Abstract
One of the emergent nutritional strategies for improving multiple features of cardiometabolic diseases is the practice of intermittent fasting (IF), which consists of alternating periods of eating and fasting. IF can reduce circulating glucose and insulin levels, fat mass, and the risk of developing age-related pathologies. IF appears to upregulate evolution-conserved adaptive cellular responses, such as stress-response pathways, autophagy, and mitochondrial function. IF was also observed to modulate the circadian rhythms of hormones like insulin or leptin, among others, which levels change in conditions of food abundance and deficit. However, some contradictory results regarding the duration of the interventions and the anterior metabolic status of the participants suggest that more and longer studies are needed in order to draw conclusions. This review summarizes the current knowledge regarding the role of IF in the modulation of mechanisms involved in type 2 diabetes, as well as the risks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several behavioural and physiological adaptations were developed through evolution to enable animals to survive to periods of food absence/scarcity [48]. The source of energy preferentially utilized and the metabolic activity of the organism are influenced by the changes in the rhythms of caloric intake and feeding [5]. Some organisms have the capacity to become dormant when food is not available for extended time periods; for example, stationary phase of yeasts, Dauer state of nematodes, and hibernation in ground squirrels and some bears [48]. In mammals, organs like the liver and the adipose tissue function as energy depots in times of food availability, informing the body about energy reserves and enabling metabolic activity during varying times of fasting/starvation [48]. In turn, chronic overfeeding promotes the development of obesity, the major risk factor for ectopic fat accumulation, insulin resistance, and type 2 diabetes (T2DM) [57]. Dietary interventions limiting energy intake such as intermittent fasting (IF) could be used to prevent such excessive accumulation. Their health benefits to treat metabolic diseases appear to be proportional to the length of the fasting period, although some contradictory results have been raised [57]. Interestingly, the benefits of energy-restrictive strategies appear to involve other mechanisms than energy restriction per se, although the modulation of such mechanisms may have opposing effects upon excessive periods of food restriction [3].
IF is defined as the practice of alternating periods of fasting and eating through several different approaches that may include or not energy restriction. It has emerged as a promising therapeutic strategy for improving multiple cardiometabolic endpoints in rodent models of disease, including ectopic fat accumulation, insulin resistance, and diabetes [67]. Available evidence in humans suggests that the benefits of IF are mostly related to energy restriction, leading to weight loss and reduced free radical production [20]. However, current studies suggest that IF can also elicit evolutionarily conserved, adaptive cellular responses that affects several organs and their crosstalk. Such mechanisms have positive contributions to energy metabolism and glucose regulation, reducing circulating insulin, fat mass, and the risk of developing age-related pathologies [20, 43, 67, 68].
Hormones like insulin, glucagon, cortisol, leptin, thyroid hormones, or growth hormone have circadian rhythms, which adjust metabolic activity and energy expenditure to food abundance or deficit, providing the needed glucose levels and energy to the organism [12, 48]. During the diurnal period, glucose is the primary source of energy for most tissues, being stimulated by insulin and used for glycolysis [8, 73]. In times of energy deficit, cells have systems to monitor changes in adenosine triphosphate (ATP) levels. The energy sensor adenosine monophosphate (AMP) activated protein kinase (AMPK) monitor changes of ATP levels, leading to an activation of ATP-producing pathways and/or a suppression of ATP-using pathways[8]. When active, AMPK shuts down anabolic pathways, such as lipogenesis (inactivates acetyl-CoA carboxylase) and transcriptional processes, and promotes catabolic pathways [73]. In such conditions, the activation of catabolic programs is capable of maintaining NADH and ATP generation in most of the organs through numerous alternative pathways, namely the oxidation of fatty and amino acids, and the utilization of ketone bodies [8]. AMPK is thought to be involved in metformin mechanism of action. In metabolic syndrome, activation of AMPK has been observed to be a promising strategy to reduce the cellular levels of non-esterified fatty acids, which are known to induce insulin resistance. Such mechanisms are particularly important in insulin-sensitive tissues, such as the skeletal muscle, the liver, and the adipose tissue. Moreover, AMPK activation is known to upregulate GLUT4 translocation to cell membrane, which further improve glycaemic control [47]
During physiological fasting, the hepatocyte coordinates peripheral responses to changes in nutrient fluxes and provides glucose through glycogenolysis and gluconeogenesis and ketone bodies as fuel for peripheral organs [32]. Fatty acids represent an alternative fuel source for the most metabolically active organs including the muscle and liver (the brain consumes mostly glucose and ketone bodies) during fasting periods [73].
Interestingly, insulin resistance is associated with reduced AMPK activity [32], extending the time needed for this metabolic shift. Thus, patients with diabetes may take longer to have this metabolic shift and to use fatty acids as energy source, turning energy expenditure more difficult [25].
Bases and types of intermittent fasting
From an evolutionary perspective, fasting is a natural phenomenon to which humans were regularly exposed, due to periodic restriction or abstention from calorie-containing foods or drinks. It has been associated with a beneficial role for increased human health and longevity [22, 23, 25, 54]. However, sustaining daily caloric restriction is very difficult. Although IF is an umbrella term used to describe different forms of fasting, it is essentially a dietary regimen that includes longer periods of intense energy restriction than the usual overnight fast (75–100% reduced caloric intake on fasting days), followed by ‘normal’ eating on non-fasting days [35]. Different protocols have been used to study the effects of IF, namely periodic fasting (PF, fasting only 1 to 2 days per week), alternate-day fasting (ADF, consuming no calories on fasting days), alternate-day modified fasting (ADMF, consuming less than 25% of baseline energy needs on fasting days), and time-restricted fasting (TRF, restricting food intake to specific time periods of the day), being non-caloric fluid intake (water, coffee and tea) permitted in all of them[13, 28]. The pattern of energy restriction and/or timing of food intake is altered, so that individuals undergo frequent and repeated periods of fasting [3, 23]. On eating days, patients are encouraged to eat a diet low in refined carbohydrates, which decreases blood glucose and insulin secretion, and an increased protein intake may or may not be recommended to help increase satiety [4, 23]. Caloric intake during non-restricted times can range from ad libitum, hypocaloric (15–30% of energy restriction), normocaloric, or hypercaloric (approximately 125% of energy requirements) [51]. From all the distinct types of IF, intermittent modified fasting comprised shorter periods of energy restriction coupled with longer periods of habitual energy intake may be more sustainable and promote better adherence than continuous daily energy restriction (Fig. 1) [27].
