tyramine effect on 2-DG uptake but not that of insulin (Table 2).
Finally, as it was the case for insulin, the impairment of tyramine-induced glucose transport by the phosphoinositide 3-kinase inhibitor wortmannin argued indirectly that a recruitment of glucose transporters at the cell surface was triggered by exposure to the amine (basal: 0.52 ± 0.04; tyramine: 0.77 ± 0.11; 1 mM tyramine + 1 µM wortmannin: 0.55 ± 06 nmol 2-DG transported/100 mg cell lipids/10 min; n = 15, p < 0.05).
Discussion
This study shows that the direct activator effect of tyramine on glucose uptake, already documented in rat adipocytes [49] and cardiomyocytes [55], can be extrapolated to human adipocytes.
Major part of the tyramine-induced glucose transport was pargyline-sensitive, agreeing with the previously reported MAO-A involvement in tyramine oxidation in human fat cells [9]. As for its antilipolytic effect in human fat cells [12], this insulin-like action of tyramine was only efficient in the sub-millimolar range and sensitive to MAO inhibitors and to antioxidants.
However, tyramine behaved slightly differently in human than in rodent fat cells. First, its modest stimulatory action in human fat cells was not potentiated by vanadate, a characteristic that can be extended to many others amine oxidase substrates, such as benzylamine, also able to stimulate glucose uptake and to inhibit lipolysis in adipocytes of both species [54, 71]. Second, tyramine activation of glucose uptake was almost insensitive to SSAO inhibitors in human adipocytes, while these inhibitors were as capable as MAO inhibitors to impair its actions in rodent adipocytes [49, 71]. However, the selective MAO-A inhibitors clorgyline and Ro 41–1049 were not overtly more efficient than MAO-B inhibitors selegiline and Ro 16–6491 in blocking the tyramine effect on 2-DG uptake in human fat cells. The resulting “mixed A/B” effect of tyramine relies with its capacity to be a substrate of both human MAO-A and MAO-B, leaving less importance to the fact that human adipocytes express more MAO-A than MAO-B [61]. On the opposite, tyramine is more readily oxidized by AOC2 [31], a minor form of the human copper-containing amine oxidases, than by the major form, encoded by AOC3 gene and known as VAP-1, equivalent to SSAO [16]. Since AOC2 is weakly expressed in human adipose cells [28], it cannot be excluded that a minor portion of the observed tyramine oxidation was mediated by this amine oxidase, therefore justifying why the combination of semicarbazide plus pargyline elicited the strongest inhibition of tyramine-induced hexose transport.
Even if the maximal inhibition with MAO-A inhibitors or with MAO-B blockers almost reached that obtained with pargyline plus semicarbazide, additional comments are necessary to complete the data interpretation. All these inhibitors have not been pre-incubated with human fat cells before the addition of tyramine, while they were co-administered together with the substrate during a 45-min incubation prior to 2-DG uptake assays and were thereby tested in a competitive situation. Consequently, any protocol including inhibitor pre-treatment would have facilitated to prevent tyramine action at lower doses. Whatsoever, the sensitivity to the various amine oxidase inhibitors tested contrasted with the lack of influence of PTX on the tyramine-induced 2-DG uptake.
The protocol of PTX treatment was identical to that previously reported to abolish the Gi-mediated antilipolytic effect of phenylisopropyladenosine, nicotinic acid, or brimonidine in human adipocytes (mediated by the activation of the Gi-coupled NIACR1, A1-adenosine and α2-adrenergic receptors, respectively) [12]. While such treatment increased basal lipolysis in human adipocytes [12], it did not modify the 2-DG uptake baseline. Similarly, PTX treatment did not affect the insulin-stimulated 2-DG uptake. This was also the case for tyramine, arguing that, similarly to its antilipolytic actions, the tyramine moderate activation of hexose uptake was not depending on Gi-coupled mechanisms. The well-recognized inhibitory effect of IBMX on glucose transport, traduced here by a tendency to inhibit basal uptake, was also unaffected by the PTX treatment. This was in agreement with the demonstration of a direct binding of the methylxanthine to the glucose transporters rather than to an inhibition of phosphodiesterases or adenosine receptors [10, 33]. In these conditions, the lack of influence of PTX treatment on tyramine ruled out any putative mediation by human TAAR8, the only trace amine receptor that is Gi-coupled [48]. Additionally, the sensitivity to amine oxidase inhibitors and antioxidants did not render a plausible mediation by TAAR1, which readily binds tyramine, but the expression of which in human adipose tissue remains under debate [21].
