Abstract
Adipose tissue is an endocrine organ which is responsible for postprandial uptake of glucose and fatty acids, consequently producing a broad range of adipokines controlling several physiological functions like appetite, insulin sensitivity and secretion, immunity, coagulation, and vascular tone, among others. Many aspects of adipose tissue pathophysiology in metabolic diseases have been described in the last years. Recent data suggest two main factors for adipose tissue dysfunction: accumulation of nonesterified fatty acids and their secondary products and hypoxia. Both of these factors are thought to be on the basis of low-grade inflammatory activation, further increasing metabolic dysregulation in adipose tissue. In turn, inflammation is involved in the inhibition of substrate uptake, alteration of the secretory profile, stimulation of angiogenesis, and recruitment of further inflammatory cells, which creates an inflammatory feedback in the tissue and is responsible for long-term establishment of insulin resistance.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Adipose Tissue Structure
Adipose tissue is a complex and heterogeneous tissue composed by cells with lipid storage functions, called adipocytes, and a stromovascular function, composed by endothelial and mesenchymal stem cells, preadipocytes, fibroblasts, and resident cells from the immune system (Rajala and Scherer 2003; Juge-Aubry et al. 2005a; Guilherme et al. 2008b; Christiaens and Lijnen 2010). During the embryonic development, the vascular network develops before adipocytes and the extracellular matrix which supports blood vessels is the first to be deposited, showing the crucial role of the vascular system in adipose tissue development (Neels et al. 2004; Christiaens and Lijnen 2010). In fact, during the embryonic development, a close communication between the stromovascular fraction and the adipocytes results in a mutual control between angiogenesis and adipogenesis. Recent data show that adipocytes may develop from capillary networks as the progenitor cells respond to pro-angiogenic stimuli in association with the expanding capillaries (Min et al. 2016).
In adult life, a well-developed vascular network is observable at the microscope and each adipocyte is surrounded by at least one capillary (Neels et al. 2004; Rutkowski et al. 2009; Christiaens and Lijnen 2010). The capillaries of the adipose tissue are fenestrated and are rich in trans-endothelial channels, which allow a close communication with the adipocytes (Christiaens and Lijnen 2010). Moreover, even in the adult life, this vascular network is very dynamic and is continuously adapting to changing nutritional fluxes. However, the mechanisms governing such remodeling are still far from being understood. Interestingly, the dynamics of vascular remodeling apparently influence adipocyte behavior during expansion. Adipocyte hypertrophy is usually associated with the formation of aberrant capillaries, while adipocyte hyperplasia is usually associated with increased angiogenesis and development of new capillaries. Hyperplasia is considered a harmless form of adipose tissue expansion, given the formation of smaller well-irrigated adipocytes with lower inflammatory activity than hypertrophic ones (Christiaens and Lijnen 2010). When the tissue is forced to expand, the formation of local phenomena of hypoxia leads to the expression of angiogenic factors which stimulate angiogenesis, including several cytokines and adipokines. The balance between these factors determines vessel density and permeability, and thus the “good” physiological or the “bad” pathophysiological expansion of the adipose tissue. The mechanisms will be detailed in the following sections.
2 Metabolic Functions of the Adipose Tissue
Although its important endocrine functions, the primary function of the adipose tissue is to store energy in the form of lipids, mainly in intracellular triglycerides droplets, also regulating lipid catabolism in different tissues due to the actions of adipokines (Rajala and Scherer 2003; Juge-Aubry et al. 2005a; Guilherme et al. 2008b). Lipid droplets are coated by a group of proteins, which the main one is Perilipin A (Per A), which prevent the contact between the stored triglycerides and the cytoplasm. In times of energetic need, such triglycerides are quickly hydrolyzed into free fatty acids and glycerol, a process called lipolysis, and released to the blood in order to feed other organs demands (Arner 2005; Guilherme et al. 2008b; Galic et al. 2010). Thus, the adipose tissue is able to recognize the metabolic state of the organism, not only by local energetic sensors, but also through different inputs coming mainly from the gut after and between meals. Moreover, adipocytes also regulate cholesterol metabolism, as they are able to produce high-density lipoproteins (HDL) in order to send excessive cholesterol to the liver.
When adipocytes accumulate excessive amounts of nonesterified fatty acids, for reasons that are currently under investigation, their metabolism and endocrine function are shifted in order to produce a broad range of proinflammatory factors, while adiponectin secretion is decreased. Such factors include cytokines and chemokines, growth factors, tumour necrosis factor (TNF)-α, interleukin (IL)-6, monocyte chemoattractant factor (MCP)-1, vascular endothelial graowth factor (VEGF), leptin, and resistin (Wellen and Hotamisligil 2005; Tilg and Moschen 2006; Guilherme et al. 2008b). In fact, a strong relationship between metabolism and innate immunity is present at the adipose tissue. Many of the intracellular pathways involved in metabolic signaling are recruited as well during an immune response and most of the adipokines and adipose tissue-derived factors, besides regulating metabolism, also have paracrine and endocrine functions in regulating the immune response. Many authors support the existence of a metabolism—immunity axis, as any metabolic change immediately induces alterations in the immune response and the regulation of metabolic fluxes usually involves the activation of intracellular inflammatory and stress pathways (Rajala and Scherer 2003; Juge-Aubry et al. 2005a; Goossens 2008; Guilherme et al. 2008b; Rutkowski et al. 2009).
2.1 Mechanisms of Nutrient Uptake and Storage in Adipocytes
Triglycerides synthesis, a process called esterification, occurs from one molecule of glucose-derived glycerol and three fatty acyl chains. Adipocytes have a limited ability to store glycogen and thus all the glucose that is not consumed in the adipocyte metabolism is transformed into glycerol and stored in the triglycerides pool (Tilg and Moschen 2006; Goossens 2008; Guilherme et al. 2008b). Esterification is mainly stimulated by insulin, which induces tyrosine kinase activity in its receptor (Fig. 1.1). This in turn leads to the tyrosine phosphorylation and activation of the insulin receptor substrate-1 (IRS-1) and initiates a signaling pathway which involves PI3K and Akt/PKB activation. Among other actions, the activation of this signaling cascade leads to the translocation to the membrane of GLUT4-containing vesicles, allowing glucose uptake (Wellen and Hotamisligil 2005; Bugianesi et al. 2005). Besides inducing glucose uptake, insulin is also responsible for lipolysis inhibition, through the inhibition of adenylate cyclase, the main enzyme involved in AMPc synthesis. AMPc activates PKA, which in turn phosphorylates and activates hormone-sensitive lipase (HSL), the main enzyme involved in triglycerides hydrolyzation (Fig. 1.1). On the other hand, in times of nutrient demand, contra-regulatory hormones, like glucagon, cortisol, growth hormone, and adrenaline, increase AMPc levels, leading to the activation of HSL and thus the release of fatty acids into the circulation (Fig. 1.1) (Tilg and Moschen 2006; Goossens 2008; Guilherme et al. 2008b). Increased lipolysis is also observed in obese individuals due to the development of insulin resistance and the increased secretion of proinflammatory cytokines that promote lipolysis (Langin 2006; Guilherme et al. 2008b). The subsequent flux of free fatty acids from the adipose tissue to the circulation may in turn cause their ectopic accumulation in other tissues, such as the liver and the skeletal muscle, mechanism which will be described in the chapter dedicated to the pathophysiology of adipose tissue.
Mechanisms of lipid storage in adipocytes and their mobilization from lipid droplets. Lipolysis is inhibited by insulin signaling and promoted by other hormones like glucagon, GH, cortisol, T3, or adrenaline, due to the stimulation of AMPc and HSL. cAMP cyclic adenosine monophosphate, FATP fatty acid binding protein, FFA free fatty acids, HSL hormone-sensitive lipase, IRS-1 insulin receptor substract-1, LPL lipoprotein lipase, PerA perilipin A, VLDL very-low density lipoprotein
Circulating lipids are derived from hepatic incorporation into VLDL or from intestinal absorption and included in chylomicrons. Such lipoproteins bind to the CD36 receptor present at the adipocyte membrane. Lipoproteins are then hydrolyzed by the lipoprotein lipase (LPL) and the fatty acids are transported to the cytoplasm through the fatty acid transporter protein (FATP/CD36). Once at the cytoplasm the nonesterified fatty acids are captured by the protein aP2, which prevent their free circulation in the cell and the consecutive activation of inflammatory and stress pathways (Ram 2003; Wellen and Hotamisligil 2005). Fatty acids are finally esterified into triglycerides and stored in lipid droplets (Fig. 1.1).
Alternatively to esterification, fatty acids may be metabolized in several mediators of intracellular signaling pathways, namely eicosanoids, which are important activators of the peroxissome proliferation activated receptor-gamma (PPARγ). This nuclear receptor controls the events involved in lipid esterification, being also activated by the pharmacological class of tiazolidinediones (TZD) (Fig. 1.2). The genes controlled by the activation of PPARγ include proteins involved in fatty acid uptake (FATP, CD36 and LPL), metabolism (PEPCK and SCD-1), storage (perilipin A), and oxidation (Adiponectin and UCP-1). PPARγ activation also inhibits cellular inflammatory pathways, including NF-κB, which will be discussed in the chapter dedicated to adipose tissue pathophysiology (Wellen and Hotamisligil 2003; Ram 2003). Thus, PPARγ promotes insulin sensitivity due to the reduction of cytoplasmatic free fatty acids and due to the inhibition of inflammatory pathways (Ram 2003; Guilherme et al. 2008b).