Types of IF that can be followed using different regimens. IF has been shown to regulate mitochondrial function, redox balance, stress response, autophagy, and circadian rhythms, which have been associated with positive impacts in metabolic disorders. IF has been shown to reduce adiposity, improve adipokine secretion, reduce insulin resistance, and improve beta-cell viability. However, IF may have the opposite effects in lean models or in more severe protocols. Also, IF apparently more efficient in reducing weight and preventing type 2 diabetes risk factors, but may account for risks in patients already with diabetes
Caloric restriction
In caloric restriction (CR), fast includes a daily 15 to 40% reduction of caloric intake without malnutrition [22]. Protocols of CR may include a reduction in the amount of daily consumed calories, provided in discrete meals, or alternating days of food consumption ad libitum (AL) with or without any food intake [49]. Recent evidence indicates that the benefits of CR may not be solely related to a reduction in calories. The period of food consumption (either feeding duration or circadian time) may also be a determinant for its impact on metabolic health, independently of total caloric intake and quality of calories [16, 22].
Time-restricted feeding
In time-restricted feeding (TRF), there is a restriction of daily food consumption to a 4- to 12-h window, followed by fasting for the remaining period [3, 22, 35]. Average fasting periods are usually 16 h and feeding periods occur during the other 8 h, generally early in the day after waking up [28, 34, 67]. Unlike most of the IF protocols, it can be practiced either with or without reducing calorie intake. Thus, it allows ad libitum energy intake of normal diet and do not limit food intake or alter dietary composition [34, 68].
Periodic fasting and alternate-day fasting
Periodic fasting and alternate-day fasting involve at least a ‘fast day’ per week, in which individuals consume 0–25% of baseline energy needs during a 24-h period, alternated with days where subjects are permitted to eat food AL [16, 69, 74]. This type of regimen may vary from 1 to 4 days of fasting per week on alternate days of feeding. One of the most common regimens is the twice-weekly fasting, where participants are required to fast 1 or 2 days per week, and allows AL food consumption for 5–6 days per week. The degree of fasting in ADF varies according to the specific protocol, with some of them suggesting no food intake on fast days [8, 16]. When a 500–600-cal meal is consumed on the fasting day, it shall be called a periodic fasting [32].
Other regimens
Ramadan is an alternative type of IF with religious motivations, which involves no CR [34]. The Islamic ritual of fasting during the month of Ramadan is a form of TRF, where food consumption is only permitted during the night time [69]. The subjects refrain from eating and drinking during the daytime, whereas food consumption is allowed in the night time [34]. In opposition to other types of IF, the hour of fasting cannot be chosen by the person, but liquids are allowed, thus avoiding dehydration risk [51].
Mechanisms involved in IF-induced modification of metabolic function
Mitochondrial function, redox state, and adaptive cellular responses to stress
Mitochondrial dysfunction and impaired redox function are central for both insulin resistance and diabetic complications, but the mechanisms are still not completely understood [29]. Excessive glucose flux increases the activity of the mitochondrial electron transport chain, leading to reactive oxygen species (ROS) overproduction [29]. ROS overproduction is a common mechanism of all the four metabolic pathways that have been implicated in the deleterious effects of hyperglycaemia (increased activity in the polyol pathway, increased production of advanced glycation end-product (AGE) precursors, protein kinase-C activation, and increased hexosamine pathway activity) [24, 29]. Although reducing mitochondrial electron transport chain activity may be beneficial in hyperglycaemia, it could be deleterious when chronically maintained [29]. A IF protocol of ADF (every-other-day fasting) during 1 month was shown to reduce mitochondrial respiratory control and ketogenesis in the rat’s liver, but not in the brain, showing a tissue-specific effect towards energy saving [63]. On the other hand, another ADF protocol also during 1 month in young control rats showed increased hepatic mitochondrial respiratory chain capacity, but higher carbonyl levels, similar to what was observed in calorie-restricted Wistar rats [61, 63]. The authors also reported increased oxidative damage in the brain of the same rats, which is in accordance with previous findings in calorie-restricted rats [11]. Nevertheless, such results were obtained in young normal models and thus the effects may not represent what would be expected in a therapeutic perspective. Baylyak et al. (2021) have shown that normal aging is associated with a decline in brain mitochondrial respiration and antioxidant enzyme levels. In such aged models, every-other-day fasting reduced oxidative stress markers and increased antioxidant enzyme levels, having however a modest effect in improving respiratory control [6]. Moderate dietary restriction was similarly observed to improve redox state and oxidative stress markers in obese patients and in the pancreas of diabetic mice [33, 75]. Such mechanisms may be associated with the increased synaptic plasticity and long-term potentiation, key events in learning and memory, observed after moderate caloric restriction in middle-aged rats [6]. Thus, current data suggest that the effects of IF in brain protection depends on the age, the type of model/patient, and the level of energy restriction of the IF protocol. In the liver, IF seems to increase metabolic activity, which was observed to be enhanced in combination with exercise [59].
Reduced oxidative damage and inflammation and improved cellular protection after IF in animal models have been associated with the activation of adaptive cellular responses [51]. IF quickly increases AMP/ATP ratio, stimulating the mitochondrial energy sensor AMPK [29]. Reduced nicotinamide adenosine dinucleotide (NAD) is also subsequent to intracellular energy deficit, activating sirtuin-1 [29]. Sirt1 has been excluded from having any involvement in IF-induced modulation of cellular metabolism, while Sirt3 was observed to mediate the effects of IF in synaptic plasticity [7, 42]. However, a recent study revealed increased Sirt1 levels and activity in the liver and retinal endothelial cells of diabetic db/db mice, being related to fasting-induced improvement of retinal microvascular lesions [30]. Both AMPK and sirtuin pathways are known to increase autophagy and PGC-1α activation, leading to a better protection to mitochondrial stress and a switch from an anabolic to a catabolic activity [29]. Glucose, lipid, and protein synthesis are inhibited, whereas fatty acid oxidation and glucose uptake are stimulated in order to restore energy balance [29]. Interestingly, Liu et al. (2020) have shown that IF with 70% caloric reduction strongly decreases long-term fatty acid levels, but induces a higher elevation of their levels and insulin resistance in the fasting days [40].