MAO are enzymes involved in biotransformation. However, all the end-products of tyramine oxidation by adipocyte amine oxidases, namely hydrogen peroxide, ammonia, and p-hydroxyphenyl acetic acid that is transformed into mandelic acid, curiously appear to be more highly reactive molecules than the tyramine itself. Regardless, only hydrogen peroxide was endowed with insulin-like activity in adipocytes [51] and was active in our experiments. A similar lack of effect of the oxidation products other than hydrogen peroxide has been already reported for the inhibition of lipolysis or for the activation of glucose uptake in rat adipocytes [30, 71]. It remains undetermined why the hydrogen peroxide effect — but not that of tyramine — was potentiated by vanadate, with regard to glucose transport activation in human fat cells. Since such difference was not present in rodent adipocytes, it remains to be established what species-specific mechanisms are involved in the cellular handling of ROS and vanadium [17].
It also appears unclear why the glucose incorporation into lipids is poorly sensitive to insulin stimulation in human adipocytes. The fact that the adipocytes of human adults exhibit less lipogenic activity from glucose than the adipocytes from young rats has already been reported [32]. We have also observed that rat adipocytes can readily lose their insulin lipogenic responsiveness with age and essential fatty acid deficiency [27]. However, at the present time, it cannot be assessed that tyramine partially reproduces the lipogenic effect of insulin in humans. Another limitation of the study is the lack of study of other insulin-like metabolic responses such as activation of lactate release, which has been observed in human adipocytes to limit lipolysis [37]. Interestingly, previous observations made on fat cells from old rats have shown that tyramine, like insulin, totally reversed the inhibitory effect of noradrenaline on lactate release [6]. However, since insulin and tyramine did not potentiate their effects in this model, it can be asked about the interaction between tyramine and insulin on carbohydrate metabolism in human fat cells. Only quantitative analyses of the fate of radiolabeled glucose and its metabolites, such as those performed on rat adipocytes [29], could determine the actual role of tyramine in the handling of glucose load by human adipocytes.
Regarding the physiological relevance of our in vitro observations, another limitation arises considering that tyramine pharmacokinetics have shown a maximal plasma concentration of approximately 0.2 µM in humans after 200 mg tyramine ingestion [68]. This issue has already been discussed when we reported the antilipolytic effect of tyramine in human adipocytes [12], which occurs in the same sub-millimolar range concentration. Not only the targets of tyramine are multiple since it is a catecholamine releaser, a substrate of various amine oxidases and an agonist at TAARs, but also its metabolic pathways are inasmuch complex once tyramine, together with other dietary amines, are ingested and facing to the intestinal microbiota [47]. These aspects are beyond the scope of the present approach limited to in vitro experiments. Nevertheless, it is worth mentioning that tyramine urinary concentrations were significantly reduced in patients with metabolic syndrome compared to controls in a recent metabolomic study on a total of 48 subjects [59]. This might suggest an indirect link between tyramine metabolism and glucose handling.