Mechanisms of PPARγ activation in response to fatty acids and their metabolites, stimulating the expression of adiponectin and proteins involved in fatty acid esterification and storage. The activity of PPARγ can be improved by the pharmacological class of TZDs. FATP fatty acid binding protein, FFA free fatty acids, LPL lipoprotein lipase, PPARγ peroxisome proliferating-activated receptor γ, VLDL very-low density lipoprotein
PPARγ is expressed in adipocytes and macrophages, controlling lipid uptake, but also preadipocyte differentiation into mature adipocytes, a process called adipogenesis (Tamori et al. 2002; Lee et al. 2011). PPARγ inhibition in obesity conducts to the inhibition of adipogenesis and to the hypertrophy of the existing adipocytes. Hypertrophic adipocytes have shown to be hypoxic and metabolically dysregulated (Trayhurn et al. 2008a; Trayhurn 2014). PPARα controls the expression of genes involved in lipid oxidation and is expressed in tissues with catabolic activity like the liver and the skeletal muscle. It is regulated by adiponectin and by drugs like fibrates and metformin (indirectly), increasing the oxidation of fatty acids on mitochondria.
2.2 Cholesterol Fluxes
The adipocyte is also an important regulator of cholesterol storage and mobilization. Excessive intracellular cholesterol is incorporated into HDL/Apo-A1 particles, being transported to the liver where it used in the synthesis of biliary acids or excreted in bile (Yin et al. 2010). Two of the most important proteins in this transport are ABCA1 and ABCG1 (“ATP-Binding Membrane Cassete Transporter A1 e G1”), being involved in the transport of cholesterol, phospholipids, and other lipophilic substances to the HDL particles (Yin et al. 2010). The expression of ABC proteins is regulated by the PPAR and by the AMPc/PKA pathway, which is important in the mobilization of stored lipids (cholesterol). However, decreased PPAR activity in dysfunctional adipocytes may compromise cholesterol mobilization into the liver (Fig. 1.3) (Yin et al. 2010). Knockout models for ABCA1 have more infiltration of inflammatory cells in several tissues, due to decreased cholesterol transport to the liver and excessive deposition in the tissues. On the other hand, ABCA1 overexpression was shown to prevent cholesterol accumulation and atherosclerosis progression. Thus, ABCA1 prevents the accumulation of cholesterol in tissues, which is known to induce the formation of foam cells from recruited macrophages. The effect of ABCA1 and ABCG1 knockout is cumulative, suggesting that they have distinct roles in regulating cholesterol efflux. It is believed that ABCA1 and ABCG1 have distinct affinities for different HDL proteins (Yin et al. 2010).
Integration of the mechanisms involved in the uptake of lipids and glucose into the adipocyte, namely the role of insulin in inducing glucose uptake and in inhibiting lipolysis, as well as the role of PPARγ in promoting fatty acid esterification and cholesterol efflux to HDL particles. FATP fatty acid binding protein, FFA free fatty acids, HDL high-density lipoprotein, HSL hormone-sensitive lipase, LPL lipoprotein lipase, PerA perilipin A, PPARγ peroxisome proliferating-activated receptor γ, VLDL very-low density lipoprotein
3 Endocrine Function of the Adipose Tissue
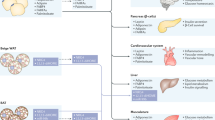
The adipose tissue secretes a broad range of factors, including adipokines, cytokines, chemokines, angiogenic factors, coagulation factors, and vasoactive factors, among others. More than 600 different factors have been identified as produced and secreted by the adipose tissue, affecting glucose and lipid metabolism, appetite, vascular function, inflammation, coagulation, or the cardiovascular function. However, the complete list of adipokines and their functions is not yet completely known (Wellen and Hotamisligil 2003; Trayhurn and Wood 2004; Guilherme et al. 2008b; Galic et al. 2010). Moreover, there are differences in the secretory profile between the different fat depots, with the visceral ones being more susceptible to nutritional signals due to their proximity to the intestine and liver.
3.1 Leptin
Leptin was the first factor originally identified as a product of adipose tissue and its discovery changed the view about this tissue, from a mere fat reserve to an endocrine organ able to control energy homeostasis. Leptin is almost exclusively produced by the adipocyte (95%) and its levels are proportional to the fat mass (Rajala and Scherer 2003; Meier and Gressner 2004; Golay and Ybarra 2005; Lorincz and Sukumar 2006; Vona-Davis and Rose 2007). Differences in leptin secretion from different fat depots were also shown, being the subcutaneous one more active (Nielsen et al. 2009). The main function of leptin is to inform the hypothalamus about nutrient availability. Leptin activates afferent nervous fibers and acts directly on the hypothalamus in order to suppress appetite and modulate energy expenditure (Fig. 1.4) (Kiess et al. 2008). Moreover, it accutely supresses insulin secretion, but increases long-term β cell survival and function. Moreover, leptin increases fatty acid uptake and oxidation in the skeletal muscle and liver, also acting as a growth factor for endothelial cells (Rajala and Scherer 2003; Meier and Gressner 2004; Bugianesi et al. 2005; Lorincz and Sukumar 2006). Although leptin levels are proportional to fat mass, its secretion may be stimulated by insulin and inhibited by increased levels of AMPc, i.e., it increases after meals and decreases in times of energetic demand (Meier and Gressner 2004). When leptin levels are lower, appetite is stimulated and thyroid hormones, thermogenesis, and immune system function are inhibited (Münzberg et al. 2005). Mutations of the leptin or leptin receptor genes are known to induce obesity. On the other hand, leptin administration leads to an increase of fatty acid oxidation and a reduction of their circulating levels. Such effects are not observed in obese patients, which are known to have a resistance to the action of the hormone (Rajala and Scherer 2003; Meier and Gressner 2004). Other functions of leptin include the stimulation of angiogenesis, the modulation of the immune response, and the expression of the key enzyme involved in estrogen synthesis, aromatase (Catalano et al. 2003, Juge-Aubry et al. 2005a, b Lorincz and Sukumar 2006).
Leptin receptor is linked to a Jak/STAT pathway, which first protein is Jak2 and leads to the activation of STAT3 and STAT5. STAT proteins are transcription factors that stimulate catabolic processes and insulin secretion in β cells (Ueki et al. 2004a, b; Laubner et al. 2005; Kaneto et al. 2010; Blüher and Mantzoros 2015) (Fig. 1.4). STAT proteins also activate SOCS proteins, involved in the negative feedback. Thus, hyperleptinemia leads not only to increased signaling but also to increased negative feedback, contributing to the leptin resistance observed in obese patients mainly in the hypothalamus (Laubner et al. 2005). SOCS proteins also inhibit insulin signaling, contributing to insulin resistance. In fact, leptin and insulin signaling have a common pathway, namely the activation of IRS-1, PI3K, and Akt (Ahima and Flier 2000; Ueki et al. 2004a; Münzberg et al. 2005; Ahima 2005; Imrie et al. 2010). Leptin also activates AMPK inducing lipid oxidation and promoting insulin sensitivity (Rajala and Scherer 2003; Meier and Gressner 2004; Ahima 2005; Juge-Aubry et al. 2005a). Moreover, leptin inhibits fatty acid synthesis, due to the suppression of the transcription factor SREBP-1c and the key proteins of the biosynthetic pathway ACC and FAS. On the other hand, such mechanisms were shown to be activated by SOCS proteins (Fig. 1.4) (Ueki et al. 2004a, b).
3.2 Adiponectin
Adiponectin is an important regulator of lipid metabolism and stimulator of its oxidation produced in the adipocyte as a consequence of the activation of PPARγ. Pharmacological activators of PPARγ, glitazones, are known to increase adiponectinemia. On the other hand, adiponectin secretion is inhibited by the activation of inflammatory pathways and cAMP (Fig. 1.5). Thus, insulin also induced adiponectin secretion by reducing cAMP levels, while its elevation in times of energy demand prevents fatty acid consume (Xu et al. 2003).
Mechanisms of adiponectin signaling in target cell, including AMPK-mediated glucose uptake and inhibition of ACC. Inhibition of ACC prevents fatty acids synthesis and prevents inhibition of fatty acid uptake by the ACC product malonyl-CoA. Activation of PPARα increases the expression of fatty acids oxidation enzymes. ACC acetyl-CoA carboxylase, Adip adiponectin, AMK AMP-activated protein kinase, COX-2 ciclooxygenas-2, PPARγ peroxisome proliferating-activated receptor γ
Adiponectin was shown to have cardioprotective effects by promoting cell viability and inhibiting apoptosis during ischemia (Ding et al. 2012; Park and Sweeney 2013; Smekal and Vaclavik 2017). As well, similar effects were demonstrated in β cells, improving insulin secretion. Adiponectin was shown to exert anti-inflammatory effects on the vessel wall preventing atherosclerosis through the inhibition of adhesion molecules expression and smooth muscle cell proliferation (Fig. 1.5) (Juge-Aubry et al. 2005a). Protective effects of adiponectin have also been reported in different pathologies like cancer and neurodegenerative diseases (Vona-Davis and Rose 2007; Pais et al. 2009; Matafome 2013; Letra et al. 2014).