Modulation of autophagy
IF has also been shown to stimulate nutrient recycling through increased autophagic function, thereby preserving organelle quality (Fig. 2) [35, 41, 65]. Such activation is impaired by insulin resistance and high-fat diet-induced metabolic syndrome [41, 65]. Papamichou et al. have shown that IF enhance autophagy, while reducing AGE levels, increasing adiponectin levels, and improving metabolic parameters in individuals without diabetes [52]. Autophagy also promotes beta-cell neogenesis during IF, since lysosomes were observed to be required for fasting-induced insulin granule degradation and beta-cell homeostasis. Moreover, IF-induced autophagy was shown to inhibit delta-notch signalling in beta-cells, which also promotes neogenesis [18]. Similar findings were made by Liu et al. (2017), showing that lysosomal activity is necessary for IF-induced Neurog-3 expression and beta-cell rescuing in high-fat diet-fed rats [41]. Such evidences indicate that stimulation of lysosome function is an integrative mechanism for achieving homeostasis in beta-cells during fasting through better organelle quality control [41].
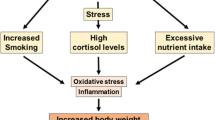
Mechanisms of IF-induced metabolic benefits in patients with overweight/obesity and metabolic syndrome. Regulation of circadian rhythms through central and peripheral clock genes may be involved in the regulation of appetite and energy expenditure. On the other hand, IF favours lipolysis and fatty acid oxidation in mitochondria, which leads to increased expression of stress and antioxidant enzymes as well as autophagy. However, long fasting periods may further increase lipolysis and lead to impaired redox balance in the mitochondria, leading to increased circulating fatty acid levels and redox imbalance. Such mechanisms may aggravate the metabolic status, especially in patients with diabetes, which are also more susceptible to IF-induced hypoglycaemia during longer fasting periods
Modulation of circadian rhythms
Over the past few years, there has been a growing interest in the concept of chrononutrition, i.e. the interaction between our circadian system and the timing of meal consumption [3]. The synchronization between the time of fasting/feeding and the circadian rhythm apparently involves the molecular mechanisms responsible for regulating meal patterns on metabolic health [22]. This perpetual self-sustained rhythm in several physiological functions leads to the expression of clock-controlled genes and molecular ‘clocks’, such as the anterior hypothalamus. Some of such clock genesregulate enzymes/enzymatic systems and metabolic regulatory factors [3, 22]. Peripheral clocks are located in several tissues vital to glucose and lipid metabolism, such as the liver, pancreas, skeletal muscle, and adipose tissue [3]. Although light is the master regulator of the central clock, peripheral clocks are responsive to other factors like food availability. Thus, temporal food restriction can reset clock gene rhythms [66]. Such rhythms permit the coordination between environmental changes (daily light–dark cycle and cyclical food availability) with the regulation of endogenous processes, such as energy balance [3]. Indeed, the perturbation of the circadian rhythm is now considered a significant contributor to the dysregulation of energy expenditure [39]. Consumption of food misaligned with the phases of the endogenous circadian cycle has been suggested as an important contributor to obesity and associated metabolic diseases [3]. Accordingly, it has been shown that the benefits of TRF beyond energy restriction may encompass a change of the endogenous circadian cycle (Fig. 2) [3]. The associated health benefits and loss of fat mass were shown to be more associated with changes of hepatic Sirt1, PPARalpha, and SREBPC1c levels in different periods of the day [60, 76]. Overnight fasting leads to a nocturnal rise in plasma free fatty acids, ghrelin, and growth hormone, as well as hepatic gluconeogenesis [65]. Interestingly, subjects submitted to diurnal fasting during Ramadan showed the opposite, namely decreased plasma ghrelin, leptin, and melatonin levels after 28 days [2]. Ajabnoor et al. suggested that loss of the circadian rhythm of cortisol in shift-workers is associated with alterations in core circadian clock [1]. Disruptions of the circadian rhythm in humans were shown to decrease insulin sensitivity and it has been shown that individuals with a misalignment of eating behaviour and sleep/wake-cycle had an increased risk to develop metabolic disorders [1, 17]. In animal models of diet-induced obesity, ADF was shown to regulate hypothalamic pathways of appetite, namely POMC and NPY, which are known targets of ghrelin and other gut hormones, and strongly regulators of feeding behaviour and circadian clocks [26, 64]. Increased hypothalamic NPY levels have been consistently observed in such models, suggesting that IF increases the sensitivity to food intake and nutrient-sensing efficiency.
Benefits and risks for metabolic syndrome and type 2 diabetes
T2DM prevalence is rising worldwide and associated with lifestyle changes [5, 41, 43]. It is characterized by hyperglycaemia as a consequence of insulin resistance and inability of pancreatic beta-cells to secrete adequate amounts of insulin [5]. Insulin resistance is driven by overnutrition and physical inactivity and promotes a compensatory effect of islet cells to increase insulin secretion, which ultimately leads to beta-cell exhaustion and hyperglycaemia. Chronic hyperglycaemia sustainedly progresses to T2DM, due to decreased beta-cell function and mass [44].
Since increased dietary intake and energetic imbalance are known to be strong risk factors for insulin resistance and T2DM, it seems obvious that restrictive interventions, such as a limited energy intake, are promising approaches for improving insulin sensitivity and reducing T2DM prevalence [5]. Although calorie restriction or complete fasting may work in the short-term, their long-term clinical implementation is difficult to sustain because of hunger and irritability [14, 22]. A growing number of studies with IF in humans show that it can be a potential strategy to improve metabolic parameters in obese and overweight subjects, namely in reducing total fat mass, also protecting against the adverse metabolic derangement of diabetes [20]. Several studies have shown that IF influence a number of biomarkers such as insulin, cytokines, and the adipokines leptin and adiponectin, although longer IF periods may have contradictory effects [48].