Even when refocusing on the direct putative effects of tyramine on adipose tissue, this issue deserves further studies, especially when considering that numerous insulin-like agents, which facilitate in vitro glucose utilization, also hamper the actions of the pancreatic hormone when combined with it. Further studies dealing with the influence of tyramine on adipose tissue are also necessary because of the discrepancy between contradictory results about its metabolic actions. Indeed, the present results contrast with those reporting a pro-lipolytic effect of 50 µM tyramine in rat adipocytes when prestimulated by forskolin or isoprenaline [62]. Similarly, the effects of tyramine on vascular reactivity around adipose depots are also controversial. The amine has been demonstrated to increase noradrenaline release from rat renal perivascular adipose tissue and to increase the contraction of renal arteries [63]. Conversely, the same perivascular adipose tissue has been proposed to contribute to an anti-contractile effect under the influence of tyramine on noradrenaline uptake and metabolism [4]. At least, the present study confirmed that the capacity of high doses of tyramine to activate glucose uptake in rodent fat cells was applicable to humans. In view of (1) the presence of tyramine in daily consumed food items or beverages [3, 39] and (2) the beneficial effects of tyramine in the metabolic and immune responses of prawns to deleterious challenges [36], it is still pertinent to unravel the roles played by this molecule on the vascular reactivity as well as on carbohydrate and lipid metabolisms in the consumers.
References
Adams F, Boschmann M, Schaller K, Franke G, Gorzelniak K, Janke J, Klaus S, Luft FC, Heer M, Jordan J (2006) Tyramine in the assessment of regional adrenergic function. Biochem Pharmacol 72:1724–1729. https://doi.org/10.1016/j.bcp.2006.09.004
Amaro CA, González-Cortazar M, Herrera-Ruiz M, Román-Ramos R, Aguilar-Santamaría L, Tortoriello J, Jiménez-Ferrer E (2014) Hypoglycemic and hypotensive activity of a root extract of Smilax aristolochiifolia, standardized on N-trans-feruloyl-tyramine. Molecules 19:11366–11384. https://doi.org/10.3390/molecules190811366
Andersen G, Marcinek P, Sulzinger N, Schieberle P, Krautwurst D (2019) Food sources and biomolecular targets of tyramine. Nutr Rev 77:107–115. https://doi.org/10.1093/nutrit/nuy036
Ayala-Lopez N, Thompson JM, Watts SW (2017) Perivascular adipose tissue’s impact on norepinephrine-induced contraction of mesenteric resistance arteries. Front Physiol 8:37. https://doi.org/10.3389/fphys.2017.00037
Aydin S, Ugur K, Aydin S (2018) Could excessive production of tyramine by the microbiota be a reason for essential hypertension? Biosci Microbiota Food Health 37:77–78. https://doi.org/10.12938/bmfh.18-010
Bairras C, Ferrand C, Atgié C (2003) Effect of tyramine, a dietary amine, on glycerol and lactate release by isolated adipocytes from old rats. J Physiol Biochem 59:161–167
Barrand MA, Fox SA (1984) Amine oxidase activities in brown adipose tissue of the rat: identification of semicarbazide-sensitive (clorgyline-resistant) activity at the fat cell membrane. J Pharm Pharmacol 36:652–658
Basile L, Pappalardo M, Guccione S, Milardi D, Ramsay RR (2014) Computational comparison of imidazoline association with the I2 binding site in human monoamine oxidases. J Chem Inf Model 54:1200–1207. https://doi.org/10.1021/ci400346k