Adiponectin circulates in three molecular forms (trimeric, hexametric, and high-molecular weight isoform (HMW) composed of several hexamers). Reduced levels of the HMW isoform have been specifically associated with metabolic disorders (Rajala and Scherer 2003; Vona-Davis and Rose 2007, 2009; Pais et al. 2009). The globular region of the adiponectin chain binds to two membrane receptors, AdipoR1 and AdipoR2. While AdipoR1 is found mainly in the skeletal muscle, AdipoR2 is more abundant in the liver (Meier and Gressner 2004). A third receptor, T-cadherin, was recently identified as an adiponectin receptor, but without intracellular signaling and mainly involved in the anchorage of the HMW isoform to the membrane (Takeuchi et al. 2007). Adiponectin binding to AdipoR1 and AdipoR2 leads to AMPK and PPARα activation, which in turn leads to increased uptake and oxidation of glucose and lipids (Fig. 1.5) (Kamon et al. 2003; Yamauchi et al. 2003; Lorincz and Sukumar 2006; Pais et al. 2009). Moreover, AMPK is involved in promoting glucose uptake through GLUT4 translocation to the membrane and in inhibiting gluconeogenesis (Juge-Aubry et al. 2005a; Nawrocki et al. 2006). Similar to leptin, adiponectin directly inhibits the enzymes involved in the fatty acids synthesis pathway, through the inhibition of acetyl-coA carboxylase (ACC), the key enzyme of such pathway (Fig. 1.5) (Ouchi et al. 2001; Xu et al. 2003; Nawrocki et al. 2006). On the other hand, the activation of PPARα increases the expression of the enzymes involved in lipid oxidation and uptake to the mitochondria (Fig. 1.5) (Kamon et al. 2003; Yamauchi et al. 2003; Gealekman et al. 2008, 2012).
3.3 Resistin
Resistin is an adipokine associated with the establishment of insulin resistance as its levels are increased in models of obesity and type 2 diabetes. Studying the role of human resistin is not easy as significant differences were found to murine resistin. Resistin gene was found in different chromosomes and human resistin is mainly produced in macrophages while murine resistin in produced in adipocytes (Lazar 2007). The activation of the immune system after metabolic dysregulation induces resistin secretion, in order to activate pathways which block nutrient uptake by hypertrophic adipocytes and promote the release of those already stored. Resistin also induces angiogenesis aiming to increase blood flow and thus adipocyte oxygenation. Importantly, resistin increases lipid uptake by macrophages, also inhibiting cholesterol efflux from these cells, in order to store adipocyte-derived lipids. However, the chronic activation of such mechanisms leads to the formation of foam cells, common in atherosclerotic lesions (Fig. 1.6) (Lazar 2007; Robertson et al. 2009).
Resistin is directly involved in blocking insulin signaling in adipocytes, but also in the liver and skeletal muscle. Resistin knockout mice were shown to be protected of obesity-related insulin resistance, suggesting that it may be an important link between the activation of the immune system and glucose metabolism (Lazar 2007; Qatanani and Szwergold 2009; Robertson et al. 2009). Moreover, resistin neutralization increases insulin sensitivity and decreases hepatic glucose production, while resistin administration induces severe insulin resistance (Bugianesi et al. 2005; Juge-Aubry et al. 2005a; Lazar 2007). The expression of human resistin specifically in the macrophages of resistin knockout mice was able to increase adipose tissue lipolysis and fatty acid ectopic accumulation in the skeletal muscle, which led to insulin resistance (Qatanani and Szwergold 2009).
Resistin is a cysteine-rich protein which forms a dimer responsible for its biological activity (Bugianesi et al. 2005; Juge-Aubry et al. 2005a; Lazar 2007; Robertson et al. 2009; Smith and Yellon 2011). Human resistin was shown to induce the proinflammatory activity of macrophages and endothelial cells, by activating the Nuclear Factor-kB (NF-kB) and inhibiting the insulin signaling (Fig. 1.6). Resistin promotes the proliferation and adhesion of endothelial cells through NF-κB activation and increased expression of the VEGF receptors (VEGFR1 and VEGFR2), matrix metalloproteinases (MMP-1 and MMP-2), and adhesion proteins (VCAM and ICAM) (Fig. 1.6) (Juge-Aubry et al. 2005a).
In macrophages, resistin promotes adipocyte-like mechanisms of lipid uptake resulting in the formation of foam cells. Such mechanisms include the upregulation of scavenger receptors (SR-A and CD36), also promoting the degradation of lipid efflux proteins in the proteasome (ABCA1, ABCG1). Foam cells are known for their proinflammatory and proangiogenic potential which is thus promoted by resistin actions (Fig. 1.6) (Lazar 2007; Lee et al. 2009; Qatanani and Szwergold 2009).
3.4 Other Adipose Tissue Products
Recently, a broad range of other adipokines has been identified, including apelin, visfatin, vaspin, omentin, and others involved in canonical mechanisms like coagulation, vascular tone, and immunity.
Visfatin was initially discovered as a factor controlling B lymphocytes development. On the other hand, an enzyme called Nampt (nicotinamide 5-fosforibosyl-1-pyrophosfate transferase) catalyzing the formation of nicotinamide adenine dinucleotide (NAD) was also described as product from the same gene (Kiess et al. 2008; Gallí et al. 2010; Saddi-Rosa et al. 2010). Thus, visfatin appears to be a factor with a simultaneous enzymatic and endocrine activity. Its circulating concentrations are correlated with the amount of visceral adipose tissue and increases during weight gain. Moreover, its synthesis was identified mostly in macrophages residing and infiltrating the tissue (Kiess et al. 2008; Gallí et al. 2010; Saddi-Rosa et al. 2010). Increased circulating visfatin levels were found in a number of pathological situations like portal inflammatory infiltration, steatohepatitis, coronary accumulation of oxidized LDL and atherosclerotic plaque instability, higher incidence of ischemic events and decreased kidney function (Saddi-Rosa et al. 2010). On the other hand, visfatin was observed to produce a rapid insulin-independent hypoglycemic effect after its administration, while animal models lacking visfatin have shown hypoinsulinemia and lower insulin-stimulated glucose uptake (Kiess et al. 2008; Gallí et al. 2010; Saddi-Rosa et al. 2010). Visfatin was also observed to increase adipocyte and myocyte glucose uptake and to suppress hepatic glucose production (Saddi-Rosa et al. 2010). In vitro and in vivo studies also demonstrated protective effects of visfatin in ischemic events and its ability to increase endothelial cell activation, promoting angiogenesis (Mocan Hognogi and Simiti 2016). Thus, contradictory results about visfatin effects have been observed. Apparently, it is a compensatory mechanism for hyperglycemia with protective effects at multiple levels (Saddi-Rosa et al. 2010). Such effects were recently attributed to its ability to synthesize NAD, which improves the activity of several enzymes involved in glucose metabolism and mitochondrial function (Gallí et al. 2010; Mocan Hognogi and Simiti 2016).
Apelin was only recently identified, after the identification of its receptor APJ. Its functions are not very clear but apparently it regulates distinct biological processes like angiogenesis, vascular tone, heart function, and insulin secretion. Apelin was shown to antagonize Angiopoietin (Ang)-2 effects of endothelial cells, increasing NO-dependent vasodilation, but it was also shown to increase smooth muscle contraction. Although these are contradictory results, apelin also has the ability to bind to antidiuretic hormone (ADH) producing neurons in the hypothalamus, decreasing its production. Thus, together with its ionotropic actions in the heart, apelin is thought to be involved in the control of blood pressure (Glassford et al. 2007; Pitkin et al. 2010; Fasshauer and Blüher 2015). Moreover, insulin was observed to increase Apelin secretion, but apelin apparently has inhibitory functions on insulin secretion, suggesting that it might be involved in a negative feedback to insulin secretion (Glassford et al. 2007; Pitkin et al. 2010; Fasshauer and Blüher 2015). Nevertheless, little is yet known about apelin and more research is needed in this field.
Vaspin is another adipose-derived hormone, initially identified as inhibitor of serine proteases, but with benefic effects on insulin sensitivity. Although the mechanisms of vaspin stimulation and secretion are unknown, it was shown to be secreted by visceral fat mass and nutritionally regulated, due to its circadian release after meals (Jeong et al. 2010; Fasshauer and Blüher 2015). Vaspin levels are decreased in type 2 diabetes and its administration was shown to improve glucose tolerance and insulin sensitivity (Fig. 1.7) (Klöting et al. 2006; Jeong et al. 2010; Fasshauer and Blüher 2015). Moreover, diet or surgery-induced weight loss leads to a decrease of vaspin levels, which may result from decreased fat mass (Klöting et al. 2006; Handisurya et al. 2010).
Omentin is mainly produced in the omental adipose tissue, but it is expressed in vascular cells and not in adipocytes (Schäffler et al. 2005; Yang et al. 2006; Yamawaki et al. 2010). Omentin was recently suggested to increase insulin-stimulated glucose uptake, being correlated with insulin sensitivity. Its concentrations were observed to be decreased in obese patients and to increase after weight loss (Fig. 1.7) (Yang et al. 2006; De Souza Batista et al. 2007; Moreno-Navarrete et al. 2010). Importantly, omentin was shown to induce eNOS-dependent vessel relaxation, although the mechanisms are still unknown (Yamawaki et al. 2010).