In a recent study, IF prevented diabetes and reduced fat percentage in rodent models of high-fat diet-induced obesity [44]. However, despite rats maintained on an ADF diet for 1 month had improved glucose tolerance, but it was observed to be impaired after 8 months. After such long period on ADF, a lower body weight than rats fed ad libitum was maintained, and the mechanism responsible for the apparent glucose intolerance was not described [19]. Similar findings were observed in another study using an ADF regimen during 12 weeks in Wistar rats, causing hyperinsulinemia and more fat mass [50]. Park et al. in 2016 tested the hypothesis that the type of diet can determine distinct adaptations of lipids and glucose metabolism in response to IF. They used two groups of rats subjected to IF with one meal a day, a group with a high-fat diet and another one with a high-protein diet [53]. They observed that ADF significantly increased total and LDL cholesterol concentrations regardless of diet type [53]. Similar findings were also observed in human populations, namely in obese adolescents during Ramadan, which have shown a transient increase in total, LDL, and HDL cholesterol [58].
Distinct results are obtained when comparing the effects of IF in individuals with and without diabetes, even when using similar IF protocols (Fig. 1) [4]. These studies observed that such distinct effects may be related to the initial differences in body weight between the studies, the presence of hyperglycaemia, or due to yet unknown factors [4]. Further evaluation of the IF-induced mechanisms in humans is needed to fully understand the possible health benefits [28]. The most immediate risk with IF is the potential for hypoglycaemia in patients who are on antidiabetic medications, specifically insulin (both postprandial and postabsorptive) and sulfonylureas, being necessary to adjust medication and fluid intake, controlling glucose levels [28].
Reduction of adiposity/improvement of adipokines
Obesity is frequently accompanied by the development of health complications such as T2DM and non-alcoholic fatty liver disease (NAFLD), so diet modifications may have a huge impact in reducing such pathologies [5, 56]. Visceral adiposity is determined by an increased waist circumference, and is considered a major risk factor for chronic inflammatory and cardiometabolic diseases [37, 38, 47]. Besides regulating metabolic homeostasis, adipose tissue is a known major endocrine organ capable of secreting various signalling and mediator proteins [47, 56]. Adipose tissue dysfunction in obesity hampers its capacity to buffer the postprandial increase of non-esterified fatty acids (NEFA) and triacylglycerol (TG), resulting in ectopic fat deposition, lipotoxicity, and insulin resistance in non-adipose tissue organs, such as the liver, skeletal muscle, and pancreas [5, 57]. Moreover, dysfunctional adipose tissue triggers cardiometabolic diseases through the dysregulated release of adipokines and pro-inflammatory cytokines (interleukin-6 (IL-6) or interleukin-1 (IL-1)), and leptin, as well as reduction of interleukin-10 (IL-10) and adiponectin [21]. IF was shown to decrease fat mass in plenty of studies, but also to remodel adipose tissue metabolism in rodent models of high-fat diet-induced obesity [45, 47, 77]. Increased expression of adiponectin and browning markers, such as UCP-1 and beta3-adrenoreceptor, and decreased expression of inflammatory mediators were observed in the adipose tissue of overweight mice following a ADF regimen during four weeks [45].
The main function of adiponectin is to promote fat oxidation and improve insulin sensitivity, also increasing glucose uptake by skeletal muscles [25]. Adiponectin has anti-inflammatory functions, being correlated with healthy metabolic profile and reduced in visceral obesity [47]. Leptin also promotes fat oxidation, besides its classical function of appetite suppression. Leptin levels are proportional to fat mass, but they increase in obesity, leading to leptin resistance, impaired appetite suppression, and continuous weight gain [47]. Adiponectin has been shown to be modified by IF and calorie restriction regimens, either to increase or decrease, depending on the type and amount of food consumed [25]. Gnanou et al. have shown significantly lower plasma adiponectin levels at the end of Ramadan fasting, although they have found an improvement in insulin sensitivity [25]. Such downregulation suggests that adiponectin levels may be directly related to body weight and fat mass changes in healthy adult subjects, reflecting their decrease after an IF protocol [25]. Nevertheless, a recent meta-analysis reported that energy restriction may lead to adiponectin raise, but this was not observed in all studies, suggesting that different IF regimens and energy restriction have distinct effects on adiponectin [71]. Regarding leptin, Lettieri-Barbato et al. revealed that its levels were significantly reduced after a 20% calorie reduction, accompanying a sustained loss of adipose tissue mass [38]. Pinto et al. obtained similar results, where reduced leptin levels reflected the comparable reduction in fat mass in middle-aged individuals under energy restriction [55]. Kroeger et al. also observed weight loss and decreased adipokine levels, such as leptin and IL-6, in individuals subjected to IF and associated such changes with reduced coronary artery disease [37]. In normal-weight animal models, Mattson et al. reported reduced circulating leptin and insulin levels following an ADF regimen [48, 70, 72]. Such results suggest that reduced fat mass after IF is associated with lower leptin levels, which may lead to lower leptin resistance.
The liver has the capacity to channel free fatty acids towards alternative metabolic pathways during fasting, namely the ketogenic pathway [5, 14]. Excessive and/or dysfunctional visceral adipose tissue results in a greater amount of fatty acids being delivered to the liver, promoting hepatic insulin resistance, inflammation, and hypertriglyceridemia [55]. Chung et al. found that TRF reduced the severity of hepatic steatosis and improved insulin resistance, despite the consumption of similar quantities of high-fat diet in female mice [14]. Nevertheless, the real impact of IF regimens on NAFLD is not known. Moreover, caloric restriction to animal models of high-fat or high-fructose feeding was shown to decrease hepatic lipogenesis, while increasing lipid oxidation markers, leading to lower steatosis and inflammation [44].
Thus, IF elicits tissue-specific metabolic adaptations, including adipose tissue remodelling (browning of white adipose tissue, increased brown adipose tissue thermogenesis, changes of adipokine secretion profile, improved lipid metabolism, and decreased inflammation), decreased liver fat content, and increased lean mass [46, 77].