Bour S, Iglesias-Osma MC, Marti L, Duro P, Garcia-Barrado MJ, Pastor MF, Prevot D, Visentin V, Valet P, Moratinos J, Carpéné C (2006) The imidazoline I2-site ligands BU 224 and 2-BFI inhibit MAO-A and MAO-B activities, hydrogen peroxide production, and lipolysis in rodent and human adipocytes. Eur J Pharmacol 552:20–30. https://doi.org/10.1016/j.ejphar.2006.09.021
Brewer PD, Romenskaia I, Mastick CC (2018) A high-throughput chemical-genetics screen in murine adipocytes identifies insulin-regulatory pathways. J Biol Chem. https://doi.org/10.1074/jbc.RA118.006986
Carpéné C, Daviaud D, Boucher J, Bour S, Visentin V, Gres S, Duffaut C, Fontana E, Testar X, Saulnier-Blache JS, Valet P (2006) Short- and long-term insulin-like effects of monoamine oxidases and semicarbazide-sensitive amine oxidase substrates in cultured adipocytes. Metabolism 55:1397–1405. https://doi.org/10.1016/j.metabol.2006.06.011
Carpéné C, Galitzky J, Belles C, Zakaroff-Girard A (2018) Mechanisms of the antilipolytic response of human adipocytes to tyramine, a trace amine present in food. J Physiol Biochem 74:623–633. https://doi.org/10.1007/s13105-018-0643-z
Carpéné C, Hasnaoui M, Balogh B, Matyus P, Fernandez-Quintela A, Rodriguez V, Mercader J, Portillo MP (2016) Dietary phenolic compounds interfere with the fate of hydrogen peroxide in human adipose tissue but do not directly inhibit primary amine oxidase activity. Oxid Med Cell Longev 2016:2427618. https://doi.org/10.1155/2016/2427618
Carpéné C, Mauriège P, Boulet N, Biron S, Grolleau JL, Garcia-Barrado MJ, Iglesias-Osma MC (2019) Methylamine activates glucose uptake in human adipocytes without overpassing action of insulin or stimulating its secretion in pancreatic islets. Medicines (Basel) 6:89. https://doi.org/10.3390/medicines6030089
Carpéné C, Schaak S, Guilbeau-Frugier C, Mercader J, Mialet-Perez J (2016) High intake of dietary tyramine does not deteriorate glucose handling and does not cause adverse cardiovascular effects in mice. J Physiol Biochem 72:539–553. https://doi.org/10.1007/s13105-015-0456-2
Elovaara H, Kidron H, Parkash V, Nymalm Y, Bligt E, Ollikka P, Smith DJ, Pihlavisto M, Salmi M, Jalkanen S, Salminen TA (2011) Identification of two imidazole binding sites and key residues for substrate specificity in human primary amine oxidase AOC3. Biochemistry 50:5507–5520. https://doi.org/10.1021/bi200117z
Fantus IG, Kadota S, Deragon G, Foster B, Posner BI (1989) Pervanadate [peroxide(s) of vanadate] mimics insulin action in rat adipocytes via activation of the insulin receptor tyrosine kinase. Biochemistry 28:8864–8871. https://doi.org/10.1021/bi00448a027
Finberg JP, Gillman K (2011) Selective inhibitors of monoamine oxidase type B and the “cheese effect.” Int Rev Neurobiol 100:169–190. https://doi.org/10.1016/b978-0-12-386467-3.00009-1
Fitzgerald DH, Tipton KF (2002) Inhibition of monoamine oxidase modulates the behaviour of semicarbazide-sensitive amine oxidase (SSAO). J Neural Transm (Vienna) 109:251–265. https://doi.org/10.1007/s007020200021
Fontana E, Boucher J, Marti L, Lizcano JM, Testar X, Zorzano A, Carpéné C (2001) Amine oxidase substrates mimic several of the insulin effects on adipocyte differentiation in 3T3 F442A cells. Biochem J 356:769–777
Gainetdinov RR, Hoener MC, Berry MD (2018) Trace amines and their receptors. Pharmacol Rev 70:549–620. https://doi.org/10.1124/pr.117.015305
Gilliam LK, Palmer JP, Taborsky GJ Jr (2007) Tyramine-mediated activation of sympathetic nerves inhibits insulin secretion in humans. J Clin Endocrinol Metab 92:4035–4038. https://doi.org/10.1210/jc.2007-0536
Gillman PK (2016) Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries 1:1–90