4 Dysregulation of Adipose Tissue Function in Obesity
Adipocyte hypertrophy is a process occurring after excessive nutrient uptake, especially in visceral adipose tissue, which is more susceptible to nutrient fluxes. Hypertrophy has been recently associated with the development of hypoxic regions, with a strong influence on the metabolic and secretory functions of the adipose tissue.
Several studies demonstrated inflammation as the link between metabolic dysregulation and hypoxia, changing the secretory profile to create a proangiogenic environment (Fig. 1.8). Inflammation blocks nutrient uptake (insulin resistance and PPARγ inhibition) in order to prevent uncontrolled cell and tissue expansion and promotes lipolysis. Dysfunctional adipose tissue secretes a broad range of cytokines, but also chemokines, which recruit circulating monocytes to the tissue. Infiltrating macrophages are then important in maintaining the inflammatory environment and in uptake of excessive lipids, leading to the formation of adipose tissue foam cells. Many authors claim the existence of a metabolism—immunity axis, given that all metabolic changes lead to an immune response and many of the inflammatory factors simultaneously regulate metabolism and the immune system (Fig. 1.8) (Rajala and Scherer 2003; Golay and Ybarra 2005; Juge-Aubry et al. 2005a; Ye et al. 2007; Goossens 2008; Guilherme et al. 2008b; Rutkowski et al. 2009; Maury and Brichard 2010).
The role of hypoxia (b) and dysregulation of the lipid metabolism (a) in activating inflammatory mechanisms (c), aiming to restore homeostasis. Inflammation inhibits nutrient uptake and promotes lipolysis (d), also increasing angiogenesis (e) and the secretion of inflammatory adipokines (e). The mechanisms involved will be discussed in the following sections according to the letters in each figure’s box
4.1 Dysregulation of Lipid Metabolism
During the process of fat accumulation, adipocytes continuously accumulate triglycerides, increasing the expression of enzymes involved in fatty acid esterification. When adipose tissue is not able anymore to store all the lipids from the diet, adipocytes release fatty acids to the circulation, which then accumulate in tissues like skeletal muscle and liver. Type 2 diabetic patients usually have hepatic and muscle steatosis, leading to insulin-resistance and morphological alterations in such tissues (Rajala and Scherer 2003; Guilherme et al. 2008b; Kawano and Cohen 2013; Lonardo et al. 2015). The increasing availability of fatty acids also increases their esterification into triglycerides and oxidation in the mitochondria (Bugianesi et al. 2005; Golay and Ybarra 2005). However, lipid oxidation is a limited process and in such conditions intermediates of fatty acid metabolism like ceramides and diacylglycerols accumulate. Such intermediates inhibit insulin signaling and glucose uptake (Fig. 1.9). In physiological condition, such mechanisms prevent excessive nutrient uptake, which would cause cell death by oxidative stress. However when such mechanisms are chronically activated they conduce to insulin resistance and contribute to the onset of type 2 diabetes (Golay and Ybarra 2005; Guilherme et al. 2008a).
Main mechanisms conducting to the activation of inflammatory pathways in adipocytes, namely the cytoplasmatic accumulation of lipid mediators and activation of membrane receptors by saturated fatty acids. FFA-Sat saturated free fatty acids, HIF-1 hypoxia inducible factor-1, JNK c-jun n-terminal kinase, MMP matrix metalloproteinase, P O2 oxygen pressure, TLR4 toll-like receptor 4, TNF-α tumor necrosis factor alpha, uPA plasminogen activator urokinase-type, VEGF vascular endothelial growth factor, VEGFR VEGF receptor
The accumulation of diacylglycerols directly activates several PKC isoforms which besides inhibiting insulin receptor activation through serine phosphorylation, also promotes the activation of the IKKβ (Fig. 1.9) (Moeschel et al. 2004; Boden et al. 2005; Guilherme et al. 2008b). IKKβ is also a serine kinase which directly inhibits the insulin receptor and leads to the activation of the NF-κB. Moreover, JNK is also activated by the PKC and by fatty acid-activated toll-like receptor 4 (TLR4), contributing to the same final objective of NF-κB activation (Qatanani and Lazar 2007; Zhang et al. 2010). In turn, NF-κB activated the expression of proinflammatory and proangiogenic molecules (MCP-1, TNF-α, VEGF, adhesion molecules, etc.), which further contribute to the inhibition of substrate uptake (Fig. 1.9).
4.2 Adipose Tissue Hypoxia
It has been demonstrated that angiogenesis is a vital process in adipose tissue expansion and proper nutrient storage. Tissue expansion is believed to lead to the formation of physiological hypoxic regions, which induce the secretion of angiogenic factors and the reestablishment of tissue homeostasis. However, chronic hypoxia has been suggested to dramatically change adipokine secretion, with a decrease of adiponectin and an increase of proinflammatory adipokines (Hosogai et al. 2007; Wang et al. 2007; Goossens 2008; Trayhurn et al. 2008b; Wood et al. 2009). Despite the reasons to the development of chronic hypoxia are not known, it was described that obese individuals lack the postprandial increase of blood supply to the adipose tissue observed in lean individuals (Trayhurn et al. 2008b; Wood et al. 2009). Hypoxic regions have been shown in several diet-induced and genetic animal models of obesity, showing deficient angiogenesis, oxygen pressure, with decreased levels of endothelial cell markers and increased accumulation of the hypoxia probe pimonidazole and lactate formation (Hosogai et al. 2007; Ye et al. 2007; Goossens 2008; Pang et al. 2008; Trayhurn et al. 2008b; Wood et al. 2009). Despite being currently controversial, a current view supports that the oxygen diffusion distance (100 μm) is lower than adipocyte diameter in obesity (150–20 μm). This would lead to hypoxia-inducible factor-1 (HIF-1) stabilization and consequent expression of GLUT1 and secretion of angiogenic factors like the VEGF (Wood et al. 2007, 2009; Ye et al. 2007; Wang et al. 2007; Rausch et al. 2008; Goossens 2008; Trayhurn et al. 2008a; He et al. 2011). However, this view is recently being abandoned and Goossens and colleagues have reported that the adipose tissue of obese individuals may even be hyperoxic under certain circumstances due to decreased metabolic activity (Goossens et al. 2011). Thus, the real causes for impaired vascular function and hypoxia (if confirmed) are currently unknown.
4.3 Activation of Stress and Inflammatory Pathways by Lipid Mediators and Hypoxia: Consequences for Inflammatory Cell Recruitment
There is no consensus about the role of adipocytes and preadipocytes in tissue response to hypoxia as current models does not discriminate the secretome change occurring in each cell type. On the other hand, cell lines do not mimic all the events observed in vivo. Nevertheless, several authors have suggested NF-κB and HIF-1 activation leading to the expression of proinflammatory cytokines like IL-6, MCP-1, plasminogen activator inhibitor-1 (PAI-1), transforming growth factor (TGF)-β, and matrix metalloproteinases (MMP), but also leptin (Ye et al. 2007; Trayhurn et al. 2008b; Halberg et al. 2009; Wood et al. 2009). NF-κB activation in hypoxia is well documented in different models of hypoxia like tumors, interacting with HIF-1 to increase the expression of inflammatory cells (Fig. 1.9) (van Uden et al. 2008). It has been described that cultured hypoxic adipocytes secrete higher levels of MCP-1, TNF-α, and VEGF and increase GLUT1 expression. Preadipocytes apparently are more susceptible to hypoxic stimuli, acquiring the ability to secrete leptin. However, hypoxia inhibits preadipocytes differentiation to adipocytes and promotes their transformation to macrophages. The release of chemoattractant factors like MCP-1 further increases the number of macrophages in the tissue (Trayhurn et al. 2008b; Wood et al. 2009).
4.3.1 Adipose Tissue Immune System: Recruitment of Inflammatory Cells and Secretion of Inflammatory Cytokines
Changes in the metabolic status are detected by the immune system, which acts in order to establish homeostasis. The activation of the immune system is an important mechanism in times of excessive nutritional fluxes, avoiding excessive nutrient uptake by the cells and maintaining cell viability and nutrient distribution by the different cells of the body. However, the low-grade inflammation observed in obesity resulting from chronic nutrient excess leads to insulin resistance in adipocytes (Galic et al. 2010). Such mechanisms include MCP-1 expression, which is involved in recruiting cells from the immune system to the tissue. Such cells, mainly monocytes-derived macrophages, further support the inflammatory feedback in the tissue, with the release of inflammatory mediators like TNF-α. In turn, inflammatory mediators further inhibit nutrient accumulation and tissue growth, showing the existence of a metabolism—immunity continuous (Schäffler et al. 2007; Galic et al. 2010).
A large number of circulating monocytes can be recruited to the adipose tissue through the action of proinflammatory cytokines and chemokines and develop into macrophages (Schäffler et al. 2007; Guilherme et al. 2008a; Surmi and Hasty 2010; Olefsky and Glass 2010). Macrophages may be derived from recruited monocytes and from differentiation from preadipocytes or mesenchymal cells and can become 50% of the total number of cells in the adipose tissue in obesity (Schäffler et al. 2007; Guilherme et al. 2008a; Surmi and Hasty 2010; Olefsky and Glass 2010). MCP-1, a major chemokine, was shown to be increased in obese patients and animal models. Its inhibition in diabetic and obese animal models improves insulin resistance and glucose tolerance (Guilherme et al. 2008a; Olefsky and Glass 2010).