Improvement in insulin sensitivity and glucose uptake
Insulin resistance develops as a protective ‘antioxidant’ adaptive mechanism [12]. Baumeier et al. showed that food intake restriction improves insulin sensitivity, presumably as a consequence of lower ectopic fat accumulation in the liver [5]. One of the premises of IF is that the repeated periods of fatty acid oxidation promote insulin sensitivity through the reduction of the ectopic lipid accumulation and their cytosolic intermediaries, which are triggers for insulin resistance development [62]. Indeed, in high-fat diet-induced obese mice, ADF during 4 weeks was shown to prevent insulin and leptin resistance, also reducing fasting glycaemia [64]. However, Koves et al. proposed that increased free fatty acid delivery to skeletal muscle conducts excessive and incomplete fatty acid oxidation, which triggers insulin resistance [36]. More severe IF protocols repeatedly exposes the tissues to high levels of fatty acid levels and oxidation in each fast-refeed cycle [3]. Indeed, all the studies (in both rodents and human subjects), reported impaired glucose tolerance, have used ADF protocols. Interestingly, insights into the time course of glycaemic changes were provided by studies in rodents, which found that despite an initial improvement after 4 weeks, glucose tolerance then deteriorated over time [10]. Based on these data, we can deduce that the effects of ADF protocols on glucose metabolism could lead to long-term harmful changes instead of beneficial ones. In humans, there is evidence that IF is effective in the short term (8 weeks to 6 months), but long-term studies are scarce, so the effects of this type of protocol remain poorly understood [3]. Together with the duration of the protocol, the percentage of energy restriction may also contribute to impaired glycaemic control. In a long-term study in patients with type 2 diabetes, continuous energy restriction did not show advantages in reducing weigh, fat mass and HbA1c in relation to intermittent energy restriction [9]. Also, severe energy restriction in young lean males was shown to impair postprandial glycaemic control [15].
The role of glucose is not limited to merely generating enough ATP (which is the case in conditions of glucose abundance and low stress), but, importantly, it is responsible for the regulation of redox potential. Reduced glucose availability restores the physiological cellular redox signalling and leads to health improvements in animal models and humans [12]. During IF, glucose levels and hepatic glycogen reserves decrease, which triggers gluconeogenesis, accompanied by a decrease in insulin secretion along with an increase in glucagon secretion [35]. In general, 12- to 24-h fasting results in a 20% or greater decrease in serum glucose and depletion of hepatic glycogen. Consequently, fatty acid–derived ketone bodies and free fatty acids are used as energy sources in humans [53]. In a study aimed to compare IF versus continuous energy intake in overweight women, lower fluctuations of glucose and insulin levels were observed, showing a higher switch towards activation of adipose tissue lipolysis and fatty acid oxidation [31]. In some previous studies, the effect of IF on blood glucose was not significant, and there were different findings according to the characteristics of participants [13]. A recent meta-analysis confirmed an improvement of fasting blood glucose, insulin resistance, and adipokine profile due to IF as compared with a non-fasting control group [13]. In T2DM, TRF protocols are more convenient and safer, since patients eat every day and the fasting periods are not long enough to significantly increase hypoglycaemia risk [51].
Improvement in insulin secretion
Obesity and insulin resistance are often characterized by hyperinsulinemia, whereas in type 1 diabetes mellitus (T1DM) and late-stage T2DM, an increase in apoptosis and hypoinsulinemia are observed [47, 51]. IF was shown to increase pancreatic beta-cell mass, insulin levels, and glucose-stimulated insulin secretion [41]. In high-fat diet-fed mice, IF decreases beta cell apoptosis as compared to ad libitum high-fat feeding, and stimulates markers of beta-cell regeneration [44]. Such effects have been suggested to derive from reduced glucolipotoxicity in models of IF [72]. Also, Quiclet et al. found that IF is an effective and non-invasive strategy to prevent pancreatic fat accumulation, probably reducing pancreatic lipotoxicity and improving beta-cell function [57]. Thus, several studies have shown that improved insulin sensitivity and glucose tolerance following IF regimens are related to preserved beta-cell mass and increased insulin β cell content in models of obesogenic diet-induced diabetes [20, 51].
Karras et al. showed that 5 weeks of TRF improved beta-cell responsiveness, insulin sensitivity, and appetite, irrespectively of weight loss in people with prediabetes [34]. Sutton et al. did a randomized study in men with prediabetes where they proved that restricting food intake to the middle of the day (TRF) reduced fasting glycaemia, insulinemia, and insulin resistance [67]. However, restricting food intake to the late afternoon or evening either produced mostly null results or worsened postprandial glucose levels and beta-cell responsiveness [67]. Altogether, available data suggest that IF may be more effective at improving insulin sensitivity and reducing insulin levels than at lowering glucose levels. More, beta-cell responsiveness varies according to the time TRF is applied. Nevertheless, assessing beta-cell function in humans is not easy and most of the studies in this topic have been performed in rodent models.
Challenges and future challenges