Gomez-Zorita S, Treguer K, Mercader J, Carpéné C (2013) Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. J Physiol Biochem 69:585–593. https://doi.org/10.1007/s13105-012-0229-0
Green A, Walters DJ, Belt SE (1997) Insulin resistance in adipocytes after downregulation of Gi subtypes. Am J Physiol 273:E254-261. https://doi.org/10.1152/ajpendo.1997.273.2.E254
Haj Ahmed W, Boulet N, Briot A, Ryan BJ, Kinsella GK, O’Sullivan J, Les F, Mercader-Barceló J, Henehan GTM, Carpéné C (2021) Novel facet of an old dietary molecule? Direct influence of caffeine on glucose and biogenic Amine handling by human adipocytes. Molecules 26:3831. https://doi.org/10.3390/molecules26133831
Harant-Farrugia I, Garcia J, Iglesias-Osma MC, Garcia-Barrado MJ, Carpéné C (2014) Is there an optimal dose for dietary linoleic acid? Lessons from essential fatty acid deficiency supplementation and adipocyte functions in rats. J Physiol Biochem 70:615–627. https://doi.org/10.1007/s13105-014-0315-6
Heniquez A, Meissonnier G, Visentin V, Prevot D, Carpéné C (2003) High expression of semicarbazide-sensitive amine oxidase genes AOC2 and AOC3, but not the diamine oxidase gene AOC1 in human adipocytes. Inflamm Res 52(Suppl 1):S74-75
Ho-Palma AC, Toro P, Rotondo F, Romero MDM, Alemany M, Remesar X, Fernández-López JA (2019) Insulin controls triacylglycerol synthesis through control of glycerol metabolism and despite increased lipogenesis. Nutrients 11:513. https://doi.org/10.3390/nu11030513
Iglesias-Osma MC, Bour S, Garcia-Barrado MJ, Visentin V, Pastor MF, Testar X, Marti L, Enrique-Tarancon G, Valet P, Moratinos J, Carpéné C (2005) Methylamine but not mafenide mimics insulin-like activity of the semicarbazide-sensitive amine oxidase-substrate benzylamine on glucose tolerance and on human adipocyte metabolism. Pharmacol Res 52:475–484. https://doi.org/10.1016/j.phrs.2005.07.008
Kaitaniemi S, Elovaara H, Grön K, Kidron H, Liukkonen J, Salminen T, Salmi M, Jalkanen S, Elima K (2009) The unique substrate specificity of human AOC2, a semicarbazide-sensitive amine oxidase. Cell Mol Life Sci 66:2743–2757. https://doi.org/10.1007/s00018-009-0076-5
Kamel AF, Norgren S, Strigård K, Thörne A, Fakhrai-Rad H, Galli J, Marcus C (2004) Age-dependent regulation of lipogenesis in human and rat adipocytes. J Clin Endocrinol Metab 89:4601–4606. https://doi.org/10.1210/jc.2003-030994
Kashiwagi A, Huecksteadt TP, Foley JE (1983) The regulation of glucose transport by cAMP stimulators via three different mechanisms in rat and human adipocytes. J Biol Chem 258:13685–13692
Krieger-Brauer HI, Medda PK, Kather H (1997) Insulin-induced activation of NADPH-dependent H2O2 generation in human adipocyte plasma membranes is mediated by Galphai2. J Biol Chem 272:10135–10143
Kubota R, Reid MJ, Lieu KL, Orme M, Diamond C, Tulberg N, Henry SH (2020) Comparison of inhibitor and substrate selectivity between rodent and human vascular adhesion protein-1. Mediators Inflamm 2020:3270513. https://doi.org/10.1155/2020/3270513
Kuo HW, Cheng W (2022) Dietary administration of tyramine upregulates on immune resistance, carbohydrate metabolism, and biogenic amines in Macrobrachium rosenbergii. Dev Comp Immunol 126:104236. https://doi.org/10.1016/j.dci.2021.104236
Langin D (2010) Adipose tissue lipolysis revisited (Again!): Lactate involvement in insulin antilipolytic action. Cell Metab 11:242–243
Langin D, Lucas S, Lafontan M (2000) Millennium fat-cell lipolysis reveals unsuspected novel tracks. Horm Metab Res 32:443–452. https://doi.org/10.1055/s-2007-978670