The reason why preadipocytes can differentiate into macrophage is because they share the same cell lineage, sharing several intracellular mechanisms of lipid uptake, storage, and metabolism (Schäffler et al. 2007). Macrophages are known to surround death adipocytes forming crown-like structures, which are typically found in the adipose tissue of obese patients and animal models (Trayhurn et al. 2008a). Such macrophages uptake and store large amounts of fat released from their lipid droplets, leading to the formation of foam cells. In turn, foam cells have an increased inflammatory activity with increased activation of the NF-κB and secretion of proinflammatory factors (Ye et al. 2007; Rutkowski et al. 2009; Maury and Brichard 2010; Galic et al. 2010). Adipose tissue hypoxic regions were shown to be strongly populated by macrophages, being strongly positive for their membrane marker F4/80, and by CD4+ and CD8+ T cells, although their role in metabolic syndrome is currently unknown (Ye et al. 2007; Rausch et al. 2008; Goossens 2008; Trayhurn et al. 2008a; Wood et al. 2009).
Two populations of macrophages may be found at the adipose tissue: M1 macrophages recruited to the tissue by the gradient of chemokines like the MCP-1 typically have a more pronounced proinflammatory secretome, originating most of the foam cells. Tissue resident M2 macrophages secreting a broad range of angiogenic and tissue remodeling factors like metalloproteinases are involved in tissue adaptation to hypoxia and have a more modest inflammatory activity (Maury and Brichard 2010; Olefsky and Glass 2010; Galic et al. 2010).
Activation of endothelial cells by inflammatory cytokines leads to the overexpression of adhesion molecules, which are important for macrophage binding and migration to the tissue matrix (Rutkowski et al. 2009; Maury and Brichard 2010). Moreover, the increased HIF-1-dependent expression of the migration inhibition factor (MIF-1) in hypoxia inhibits macrophage exit from the tissue, contributing to their accumulation (Ye et al. 2007). Most of the cytokines released by the adipose tissue in obesity, namely TNF-α, IL-6, and resistin, are derived from the infiltrating macrophages. TNF-α acts on adipocytes in order to inhibit insulin receptor and the PPARγ and to activate NF-κB, completing the inflammatory feedback in the tissue aiming to block further nutrient uptake and to increase angiogenesis and tissue remodeling (Wellen and Hotamisligil 2003; Moeschel et al. 2004; Sandu et al. 2005; Qatanani and Lazar 2007; Guilherme et al. 2008a; Olefsky and Glass 2010; Min et al. 2016). On the other hand, TNF-α and MCP-1 inhibition in models of diet-induced insulin resistance was shown to improve insulin sensitivity (Wellen and Hotamisligil 2003; Bugianesi et al. 2005; Guilherme et al. 2008b).
4.4 Inhibition of Nutrient Uptake by Inflammatory Stimuli: Insulin Resistance and PPARγ Inhibition
Insulin resistance is characterized by a deficient cell response to physiological insulin concentrations, leading to increased β-cell insulin secretion and compensatory hyperinsulinemia (Yki-Järvinen 2005, 2015; Yki-Järvinen and Westerbacka 2005). Insulin resistance is not associated with the body mass index (BMI), but depends on impaired adipose tissue lipid storage and activation of inflammatory pathways (Yki-Järvinen 2005, 2015; Yki-Järvinen and Westerbacka 2005). Anti-inflammatory molecules like PPARγ agonists (thiazolidinediones and polyunsaturated fatty acids) and salicylates are known to improve insulin sensitivity. As well, physical exercise is known to decrease inflammation and to improve insulin sensitivity (Bugianesi et al. 2005; Guilherme et al. 2008b; Oliveira et al. 2011).
Fatty acids are known to directly cause insulin resistance. Even nondiabetic individuals were shown to decrease insulin sensitivity after an acute exposure to fatty acids, which is a mechanism important to prevent excessive accumulation of nonesterified fatty acids in the cytoplasm (Golay and Ybarra 2005; Einstein et al. 2010). Circulating free fatty acids are also strong inducers of insulin resistance acting through the membrane receptor TLR4 (Qatanani and Lazar 2007; Guilherme et al. 2008a; Zhang et al. 2010; Olefsky and Glass 2010).
Hypoxia is a strong inhibitor of nutrient uptake. In cultured adipocytes hypoxia-dependent HIF-1 activation leads to the upregulation of stress pathways that blunts insulin signaling (Regazzetti et al. 2009; Ye 2009; Wood et al. 2009; Zhang et al. 2010). As well, the same mechanisms also inhibit PPARγ activity, leading to decreased secretion of adiponectin (Gentil et al. 2006; Chen et al. 2006; Goossens 2008; Ye 2009). However, it was shown that the overexpression of an inactive form of HIF-1 during a high-fat diet challenge aggravates insulin resistance, suggesting distinct acute and chronic effects of hypoxia in adipose tissue. Such evidences support the idea that acute activation of hypoxia response mechanisms are important regulators of nutrient uptake be adipocytes, but long-term activation of such mechanisms is important to the regulation of mitochondrial biogenesis and angiogenesis (Zhang et al. 2010; He et al. 2011). In fact, adipose tissue vascular density was shown to correlate with insulin ability to suppress lipolysis, i.e., insulin sensitivity (Pasarica et al. 2010).
A main contributor to the long-term activation of such mechanisms is macrophage-derived inflammatory signals. Activation of inflammatory signals results not only in the inhibition of nutrient uptake but also the release of those already accumulated, namely through the stimulation of lipolysis. Cytokines like the TNF-α and IL-6 are known to be mostly produced in adipose tissue infiltrating macrophages. These cytokines were shown to activate NF-κB through serine kinases like JNK and IKK, leading to the transcription of inflammatory factors and proteins involved in stress and inflammatory pathways (Fig. 1.10) (Tilg and Moschen 2006; Kiess et al. 2008; Olefsky and Glass 2010). Moreover, serine kinases phosphorylate the insulin receptor and its substrate (IRS-1) in serine residues instead of the stimulatory tyrosine phosphorylation, leading to the inactivation of the pathway (Fig. 1.10) (Moeschel et al. 2004; Boden et al. 2005; Wellen and Hotamisligil 2005; Qatanani and Lazar 2007; Guilherme et al. 2008a; Wang et al. 2009; Kaneto et al. 2010; Maury and Brichard 2010; Olefsky and Glass 2010; Galic et al. 2010). JNK was also shown to increase the expression of SOCS proteins, which are known to bind to and signalize IRS-1 to proteasome degradation (Ueki et al. 2004a; Howard and Flier 2006). Such mechanisms perpetuate the inhibition of insulin signaling in response to inflammatory cytokines.
Consequences of adipocyte inflammatory mechanisms for inhibition of insulin signaling and PPARγ activity, inhibiting nutrient uptake and promoting lipolysis. FFA-Sat saturated free fatty acids, IRS-1 insulin receptor substract-1, JNK c-jun n-terminal kinase, NF-kB nuclear factor kappa B, PerA perilipin A, PKC protein kinase C, PPARγ peroxisome proliferating-activated receptor γ, Ser serine, TLR4 toll-like receptor 4, TNF-α tumor necrosis factor alpha
Regarding lipid storage, TNF-α was shown to inhibit PPARγ activity, causing the downregulation of key enzymes in fatty acid uptake (LPL and FATP) and esterification, as well as adiponectin (Boden et al. 2005; Juge-Aubry et al. 2005a; Qatanani and Lazar 2007; Guilherme et al. 2008b). TNF-α was also shown to increase intracellular levels of cAMP, which is known to activate hormone-sensitive lipase (HSL) and thus to induce lipolysis (Fig. 1.10) (Wellen and Hotamisligil 2003; Juge-Aubry et al. 2005a; Guilherme et al. 2008a; Maury and Brichard 2010; Galic et al. 2010). Interestingly, hypoxia is also associated with decreased levels of ATP and increased cAMP levels, also contributing to lipolysis (Ye 2009).
4.5 Activation of Angiogenesis
The formation of new blood vessels is a highly regulated process involving several cell types, which produce a broad range of factors controlling vessel permeability and stability. The plasticity of the vascular network is also modulated by a series of hormones involved in the metabolism, showing that the nutritional status has a major influence in the angiogenic stimulus. The activation of the immune system in metabolic syndrome dramatically changes the stimulus to endothelial cells, promoting vascularization as an attempt to restore homeostasis.
Obese patients with adipose tissue hypoxia were shown to have normal oxygen pressure and hemoglobin concentration and saturation, suggesting that hypoxia derives from local changes in blood supply (Goossens 2008; Rutkowski et al. 2009; Corvera and Gealekman 2014). Obese individuals may have a 30–40% reduction of blood supply than lean ones and a similar reduction was observed in obese Zucker rats (Ye 2009). Moreover, obese and type 2 diabetic animal models have been shown to have a decrease of the endothelial cells marker CD31, which may possibly be associated with the development of hypoxia (Goossens 2008; Ye 2009). Moreover, recent studies also demonstrated a reduction of endothelial progenitor cells, which may contribute to impaired angiogenic function (Neels et al. 2004; Gealekman et al. 2008; Tam et al. 2009; Corvera and Gealekman 2014).