The benefits of fasting may be mediated by highly conserved nutrient-sensing pathways that control circadian rhythms of appetite and energy expenditure, and stress-response pathways that upregulate cell defences like antioxidant enzymes. During extended periods of food unavailability, organismal metabolism switches from storage to mobilization through production and utilization of fatty acid–derived ketones that serve to preserve the brain and muscle function, as well as by increasing nutrient recycling at the cellular level through autophagy. Taking into account the increasing prevalence of T2DM, it is of particular importance to develop preventive strategies. Several studies have demonstrated that IF improves insulin sensitivity and increases log-term glycaemic control in T2DM. However, some contradictory results regarding the duration of the interventions and the anterior metabolic status of the participants suggest that more studies are needed and of longer duration in order to draw conclusions. There is a growing evidence demonstrating the benefits of IF over the short-to-medium term on adiposity, insulin sensitivity, and glucose and lipid homeostasis. However, long-term studies are also needed, which may yet uncover the potential for adverse health consequences. Of relevance is also the type of protocol used, since TRF or moderate energy restriction has shown better results than more severe protocols, which have been commonly associated with increased oxidative stress and fasting glycaemia. This is particularly observed in lean models, where IF use is questionable. Thus, the available evidences suggest that moderate IF regimens may be useful in conditions of obesity/overweight in order to prevent associated pathologies like type 2 diabetes, but the use of severe protocols may produce the opposite results.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Ajabnoor GMA, Bahijri S, Shaik NA et al (2017) Ramadan fasting in Saudi Arabia is associated with altered expression of CLOCK, DUSP and IL-1alpha genes, as well as changes in cardiometabolic risk factors. PLoS ONE 12:1–12. https://doi.org/10.1371/journal.pone.0174342
Al-Rawi N, Madkour M, Jahrami H et al (2020) Effect of diurnal intermittent fasting during Ramadan on ghrelin, leptin, melatonin, and cortisol levels among overweight and obese subjects: a prospective observational study. PLoS ONE 15:1–15. https://doi.org/10.1371/journal.pone.0237922
Antoni R, Johnston KL, Collins AL, Robertson MD (2017) Effects of intermittent fasting on glucose and lipid metabolism. Proc Nutr Soc 76(3):361–368. https://doi.org/10.1017/S0029665116002986
Arnason T, Bowen M, Mnasell K (2017) Effects of intermittent fasting on health markers in those with type 2 diabetes: a pilot study. World J Diabetes 8(4):154–164
Baumeier C, Kaiser D, Heeren J et al (2015) Caloric restriction and intermittent fasting alter hepatic lipid droplet proteome and diacylglycerol species and prevent diabetes in NZO mice. Biochim Biophys Acta - Mol Cell Biol Lipids 1851:566–576. https://doi.org/10.1016/j.bbalip.2015.01.013
Bayliak MM, Sorochynska OM, Kuzniak OV et al (2021) Middle age as a turning point in mouse cerebral cortex energy and redox metabolism: modulation by every-other-day fasting. Exp Gerontol 145:111182. https://doi.org/10.1016/j.exger.2020.111182
Boutant M, Kulkarni SS, Joffraud M et al (2016) SIRT1 gain of function does not mimic or enhance the adaptations to intermittent fasting. Cell Rep 14:2068–2075. https://doi.org/10.1016/j.celrep.2016.02.007
Carling D (2017) AMPK signalling in health and disease. Curr Opin cel Biol 45:31–37
Carter S, Clifton PM, Keogh JB (2018) Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw open 1(3):e180756. https://doi.org/10.1001/jamanetworkopen.2018.0756
Cerqueira FM, Cunha FM, Caldeira CC et al (2011) Long-term intermittent feeding, but not caloric restriction, leads to redox imbalance, insulin receptor nitration, and glucose intolerance. Free Radic Biol Med 51:1454–1460. https://doi.org/10.1016/j.freeradbiomed.2011.07.006
Chausse B, Vieira-Lara MA, Sanchez AB et al (2015) Intermittent fasting results in tissue-specific changes in bioenergetics and redox state. PLoS ONE 10:1–13. https://doi.org/10.1371/journal.pone.0120413
Cherkas A, Holota S, Mdzinarashvili T et al (2020) Glucose as a major antioxidant: when, what for and why it fails? Antioxidants 9:1–20. https://doi.org/10.3390/antiox9020140
Cho Y, Hong N, Kim K et al (2019) The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: a systematic review and meta-analysis. J Clin Med 8(10):1645. https://doi.org/10.3390/jcm8101645
Chung H, Chou W, Sears D et al (2016) Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism 65(12):1743–1754. https://doi.org/10.1016/j.metabol.2016.09.006
Clayton DJ, Biddle J, Maher T et al (2018) 24-H severe energy restriction impairs postprandial glycaemic control in young, lean males. Br J Nutr 120:1107–1116. https://doi.org/10.1017/S0007114518002568
Crupi AN, Haase J, Brandhorst S, Longo VD (2020) Periodic and intermittent fasting in diabetes and cardiovascular disease. Springer Sci Media 20(12):83
Dedual M, Wueest S, Borsigova M, Daniel K (2019) Intermittent fasting improves metabolic flexibility in short-term high-fat diet-fed mice. Am J Physiol 317(5):E773–E782
Dinicolantonio JJ, Mccarty M (2019) Autophagy-induced degradation of Notch1, achieved through intermittent fasting, may promote beta cell neogenesis: implications for reversal of type 2 diabetes. Open Hear 6:1–10. https://doi.org/10.1136/openhrt-2019-001028
Dorighello GG, Rovani JC, Luhman CJF et al (2013) Food restriction by intermittent fasting induces diabetes and obesity and aggravates spontaneous atherosclerosis development in hypercholesterolaemic mice. Br J Nutr 111(6):979–986. https://doi.org/10.1017/S0007114513003383
Faris MAI, Jahrami H, BaHammam A et al (2020) A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during Ramadan on glucometabolic markers in healthy subjects. Diabetes Res Clin Pract 165:1–76. https://doi.org/10.1016/j.diabres.2020.108226
Faris MAIE, Madkour MI, Obaideen AK et al (2019) Effect of Ramadan diurnal fasting on visceral adiposity and serum adipokines in overweight and obese individuals. Diabetes Res Clin Pract 153:166–175. https://doi.org/10.1016/j.diabres.2019.05.023
Di FA, Di GC, Bernier M, De CR (2018) A time to fast Diet Heal 775:770–775
Furmli S, Elmasry R, Ramos M, Fung J (2018) Therapeutic use of intermittent fasting for people with type 2 diabetes as an alternative to insulin. BMJ Case Rep 2018:bcr-2017-221854. https://doi.org/10.1136/bcr-2017-221854
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070. https://doi.org/10.1161/CIRCRESAHA.110.223545
Gnanou JV, Caszo BA, Khalil KM et al (2015) Effects of Ramadan fasting on glucose homeostasis and adiponectin levels in healthy adult males. J Diabetes Metab Disord 14:55. https://doi.org/10.1186/s40200-015-0183-9