Latorre-Moratalla ML, Comas-Basté O, Bover-Cid S, Vidal-Carou MC (2017) Tyramine and histamine risk assessment related to consumption of dry fermented sausages by the Spanish population. Food Chem Toxicol 99:78–85. https://doi.org/10.1016/j.fct.2016.11.011
Leroux M, Lemery T, Boulet N, Briot A, Zakaroff A, Bouloumié A, Andrade F, Pérez-Matute P, Arbones-Mainar JM, Carpéné C (2019) Effects of the amino acid derivatives, β-hydroxy-β-methylbutyrate, taurine, and N-methyltyramine, on triacylglycerol breakdown in fat cells. J Physiol Biochem 75:263–273. https://doi.org/10.1007/s13105-019-00677-5
Les F, Deleruyelle S, Cassagnes LE, Boutin JA, Balogh B, Arbones-Mainar JM, Biron S, Marceau P, Richard D, Nepveu F, Mauriège P, Carpéné C (2016) Piceatannol and resveratrol share inhibitory effects on hydrogen peroxide release, monoamine oxidase and lipogenic activities in adipose tissue, but differ in their antilipolytic properties. Chem Biol Interact 258:115–125. https://doi.org/10.1016/j.cbi.2016.07.014
Lino CdS, Sales TdP, Gomes PB, Amaral JFd, Alexandre FSA, Silveira ER, Ferreira JM, de Sousa DF, Queiroz MGRd, Sousa FCFd, Brito GAC, Brito SMRC, Viana GSB (2007) Anti-diabetic activity of a fraction from Cissus verticillata and tyramine, its main bioactive constituent, in alloxan-induced diabetic rats. Am J Pharmacol Toxicol 2:178–188. https://doi.org/10.3844/ajptsp.2007.178.188
Little SA, de Haën C (1980) Effects of hydrogen peroxide on basal and hormone-stimulated lipolysis in perifused rat fat cells in relation to the mechanism of action of insulin. J Biol Chem 255:10888–10895
Lönnroth P, Eriksson JW, Posner BI, Smith U (1993) Peroxovanadate but not vanadate exerts insulin-like effects in human adipocytes. Diabetologia 36:113–116. https://doi.org/10.1007/bf00400690
Lopes de Carvalho L, Bligt-Linden E, Ramaiah A, Johnson MS, Salminen TA (2019) Evolution and functional classification of mammalian copper amine oxidases. Mol Phylogenet Evol 139:106571. https://doi.org/10.1016/j.ympev.2019.106571
Lyles GA (1995) Substrate-specificity of mammalian tissue-bound semicarbazide-sensitive amine oxidase. Prog Brain Res 106:293–303
Lyte JM (2018) Eating for 3.8 x 10(13): Examining the impact of diet and nutrition on the microbiota-gut-brain axis through the lens of microbial endocrinology. Front Endocrinol (Lausanne) 9:796. https://doi.org/10.3389/fendo.2018.00796
Maguire JJ, Parker WA, Foord SM, Bonner TI, Neubig RR, Davenport AP (2009) International Union of Pharmacology. LXXII. Recommendations for trace amine receptor nomenclature. Pharmacol Rev 61:1–8. https://doi.org/10.1124/pr.109.001107
Marti L, Morin N, Enrique-Tarancon G, Prevot D, Lafontan M, Testar X, Zorzano A, Carpéné C (1998) Tyramine and vanadate synergistically stimulate glucose transport in rat adipocytes by amine oxidase-dependent generation of hydrogen peroxide. J Pharmacol Exp Ther 285:342–349
Marttila-Ichihara F, Smith DJ, Stolen C, Yegutkin GG, Elima K, Mercier N, Kiviranta R, Pihlavisto M, Alaranta S, Pentikäinen U, Pentikäinen O, Fülöp F, Jalkanen S, Salmi M (2006) Vascular amine oxidases are needed for leukocyte extravasation into inflamed joints in vivo. Arthritis Rheum 54:2852–2862. https://doi.org/10.1002/art.22061
May JM, de Haen C (1979) The insulin-like effect of hydrogen peroxide on pathways of lipid synthesis in rat adipocytes. J Biol Chem 254:9017–9021
Moldes M, Fève B, Pairault J (1999) Molecular cloning of a major mRNA species in murine 3T3 adipocyte lineage. differentiation-dependent expression, regulation, and identification as semicarbazide-sensitive amine oxidase. J Biol Chem 274:9515–9523