Inhibition of angiogenesis was initially suggested as an effective strategy to prevent adipose tissue growth (Lijnen 2008). However, this hypothesis has been abandoned given that the vascular network was shown to be essential for proper tissue oxygenation and storage and metabolic functions and blood supply was shown to correlate with insulin sensitivity (Mannerås-Holm and Krook 2012). Moreover, it was shown that adipose tissue expansion is necessary for nutrient storage and to prevent ectopic lipid deposition in liver and skeletal muscle.
The formation of hypoxic regions activates the secretion of angiogenic factors which ultimately restore homeostasis (Cao 2007; Goossens 2008; Rutkowski et al. 2009). However, if angiogenesis is not enough, hypoxia is maintained and HIF-1 levels increase in order to stimulate the expression of VEGF, angiopoietins, leptin, and matrix-remodeling factors (Minet et al. 2001; Yamakawa et al. 2003; Cao 2007; Hosogai et al. 2007; Goossens 2008). However, HIF-1 chronic activation also leads to the secretion of fibrotic factors (Rutkowski et al. 2009; Wood et al. 2009; Suga et al. 2010).
4.5.1 Pro and Antiangiogenic Factors
Adipose tissue angiogenesis is regulated by the balance between pro and antiangiogenic factors derived from all the cells of the tissue (Neels et al. 2004; Cao 2007; Rutkowski et al. 2009; Tinahones et al. 2012; Corvera and Gealekman 2014).
VEGF is the main endothelial cell growth factor, being associated with the development of obesity (Cao 2007; Rutkowski et al. 2009; Tinahones et al. 2012; Corvera and Gealekman 2014). Animal models of obesity and obese individuals have increased VEGF levels, which were shown to decrease after weight loss (Gómez-Ambrosi et al. 2010). VEGF is mostly produced by stroma cells and by mature adipocytes, acting in adjacent capillaries (Fig. 1.11) (Neels et al. 2004; Christiaens and Lijnen 2010). During adipose tissue expansion, a significant part of VEGF is produced by infiltrating inflammatory cells. VEGFR2 inhibition prevents diet-induced tissue expansion and preadipocyte differentiation, showing that angiogenesis is crucial to the development of new adipocytes (Tam et al. 2009; Tran et al. 2012; Min et al. 2016). The major regulators of VEGF expression are hypoxia and insulin, while inflammatory cytokines can also induce its expression. VEGF inhibition as a strategy to prevent obesity is still controversial. VEGF was shown to increase in genetic models of obesity, but not in models of metabolic syndrome, suggesting that hypoxia-induced VEGF overexpression may be lost in hyperglycemia (Trayhurn et al. 2008b; Wood et al. 2009). Such observations derive from impaired HIF-1 stabilization in hyperglycemic models, resulting in impaired VEGF expression and dysregulation of the angiogenic process (Bento et al. 2010b). Moreover, insulin resistance may also contribute to impaired VEGF expression in type 2 diabetes. Thus, the initial idea that inhibiting VEGF could be an effective strategy to prevent obesity is currently being changed and is now believed that VEGF in necessary for proper adipose tissue angiogenesis and function (Treins et al. 2002; Hausman and Richardson 2004; Ye 2009; Vona-Davis and Rose 2009).
Causes and consequences of inflammatory processes in adipose tissue. Inflammation directly inhibits excessive nutrient uptake by adipocytes and their continuous hypertrophy. Moreover, such mechanisms also stimulate angiogenic function in order to reestablish homeostasis. HIF-1 hypoxia inducible factor-1, MCP-1 monocyte chemoattractant protein-1, MMP matrix metalloproteinases, NF-kB nuclear factor-kappa B, P O2 oxygen pressure, PPARγ peroxisome proliferating-activated receptor γ, TNF-α tumor necrosis factor alpha, VEGF vascular endothelial growth factor
VEGF effects on the vascular network depend on its interaction with angiopoietins (Ang). Ang-1 and Ang-2 have opposite effects on the vasculature, although both were shown to be necessary for embryonic development. While Ang-1 is involved in vessel stabilization, increasing cell-cell adhesion, Ang-2 induces destabilization of cell-cell and cell-matrix interactions in order to allow cell proliferation and migration (Cao 2007; Christiaens and Lijnen 2010; Corvera and Gealekman 2014). Ang-1 promotes vessels stiffness through modulation of TGFβ activity and deposition of extracellular matrix proteins (Cao 2007; Rutkowski et al. 2009; Christiaens and Lijnen 2010). On the other hand, Ang-2 expression is induced by hypoxia like VEGF and its receptors (Tie-2) are often co-localized with VEGFR2 (Christiaens and Lijnen 2010; Bento et al. 2010a). Ang-2 action in order to induce destabilization of cell interactions is necessary for VEGF-induced proliferation (Christiaens and Lijnen 2010; Bento et al. 2010a). The absence of VEGF after Ang-2 induced destabilization of endothelial cells leads to the formation of aberrant blood vessels. Such mechanisms have been suggested to be involved in the development of diabetic vascular complications and to be present in adipose tissue during the process of metabolic dysregulation (Bento et al. 2010a; Matafome et al. 2012).
Leptin was also shown to be proangiogenic, sharing many mechanisms with insulin, namely MAPK activation (Cao 2007; Vona-Davis and Rose 2007; Lijnen 2008; Christiaens and Lijnen 2010; Bento et al. 2010a). Leptin and insulin promote endothelial cells migration, proliferation, and survival, causing the increased VEGF secretion and VEGFR2 expression. Moreover, leptin was shown to increase MMP-dependent matrix remodeling (Cao 2007; Vona-Davis and Rose 2007; Lijnen 2008; Christiaens and Lijnen 2010; Bento et al. 2010a). On the other hand, adiponectin was shown to counteract all these effects, by inhibiting VEGF actions in endothelial cells (Vona-Davis and Rose 2009). Adiponectin is mostly produced by small and non-inflamed adipocytes. Thus, when adipose tissue blood supply is enough, adiponectin prevents excessive blood vessel growth. On the other hand, leptin is produced proportionally to fat mass and thus its angiogenic functions are necessary to sustain tissue angiogenesis.
Other factors that have been suggested to be involved in the regulation of adipose tissue angiogenesis are fibroblast growth factor (FGF) (Lijnen 2008; Christiaens and Lijnen 2010), hepatic growth factor (HGF), and platelet-derived growth factor (PDGF) (Lijnen 2008; Pang et al. 2008; Christiaens and Lijnen 2010). As well, fibrotic and inflammatory factors like TGF-β and TNF-α have been shown to exert direct effects on adipose tissue endothelial cells. While TNF-α is involved in endothelial cell proliferation, TGF-β is important for the deposition of the extracellular matrix and vessel stabilization (Lijnen 2008; Niu et al. 2008; Christiaens and Lijnen 2010).
Angiogenesis is not only regulated by endothelial cell proliferation and migration, but also by matrix remodeling. Extracellular matrix degradation is a highly regulated process by the plasminogen system and by MMPs. The plasminogen system is known for its role on blood clotting but it is also shown to be involved in the regulation of extracellular matrix degradation (Lijnen 2008; Carter and Church 2009; Christiaens and Lijnen 2010). Animal models of obesity and metabolic syndrome have been shown to have increased levels of the inhibitor of plasminogen activator (PAI-1). Transgenic models of adipocyte PAI-1 expression have shown lack of visceral adipose tissue, showing that PAI-1 inhibits matrix degradation and vessel growth. However, although PAI-1 is apparently involved in preventing matrix degradation, its expression is increased by hypoxia and its levels are correlated with the body mass index (BMI) and insulin resistance, suggesting that its function in adipose tissue may be far more complex. Regarding MMPs, they are involved in matrix degradation, allowing endothelial cell migration. Their levels are increased in obesity and their overexpression results in decreased capillary density in adipose tissue. However, the mechanisms of their regulation and their real impact in adipose tissue pathophysiology are currently unknown (Lijnen 2008; Christiaens and Lijnen 2010; Corvera and Gealekman 2014).
4.6 Inflammatory Feedback in Adipose Tissue: Unifying Mechanism
The dysregulation of adipocyte metabolism occurs with the accumulation of fatty acids and their intermediates and with the development of hypoxia. All these conditions lead to the activation of stress pathways resulting in inhibition of nutrient uptake and increased expression of chemokines involved in the recruitment of inflammatory cells to the tissue (Galic et al. 2010). PPARγ inhibition also leads to decreased adiponectin secretion, which contributes to the inflammatory feedback in the tissue. Monocyte recruitment and their differentiation into macrophages further increase the release of inflammatory chemokines, cytokines like TNF-α, and angiogenic factors. Such events further chronically inhibit insulin signaling and fatty acid uptake, also increasing lipolysis (Fig. 1.11) (Qatanani and Lazar 2007; Olefsky and Glass 2010).