Gotthardt JD, Verpeut JL, Yeomans BL et al (2016) Intermittent fasting promotes fat loss with lean mass retention, increased hypothalamic norepinephrine content, and increased neuropeptide Y gene expression in diet-induced obese male mice. Endocrinology 157:679–691. https://doi.org/10.1210/en.2015-1622
Gow ML, Garnett SP, Baur LA, Lister NB (2016) The effectiveness of different diet strategies to reduce type 2 diabetes risk in youth. Nutrients 8:1–13. https://doi.org/10.3390/nu8080486
Grajower MM, Horne BD (2019) Clinical management of intermittent fasting in patients with diabetes mellitus. Nutrients 11(4):873
Hallan S, Sharma K (2016) The role of mitochondria in diabetic kidney disease. Curr Diab Rep 16:1–9. https://doi.org/10.1007/s11892-016-0748-0
Hammer SS, Vieira CP, McFarland D et al (2021) Fasting and fasting-mimicking treatment activate SIRT1/LXRα and alleviate diabetes-induced systemic and microvascular dysfunction. Diabetologia 64:1674–1689. https://doi.org/10.1007/s00125-021-05431-5
Hutchison AT, Liu B, Wood RE et al (2019) Effects of intermittent versus continuous energy intakes on insulin sensitivity and metabolic risk in women with overweight. Obesity 27:50–58. https://doi.org/10.1002/oby.22345
Jeon SM (2016) Regulation and function of AMPK in physiology and diseases. Exp Mol Med 48:e245. https://doi.org/10.1038/emm.2016.81
Kanda Y, Hashiramoto M, Shimoda M et al (2015) Dietary restriction preserves the mass and function of pancreatic β cells via cell kinetic regulation and suppression of oxidative/ER stress in diabetic mice. J Nutr Biochem 26:219–226. https://doi.org/10.1016/j.jnutbio.2014.10.007
Karras SN, Koufakis T, Adamidou L et al (2020) Effects of orthodox religious fasting versus combined energy and time restricted eating on body weight, lipid concentrations and glycaemic profile. Int J Food Sci Nutr 72:1–11. https://doi.org/10.1080/09637486.2020.1760218
Khedkar PH (2020) Intermittent fasting—the new lifestyle? Acta Physiol 229(4):e13518. https://doi.org/10.1111/apha.13518
Koves TR, Ussher JR, Noland RC et al (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7:45–56. https://doi.org/10.1016/j.cmet.2007.10.013
Kroeger CM, Klempel MC, Bhutani S et al (2012) Improvement in coronary heart disease risk factors during an intermittent fasting/calorie restriction regimen: relationship to adipokine modulations. Nutr Metab 9:1–8. https://doi.org/10.1186/1743-7075-9-98
Lettieri-Barbato D, Giovannetti E, Aquilano K (2016) Effects of dietary restriction on adipose mass and biomarkers of healthy aging in human. Aging (Albany NY) 8:3341–3355. https://doi.org/10.18632/aging.101122
Li G, Xie C, Lu S et al (2018) Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab 26:672–685. https://doi.org/10.1016/j.cmet.2017.08.019.Intermittent
Liu B, Hutchison A, Thompson C et al (2020) Effects of intermittent fasting or calorie restriction on markers of lipid metabolism in human skeletal muscle. J Clin Endocrinol Metab 06(3):e1389–e1399
Liu H, Javaheri A, Godar RJ et al (2017) Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy 13:1952–1968. https://doi.org/10.1080/15548627.2017.1368596
Liu Y, Cheng A, Li YJ et al (2019) SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat Commun 10:1–11. https://doi.org/10.1038/s41467-019-09897-1
Liu Z, Dai X, Zhang H et al (2020) Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun 11:1–14. https://doi.org/10.1038/s41467-020-14676-4
de Souza Marinho T, Borges CC, Aguila MB, Mandarim-de-Lacerda CA (2020) Intermittent fasting benefits on alpha- and beta-cell arrangement in diet-induced obese mice pancreatic islet. J Diabetes Complications 34:107497. https://doi.org/10.1016/j.jdiacomp.2019.107497
de Souza Marinho T, Ornellas F, Aguila MB, Mandarim-de-Lacerda CA (2020) Browning of the subcutaneous adipocytes in diet-induced obese mouse submitted to intermittent fasting. Mol Cell Endocrinol 513:1–9. https://doi.org/10.1016/j.mce.2020.110872
de Souza Marinho T, Ornellas F, Barbosa-da-Silva S et al (2019) Beneficial effects of intermittent fasting on steatosis and inflammation of the liver in mice fed a high-fat or a high-fructose diet. Nutrition 65:103–112. https://doi.org/10.1016/j.nut.2019.02.020
Matafome P, Seiça R (2017) Function and dysfunction of adipose tissue. Adv Neurobiol 19:3–31. https://doi.org/10.1007/978-3-319-63260-5_1
Mattson M, Longo V, Harvie M (2017) Impact of intermittent fasting on health and disease processes. Ageing Res Rev 76:139–148. https://doi.org/10.1016/j.physbeh.2017.03.040
Mitchell SJ, Bernier M, Mattison JA et al (2019) Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab 29:221–228. https://doi.org/10.1016/j.cmet.2018.08.011.Daily
Munhoz AC, Vilas-boas EA, Panveloski-costa AC et al (2020) Intermittent fasting for twelve weeks leads to increases in fat mass and hyperinsulinemia in young female Wistar rats. Nutrients 12(4):1029
Muñoz-Hernández L, Márquez-López Z, Mehta R, Aguilar-Salinas CA (2020) Intermittent fasting as part of the management for T2DM: from animal models to human clinical studies. Curr Diab Rep 20:1–10. https://doi.org/10.1007/s11892-020-1295-2
Papamichou D, Panagiotakos DB, Itsiopoulos C (2019) Dietary patterns and management of type 2 diabetes: a systematic review of randomised clinical trials. Nutr Metab Cardiovasc Dis 29:531–543. https://doi.org/10.1016/j.numecd.2019.02.004
Park S, Yoo KM, Hyun JS, Kang S (2017) Intermittent fasting reduces body fat but exacerbates hepatic insulin resistance in young rats regardless of high protein and fat diets. J Nutr Biochem 40:14–22. https://doi.org/10.1016/j.jnutbio.2016.10.003
Persynaki A, Karras S, Pichard C (2017) Unraveling the metabolic health benefits of fasting related to religious beliefs: a narrative review. Nutrition 35:14–20. https://doi.org/10.1016/j.nut.2016.10.005
Pinto AM, Bordoli C, Buckner LP et al (2019) Intermittent energy restriction is comparable to continuous energy restriction for cardiometabolic health in adults with central obesity : A randomized controlled trial; the Met-IER study. Clin Nutr 39(6):1753–1763. https://doi.org/10.1016/j.clnu.2019.07.014
Qiao Q, Bouwman FG, Van Baak MA et al (2019) Glucose restriction plus refeeding in vitro induce changes of the human adipocyte secretome with an impact on complement factors and cathepsins. Int J Mol Sci 20:1–17. https://doi.org/10.3390/ijms20164055