Moody AJ, Stan MA, Stan M, Gliemann J (1974) A simple free fat cell bioassay for insulin. Horm Metab Res 6:12–16. https://doi.org/10.1055/s-0028-1093895
Morin N, Lizcano JM, Fontana E, Marti L, Smih F, Rouet P, Prévot D, Zorzano A, Unzeta M, Carpéné C (2001) Semicarbazide-sensitive amine oxidase substrates stimulate glucose transport and inhibit lipolysis in human adipocytes. J Pharmacol Exp Ther 297:563–572
Morin N, Visentin V, Calise D, Marti L, Zorzano A, Testar X, Valet P, Fischer Y, Carpéné C (2002) Tyramine stimulates glucose uptake in insulin-sensitive tissues in vitro and in vivo via its oxidation by amine oxidases. J Pharmacol Exp Ther 303:1238–1247. https://doi.org/10.1124/jpet.102.040592
Morris NJ, Ducret A, Aebersold R, Ross SA, Keller SR, Lienhard GE (1997) Membrane amine oxidase cloning and identification as a major protein in the adipocyte plasma membrane. J Biol Chem 272:9388–9392. https://doi.org/10.1074/jbc.272.14.9388
Mueller PS, Horwitz D (1962) Plasma free fatty acid and blood glucose responses to analogues of norepinephrine in man. J Lipid Res 3:251–255
Muller G, Wied S, Straub J, Jung C (2008) Coordinated regulation of esterification and lipolysis by palmitate, H2O2 and the anti-diabetic sulfonylurea drug, glimepiride, in rat adipocytes. Eur J Pharmacol 597:6–18. https://doi.org/10.1016/j.ejphar.2008.08.034
Patel A, Thompson A, Abdelmalek L, Adams-Huet B, Jialal I (2019) The relationship between tyramine levels and inflammation in metabolic syndrome. Horm Mol Biol Clin Investig 40:1. https://doi.org/10.1515/hmbci-2019-0047
Paulsen P, Grossgut R, Bauer F, Rauscher-Gabernig E (2012) Estimates of maximum tolerable levels of tyramine content in foods in Austria. J Food Nutr Res 51:52–59
Pizzinat N, Marti L, Remaury A, Leger F, Langin D, Lafontan M, Carpéné C, Parini A (1999) High expression of monoamine oxidases in human white adipose tissue: evidence for their involvement in noradrenaline clearance. Biochem Pharmacol 58:1735–1742
Raimondi L, Banchelli G, Sgromo L, Pirisino R, Ner M, Parini A, Cambon C (2000) Hydrogen peroxide generation by monoamine oxidases in rat white adipocytes: role on cAMP production. Eur J Pharmacol 395:177–182. https://doi.org/10.1016/s0014-2999(00)00181-3
Restini CBA, Ismail A, Kumar RK, Burnett R, Garver H, Fink GD, Watts SW (2018) Renal perivascular adipose tissue: form and function. Vascul Pharmacol 106:37–45. https://doi.org/10.1016/j.vph.2018.02.004
Rumore MM, Roth M, Orfanos A (2010) Dietary tyramine restriction for hospitalized patients on linezolid: an update. Nutr Clin Pract 25:265–269. https://doi.org/10.1177/0884533610368711
Shen SH, Wertz DL, Klinman JP (2012) Implication for functions of the ectopic adipocyte copper amine oxidase (AOC3) from purified enzyme and cell-based kinetic studies. PLoS ONE 7:e29270. https://doi.org/10.1371/journal.pone.0029270
Steinfelder HJ, Petho-Schramm S (1990) Methylxanthines inhibit glucose transport in rat adipocytes by two independent mechanisms. Biochem Pharmacol 40:1154–1157. https://doi.org/10.1016/0006-2952(90)90508-i
Stolen CM, Madanat R, Marti L, Kari S, Yegutkin GG, Sariola H, Zorzano A, Jalkanen S (2004) Semicarbazide sensitive amine oxidase overexpression has dual consequences: insulin mimicry and diabetes-like complications. Faseb j 18:702–704. https://doi.org/10.1096/fj.03-0562fje
VanDenBerg CM, Blob LF, Kemper EM, Azzaro AJ (2003) Tyramine pharmacokinetics and reduced bioavailability with food. J Clin Pharmacol 43:604–609