References
Ahima RS (2005) Central actions of adipocyte hormones. Trends Endocrinol Metab 16:307–313
Ahima RS, Flier JS (2000) Leptin. Annu Rev Physiol 62:413–437
Arner P (2005) Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab 19:471–482
Bento CF, Fernandes R, Matafome P, Sena C, Seiça R, Pereira P (2010a) Methylglyoxal-induced imbalance in the ratio of vascular endothelial growth factor to angiopoietin 2 secreted by retinal pigment epithelial cells leads to endothelial dysfunction. Exp Physiol 95:955–970
Bento CF, Fernandes R, Ramalho J, Marques C, Shang F, Taylor A, Pereira P (2010b) The chaperone-dependent ubiquitin ligase CHIP targets HIF-1α for degradation in the presence of methylglyoxal. PLoS One 5:1–13
Blüher M, Mantzoros CS (2015) From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism 64(1):131–145
Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, Xiang X, Luo Z, Ruderman N (2005) Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-κb pathway in rat liver. Diabetes 54:3458–3465
Bugianesi E, McCullough AJ, Marchesini G (2005) Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology 42:987–1000
Cao Y (2007) Angiogenesis modulates adipogenesis and obesity. J Clin Invest 117:2362–2368
Carter JC, Church FC (2009) Obesity and breast cancer: the roles of peroxisome proliferator-activated receptor-gamma and plasminogen activator inhibitor-1. PPAR Res 2009:345320
Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, Andò S (2003) Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem 278:28668–28676
Chen B, Lam KSL, Wang Y, Wu D, Lam MC, Shen J et al (2006) Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun 341:549–556
Christiaens V, Lijnen HR (2010) Angiogenesis and development of adipose tissue. Mol Cell Endocrinol 318:2–9
Corvera S, Gealekman O (2014) Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta 1842:463–472
De Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, DZ Y, Pray J et al (2007) Omentin plasma levels and gene expression are decreased in obesity. Diabetes 56:1655–1661
Ding M, Rzucidlo EM, Davey JC, Xie Y, Liu R, Jin Y, Stavola L, Martin KA (2012) Adiponectin in the heart and vascular system. Vitam Horm 90:289–319
Einstein FH, Huffman DM, Fishman S, Jerschow E, Heo HJ, Atzmon G, Schechter C, Barzilai N, Muzumdar RH (2010) Aging per se increases the susceptibility to free fatty acid-induced insulin resistance. J Gerontol A Biol Sci Med Sci 65(8):800–808
Fasshauer M, Blüher M (2015) Adipokines in health and disease. Trends Pharmacol Sci 36(7):461–470
Galic S, Oakhill JS, Steinberg GR (2010) Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316(2):129–139
Gallí M, Van Gool F, Rongvaux A, Andris F, Leo O (2010) The nicotinamide phosphoribosyltransferase: a molecular link between metabolism, inflammation, and cancer. Cancer Res 70(1):8–11
Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S (2008) Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab 295(5):E1056–E1064
Gealekman O, Guseva N, Gurav K, Gusev A, Hartigan C, Thompson M, Malkani S, Corvera S (2012) Effect of rosiglitazone on capillary density and angiogenesis in adipose tissue of normoglycaemic humans in a randomised controlled trial. Diabetologia 55:2794–2799
Gentil C, Le Jan S, Philippe J, Leibowitch J, Sonigo P, Germain S, Piétri-Rouxel F (2006) Is oxygen a key factor in the lipodystrophy phenotype? Lipids Health Dis 5:1–11
Glassford AJ, Yue P, Sheikh AY, Chun HJ, Zarafshar S, Chan DA, Reaven GM, Quertermous T, Tsao PS (2007) HIF-1 regulates hypoxia- and insulin-induced expression of apelin in adipocytes. Am J Physiol Endocrinol Metab 293(6):E1590–E1596
Golay A, Ybarra J (2005) Link between obesity and type 2 diabetes. Best Pract Res Clin Endocrinol Metab 19(4):649–663
Gómez-Ambrosi J, Catalán V, Rodríguez A, Ramírez B, Silva C, Gil MJ, Salvador J, Frühbeck G (2010) Involvement of serum vascular endothelial growth factor family members in the development of obesity in mice and humans. J Nutr Biochem 21(8):774–780
Goossens GH (2008) The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav 94(2):206–218
Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clément K, Blaak EE (2011) Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 124(1):67–76
Guilherme A, Virbasius J, Puri V, Czech MP (2008a) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9(5):367–377
Guilherme A, Virbasius JV, Puri V, Czech MP (2008b) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 114:715–728
Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S et al (2009) Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29(16):4467–4483
Handisurya A, Riedl M, Vila G, Maier C, Clodi M, Prikoszovich T et al (2010) Serum vaspin concentrations in relation to insulin sensitivity following RYGB-induced weight loss. Obes Surg 20(2):198–203
Hausman GJ, Richardson RL (2004) Adipose tissue angiogenesis. J Anim Sci 82:925–934
He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J (2011) Regulation of HIF-1{alpha} activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab 300(5):E877–E885
Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K et al (2007) Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56:901–911
Howard JK, Flier JS (2006) Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab 17(9):365–371
Imrie H, Abbas A, Kearney M (2010) Insulin resistance, lipotoxicity and endothelial dysfunction. Biochim Biophys Acta 1801(3):320–326
Jeong E, Youn BS, Kim DW, Kim EH, Park JW, Namkoong C et al (2010) Circadian rhythm of serum vaspin in healthy male volunteers: relation to meals. J Clin Endocrinol Metab 95(4):1869–1875
Juge-Aubry CE, Henrichot E, C a M (2005a) Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab 19(4):547–566
Juge-Aubry CE, Somm E, Pernin A, Alizadeh N, Giusti V, Dayer J-M, Meier C (2005b) Adipose tissue is a regulated source of interleukin-10. Cytokine 29:270–274
Kamon J, Yamauchi T, Terauchi Y, Kubota N, Kadowaki T (2003) The mechanisms by which PPARgamma and adiponectin regulate glucose and lipid metabolism. Nihon yakurigaku zasshi Folia pharmacologica Japonica 122(4):294–300
Kaneto H, Katakami N, Matsuhisa M, Matsuoka T (2010) Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediat Inflamm 2010:1–11
Kawano Y, Cohen DE (2013) Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol 48(4):434–441
Kiess W, Petzold S, Töpfer M, Garten A, Blüher S, Kapellen T, Körner A, Kratzsch J (2008) Adipocytes and adipose tissue. Best Pract Res Clin Endocrinol Metab 22(1):135–153
Klöting N, Berndt J, Kralisch S, Kovacs P, Fasshauer M, Schön MR, Stumvoll M, Blüher M (2006) Vaspin gene expression in human adipose tissue: association with obesity and type 2 diabetes. Biochem Biophys Res Commun 339(1):430–436
Langin D (2006) Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res 53(6):482–491
Laubner K, Kieffer TJ, Lam NT, Niu X, Jakob F, Seufert J (2005) Inhibition of preproinsulin gene expression by leptin induction of suppressor of cytokine signaling 3 in pancreatic beta-cells. Diabetes 54:3410–3417
Lazar MA (2007) Resistin- and obesity-associated metabolic diseases. Horm Metab Res 39(10):710–716
Lee TS, Lin CY, Tsai JY, YL W, KH S, KY L et al (2009) Resistin increases lipid accumulation by affecting class a scavenger receptor, CD36 and ATP-binding cassette transporter-A1 in macrophages. Life Sci 84:97–104
Lee J-Y, Hashizaki H, Goto T, Sakamoto T, Takahashi N, Kawada T (2011) Activation of peroxisome proliferator-activated receptor-α enhances fatty acid oxidation in human adipocytes. Biochem Biophys Res Commun 407(4):818–822
Letra L, Santana I, Seiça R (2014) Obesity as a risk factor for Alzheimer’s disease: the role of adipocytokines. Metab Brain Dis 29:563–568
Lijnen HR (2008) Angiogenesis and obesity. Cardiovasc Res 78:286–293
Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P (2015) Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis 47(3):181–190
Lorincz AM, Sukumar S (2006) Molecular links between obesity and breast cancer. Endocr Relat Cancer 13(2):279–292
Mannerås-Holm L, Krook A (2012) Targeting adipose tissue angiogenesis to enhance insulin sensitivity. Diabetologia 55:2562–2564
Matafome P (2013) Common mechanisms of dysfunctional adipose tissue and obesity-related cancers. Diabetes Metab Res Rev 29(4):285–295
Matafome P, Santos-Silva D, Crisóstomo J, Rodrigues T, Rodrigues L, Sena CM, Pereira P, Seiça R (2012) Methylglyoxal causes structural and functional alterations in adipose tissue independently of obesity. Arch Physiol Biochem 118(2):58–68
Maury E, Brichard SM (2010) Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol 314(1):1–16
Meier U, Gressner AM (2004) Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem 50(9):1511–1525
Min SY, Kady J, Nam M, Rojas-Rodriguez R, Berkenwald A, Kim JH et al (2016) Human “brite/beige” adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med 22(3):312–318
Minet E, Michel G, Mottet D (2001) Transduction pathways involved in hypoxia-inducible factor-1 phosphorylation and activation. Free Radic Biol Med 31(7):847–855
Mocan Hognogi LD, Simiti LV (2016) The cardiovascular impact of visfatin—an inflammation predictor biomarker in metabolic syndrome. Clujul Medical 89:322
Moeschel K, Beck A, Weigert C, Lammers R, Kalbacher H, Voelter W, Schleicher ED, Häring H-U, Lehmann R (2004) Protein kinase C-zeta-induced phosphorylation of Ser318 in insulin receptor substrate-1 (IRS-1) attenuates the interaction with the insulin receptor and the tyrosine phosphorylation of IRS-1. J Biol Chem 279(24):25157–25163