Quiclet C, Dittberner N, Gässler A et al (2019) Pancreatic adipocytes mediate hypersecretion of insulin in diabetes-susceptible mice. Metabolism 97:9–17. https://doi.org/10.1016/j.metabol.2019.05.005
Radhakishun N, Blokhuis C, Van Vliet M et al (2014) Intermittent fasting during Ramadan causes a transient increase in total, LDL, and HDL cholesterols and hs-CRP in ethnic obese adolescents. Eur J Pediatr 173:1103–1106. https://doi.org/10.1007/s00431-014-2276-8
Real-Hohn A, Navegantes C, Ramos K et al (2018) The synergism of high-intensity intermittent exercise and every-other-day intermittent fasting regimen on energy metabolism adaptations includes hexokinase activity and mitochondrial efficiency. PLoS ONE 13(12):e0202784. https://doi.org/10.1371/journal.pone.0202784
Réda A, Wassil M, Mériem M et al (2020) Food timing, circadian rhythm and chrononutrition: a systematic review of time-restricted eating’s effects on human health. Nutrients 12:1–15. https://doi.org/10.3390/nu12123770
Rodrigues L, Crisóstomo J, Matafome P et al (2011) Dietary restriction improves systemic and muscular oxidative stress in type 2 diabetic Goto-Kakizaki rats. J Physiol Biochem 67:613–619. https://doi.org/10.1007/s13105-011-0108-0
Shulman G (2000) Cellular mechanisms of insulin resistance. J Clin Invest 106:171–176. https://doi.org/10.1111/j.1464-5491.2005.01566.x
Sorochynska OM, Bayliak MM, Gospodaryov DV et al (2019) Every-other-day feeding decreases glycolytic and mitochondrial energy-producing potentials in the brain and liver of young mice. Front Physiol 10:1–15. https://doi.org/10.3389/fphys.2019.01432
Spezani R, da Silva RR, Martins FF et al (2020) Intermittent fasting, adipokines, insulin sensitivity, and hypothalamic neuropeptides in a dietary overload with high-fat or high-fructose diet in mice. J Nutr Biochem 83:108419. https://doi.org/10.1016/j.jnutbio.2020.108419
Stockman MC, Thomas D, Burke J, Apovian CM (2018) Intermittent fasting: is the wait worth the weight? Curr Obes Rep 7:172–185. https://doi.org/10.1007/s13679-018-0308-9
St-Onge MP, Ard J, Baskin ML et al (2017) Meal timing and frequency: implications for cardiovascular disease prevention. Circulation 135:e96–e121. https://doi.org/10.1161/CIR.0000000000000476
Sutton E, Beyl R, Early K et al (2019) Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 27:1212–1221. https://doi.org/10.1016/j.cmet.2018.04.010.Early
Theron G, Peter J, Van Z-S et al (2018) Time-restricted feeding of a high fat diet in C57BL/6 male mice reduces adiposity, but does not protect against increased systemic inflammation. Appl Physiol Nutr Metab 184(1):132–140. https://doi.org/10.1164/rccm.201101-0056OC
Varady KA (2016) Impact of intermittent fasting on glucose homeostasis. Curr Opin Clin Nutr Metab Care 19(4):300–302. https://doi.org/10.1097/MCO.0000000000000291
Varady KA, Allister CA, Roohk DJ, Hellerstein MK (2010) Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus calorie restriction. J Nutr Biochem 21:188–195. https://doi.org/10.1016/j.jnutbio.2008.11.001
VarkanehKord H, Tinsley GM, Santos HO et al (2020) The influence of fasting and energy-restricted diets on leptin and adiponectin levels in humans: a systematic review and meta-analysis. Clin Nutr 40(4):1811–1821. https://doi.org/10.1016/j.clnu.2020.10.034
Wan R, Ahmet I, Brown M et al (2010) Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem 21:413–417. https://doi.org/10.1016/j.jnutbio.2009.01.020
Wang P, Zhang RY, Song J et al (2012) Loss of AMP-activated protein kinase-α2 impairs the insulin-sensitizing effect of calorie restriction in skeletal muscle. Diabetes 61:1051–1061. https://doi.org/10.2337/db11-1180
Wilson RA, Deasy W, Stathis CG et al (2018) Intermittent fasting with or without exercise prevents weight gain and improves lipids in diet-induced obese mice. Nutrients 10:1–15. https://doi.org/10.3390/nu10030346
Wycherley TP, Brinkworth GD, Noakes M et al (2008) Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes Metab 10:1062–1073. https://doi.org/10.1111/j.1463-1326.2008.00863.x
Ye Y, Xu H, Xie Z et al (2020) Time-restricted feeding reduces the detrimental effects of a high-fat diet, possibly by modulating the circadian rhythm of hepatic lipid metabolism and gut microbiota. Front Nutr 7:596285. https://doi.org/10.3389/fnut.2020.596285
Zhu S, Surampudi P, Rosharavan B, Chondronikola M (2020) Intermittent fasting as a nutrition approach against obesity and metabolic disease. Curr Opin Clin Nutr Metab Care 23:387–394. https://doi.org/10.1097/MCO.0000000000000694
Acknowledgements
The authors are grateful to Coimbra Health School (ESTeSC) for the equipment used.
Funding
This work was supported by the Portuguese Science and Technology Foundation (FCT): Strategic Project UIDB/04539/2020 (CIBB).
Author information
Authors and Affiliations
Contributions
Lisandra Joaquim was responsible for literature search and for the first draft. Ana Faria and Helena Loureiro were involved in manuscript reading and correction, while Paulo Matafome was responsible for the last version of the manuscript and is the senior author. ‘The authors declare that all data were generated in-house and that no paper mill was used’.
Corresponding author
Ethics declarations
Research involving human participants and/or animals
No.
Informed consent
Non-applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
- IF is an emergent nutritional strategy for improving multiple features of cardiometabolic and age-related pathologies, due to weight and insulin resistance reduction.
- IF upregulates evolution-conserved adaptive cellular responses, such as stress-response pathways, autophagy, and mitochondrial function
- IF regulates the circadian rhythms of hormones like insulin or leptin, among others.
- Too long interventions, severe energy restriction, and the anterior metabolic status of the participants may conduce to the opposite effects.
Rights and permissions
About this article
Cite this article
Joaquim, L., Faria, A., Loureiro, H. et al. Benefits, mechanisms, and risks of intermittent fasting in metabolic syndrome and type 2 diabetes. J Physiol Biochem 78, 295–305 (2022). https://doi.org/10.1007/s13105-021-00839-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-021-00839-4