Visentin V, Bour S, Boucher J, Prévot D, Valet P, Ordener C, Parini A, Carpéné C (2005) Glucose handling in streptozotocin-induced diabetic rats is improved by tyramine but not by the amine oxidase inhibitor semicarbazide. Eur J Pharmacol 522:139–146. https://doi.org/10.1016/j.ejphar.2005.08.051
Visentin V, Marq P, Bour S, Subra C, Prévot D, Morin N, Valet P, Monje MC, Nepveu F, Carpéné C (2003) Effect of prolonged treatment with tyramine on glucose tolerance in streptozotocin-induced diabetic rats. J Physiol Biochem 59:225–232
Visentin V, Prevot D, Marti L, Carpéné C (2003) Inhibition of rat fat cell lipolysis by monoamine oxidase and semicarbazide-sensitive amine oxidase substrates. Eur J Pharmacol 466:235–243
Wang CC, Billett E, Borchert A, Kuhn H, Ufer C (2013) Monoamine oxidases in development. Cell Mol Life Sci 70:599–630. https://doi.org/10.1007/s00018-012-1065-7
Wang L, Hayashi H, Kishi K, Huang L, Hagi A, Tamaoka K, Hawkins PT, Ebina Y (2000) Gi-mediated translocation of GLUT4 is independent of p85/p110alpha and p110gamma phosphoinositide 3-kinases but might involve the activation of Akt kinase. Biochem J 345(Pt 3):543–555
Yu PH, Wang M, Fan H, Deng Y, Gubisne-Haberle D (2004) Involvement of SSAO-mediated deamination in adipose glucose transport and weight gain in obese diabetic KKAy mice. Am J Physiol Endocrinol Metab 286:E634-641. https://doi.org/10.1152/ajpendo.00272.2003
Acknowledgements
The authors are grateful to all members of CTPIOD mini-network for the helpful discussions (http://obesitydiabetesinctp.weebly.com). All our thanks to Anne Bouloumié, Jean Galitzky, and Pauline Descaunes for facilitating connection with the surgical Dept. Also, the authors are grateful for the expert technical assistance of Danielle Prévot, for the radioactive protection managed by Magali Diette, and for the assistance of Marc Jousseaume.
Funding
This study was partly supported by recurrent INSERM funds and by FEDER funding via grants of the French-Spanish POLYFrEsNOL/Refbio project.
Author information
Authors and Affiliations
Contributions
Conceptualization, CC; biological resources, J-LG, NB, and AB; in vitro investigations and data acquisition, CC, FL, and J.M-B; data analysis, FL; data curation, CC; writing, reviewing and editing, JM-B, and CC. All the authors read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
Animal procedures were approved with code 12–1048-03–15, by the Animal Ethics Committee of the INSERM unit US006 CREFRE (Centre Régional d’Exploration Fonctionnelle et Ressources Expérimentales, Toulouse, France).
Consent to participate
All individuals gave their informed consent for their participation to the study as validated by the local ethic committee for the protection of individuals (Comité de Protection des Personnes Sud Ouest et Outre Mer II) under the reference: DC-2014–2039.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
Human fat cells have the enzymatic ability to catalyze the oxidative deamination of tyramine
Oxidation of 0.1–1 mM tyramine results in activation of glucose uptake in fat cells
Stimulation of hexose transport by tyramine is weaker than that supported by insulin
Monoamine oxidase inhibitors impair tyramine activation of glucose transport
Rights and permissions
About this article
Cite this article
Carpéné, C., Les, F., Mercader-Barceló, J. et al. High doses of tyramine stimulate glucose transport in human fat cells. J Physiol Biochem 78, 543–556 (2022). https://doi.org/10.1007/s13105-021-00864-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-021-00864-3