Moreno-Navarrete JM, Catalán V, Ortega F, Gómez-Ambrosi J, Ricart W, Frühbeck G, Fernández-Real JM (2010) Circulating omentin concentration increases after weight loss. Nutr Metab (Lond) 7:27
Münzberg H, Björnholm M, Bates SH, Myers MG (2005) Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci 62(6):642–652
Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME et al (2006) Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 281(5):2654–2660
Neels JG, Thinnes T, Loskutoff DJ (2004) Angiogenesis in an in vivo model of adipose tissue development. FASEB J 18:983–1002
Nielsen NB, Højbjerre L, Sonne MP, Alibegovic AC, Vaag A, Dela F, Stallknecht B (2009) Interstitial concentrations of adipokines in subcutaneous abdominal and femoral adipose tissue. Regul Pept 155(1–3):39–45
Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE (2008) Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP). J Biol Chem 283(21):14542–14551
Olefsky JM, Glass CK (2010) Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72:219–246
Oliveira AG, Carvalho BM, Tobar N, Ropelle ER, Pauli JR, R a B, Guadagnini D, Carvalheira JBC, Saad MJA (2011) Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. Diabetes 60:784–796
Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y et al (2001) Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class a scavenger receptor expression in human monocyte-derived macrophages. Circulation 103:1057–1063
Pais R, Silaghi H, Silaghi AC, Rusu ML, Dumitrascu DL (2009) Metabolic syndrome and risk of subsequent colorectal cancer. World J Gastroenterol 15(41):5141–5148
Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J (2008) Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am J Physiol Endocrinol Metab 295(2):E313–E322
Park M, Sweeney G (2013) Direct effects of adipokines on the heart: focus on adiponectin. Heart Fail Rev 18(5):631–644
Pasarica M, Rood J, Ravussin E, Schwarz J-M, Smith SR, Redman LM (2010) Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab 95(8):4052–4055
Pitkin SL, Maguire JJ, Bonner TI, Davenport AP (2010) International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev 62(3):331–342
Qatanani M, Lazar MA (2007) Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev 21(12):1443–1455
Qatanani M, Szwergold N (2009) Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest 119(3):531–539
Rajala MW, Scherer PE (2003) Minireview: the adipocyte—at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 144:3765–3773
Ram VJ (2003) Therapeutic significance of peroxisome proliferator-activated receptor modulators in diabetes. Drug Today 39:609–632
Rausch ME, Weisberg S, Vardhana P, Tortoriello D V (2008) Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes 32:451–463
Regazzetti C, Peraldi P, Grémeaux T, Najem-Lendom R, Ben-Sahra I, Cormont M et al (2009) Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes 58:95–103
Robertson SA, Rae CJ, Graham A (2009) Resistin: TWEAKing angiogenesis. Atherosclerosis 203:34–37
Rutkowski JM, Davis KE, Scherer PE (2009) Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J 276:5738–5746
Saddi-Rosa P, Oliveira CS, Giuffrida FM, Reis AF (2010) Visfatin, glucose metabolism and vascular disease: a review of evidence. Diabetol Metab Syndr 2:21
Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H (2005) Insulin resistance and type 2 diabetes in high-fat—fed mice are linked to high glycotoxin intake. Diabetes 54(8):2314–2319
Schäffler A, Neumeier M, Herfarth H, Fürst A, Schölmerich J, Büchler C (2005) Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim Biophys Acta 1732:96–102
Schäffler A, Schölmerich J, Salzberger B (2007) Adipose tissue as an immunological organ: toll-like receptors, C1q/TNFs and CTRPs. Trends Immunol 28:393–399
Smekal A, Vaclavik J (2017) Adipokines and cardiovascular disease: a comprehensive review. Biomed Pap 161:31–40
Smith CCT, Yellon DM (2011) Adipocytokines, cardiovascular pathophysiology and myocardial protection. Pharmacol Ther 129:206–219
Suga H, Eto H, Aoi N, Kato H, Araki J, Doi K, Higashino T, Yoshimura K (2010) Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg 126:1911–1923
Surmi BK, Hasty AH (2010) The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vasc Pharmacol 52:27–36
Takeuchi T, Adachi Y, Ohtsuki Y, Furihata M (2007) Adiponectin receptors, with special focus on the role of the third receptor, T-cadherin, in vascular disease. Med Mol Morphol 40:115–120
Tam J, Duda DG, Perentes JY, Quadri RS, Fukumura D, Jain RK (2009) Blockade of VEGFR2 and not VEGFR1 can limit diet-induced fat tissue expansion: role of local versus bone marrow-derived endothelial cells. PLoS One 4:e4974
Tamori Y, Masugi J, Nishino N, Kasuga M (2002) Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes 51:2045–2055
Tilg H, Moschen AR (2006) Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6:772–783
Tinahones FJ, Coín-Aragüez L, Mayas MD, Garcia-Fuentes E, Hurtado-Del-Pozo C, Vendrell J et al (2012) Obesity-associated insulin resistance is correlated to adipose tissue vascular endothelial growth factors and metalloproteinase levels. BMC Physiol 12:4
Tran K, Gealekman O, Frontini A, Zingaretti M, Morroni M, Giordano A et al (2012) The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab 15:222–229
Trayhurn P (2014) Hypoxia and adipocyte physiology: implications for adipose tissue dysfunction in obesity. Annu Rev Nutr 34:207–236
Trayhurn P, Wood IS (2004) Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92:347–355
Trayhurn P, Wang B, Wood IS (2008a) Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr 100:227–235
Trayhurn P, Wang B, Wood IS (2008b) Hypoxia and the endocrine and signalling role of white adipose tissue. Arch Physiol Biochem 114:267–276
Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E (2002) Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem 277:27975–27981
van Uden P, Kenneth NS, Rocha S, Van UP (2008) Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J 412:477–484
Ueki K, Kondo T, Kahn C (2004a) Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins. Mol Cell Biol 24:5434–5446
Ueki K, Kondo T, Tseng Y-H, Kahn CR (2004b) Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci U S A 101:10422–10427
Vona-Davis L, Rose DP (2007) Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 14:189–206
Vona-Davis L, Rose DP (2009) Angiogenesis, adipokines and breast cancer. Cytokine Growth Factor Rev 20:193–201
Wang B, Wood IS, Trayhurn P (2007) Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch: Eur J Physiol 455:479–492
Wang Y, Nishina P, Naggert J (2009) Degradation of IRS1 leads to impaired glucose uptake in adipose tissue of the type 2 diabetes mouse model TALLYHO/Jng. J Endocrinol 203:65–74
Wellen K, Hotamisligil G (2003) Obesity-induced inflammatory changes in adipose tissue. J Clin Investig 112:1785–1788
Wellen K, Hotamisligil G (2005) Inflammation, stress, and diabetes. J Clin Investig 115:1111–1119
Wood IS, Wang B, Lorente-cebrián S, Trayhurn P (2007) Hypoxia increases expression of selective facilitative glucose transporters (GLUT) and 2-deoxy-D-glucose uptake in human adipocytes. Biochem Biophys Res Commun 361:468–473
Wood IS, Heredia FP, Wang B, Trayhurn P (2009) Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc 68:370–377
Xu A, Wang Y, Keshaw H, Xu L, Lam K, Cooper G (2003) The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Investig 112:91–100
Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY et al (2003) Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res 93:664–673
Yamauchi T, Hara K, Kubota N, Terauchi Y, Tobe K, Froguel P, Nagai R, Kadowaki T (2003) Dual roles of adiponectin/Acrp30 in vivo as an anti-diabetic and anti-atherogenic adipokine. Curr Drug Targets Immune Endocr Metabol Disord 3:243–254
Yamawaki H, Tsubaki N, Mukohda M, Okada M, Hara Y (2010) Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem Biophys Res Commun 393:668–672
Yang R-Z, Lee M-J, Hu H, Pray J, H-B W, Hansen BC et al (2006) Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab 290:E1253–E1261
Ye J (2009) Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes 33:54–66
Ye J, Gao Z, Yin J, He Q (2007) Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293:E1118–E1128
Yin K, Liao D, Tang C (2010) ATP-binding membrane cassette transporter A1 (ABCA1): a possible link between inflammation and reverse cholesterol transport. Mol Med 16:438–449
Yki-Järvinen H (2005) Fat in the liver and insulin resistance. Ann Med 37:347–356
Yki-Järvinen H (2015) Nutritional modulation of non-alcoholic fatty liver disease and insulin resistance. Forum Nutr 7:9127–9138
Yki-Järvinen H, Westerbacka J (2005) The fatty liver and insulin resistance. Curr Mol Med 5:287–295
Zhang X, Lam K, Ye H, Chung S, Zhou M, Wang Y, Xu A (2010) Adipose tissue-specific inhibition of hypoxia-inducible factor 1α induces obesity and glucose intolerance by impeding energy expenditure in mice. J Biol Chem 285:32869–32877
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Matafome, P., Seiça, R. (2017). Function and Dysfunction of Adipose Tissue. In: Letra, L., Seiça, R. (eds) Obesity and Brain Function. Advances in Neurobiology, vol 19. Springer, Cham. https://doi.org/10.1007/978-3-319-63260-5_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-63260-5_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-63259-9
Online ISBN: 978-3-319-63260-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)