Abstract
Narcissus tazetta (Amaryllidaceae) is a medicinal plant widely used for cut flowers and potted ornamental plant in Tunisia flora. The current study evaluated the phenolic composition and antioxidant properties of its flower extracts and investigated its potential protective activity against cadmium chloride (CdCl2)–induced hepatotoxicity in mice. Mice were divided into six groups of six each: group 1, serving as negative controls, received by intraperitoneal way only distilled water; group 2 received by intraperitoneal way CdCl2 (0.16 mg/kg bw); groups 3 and 4 received CdCl2 at the same dose of group 2 and 100 or 200 mg/kg bw of Narcissus tazetta flower extracts via oral route; groups 5 and 6, serving as positive controls, received only Narcissus tazetta flower extracts. Polyphenolic compounds of the extract were analyzed by colorimetric and high-performance liquid chromatography-mass spectrometry (HPLC-MS) methods. Total antioxidant activity and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging potential of the extract were estimated using colorimetric method. Results indicated that ethanolic flower extract contained high levels of total phenolic and flavonoid along with a strong total antioxidant and DPPH free radical scavenging activities. HPLC-MS analysis identified eight phenolic compounds, including rutin, kaempferol glycosides, and chlorogenic acids. The extract also exhibited marked hepatoprotective effects against CdCl2 toxicity by reducing hepatic levels of malondialdehyde, advanced oxidation protein products, hydrogen peroxide, metallothioneins, and DNA degradation. Additionally, co-administration of Narcissus tazetta flower extracts lowered the plasma activities of transaminases, gamma glutamyl transpeptidase, and lactate dehydrogenase and increased hepatic levels of reduced glutathione, nonprotein thiols, vitamin C, and catalase activity. The hepatoprotective effects of the extract were demonstrated by histopathological improvement of liver disorders. The current study provided ethnopharmacological application of Narcissus tazetta flower extracts against CdCl2-induced oxidative stress, suggesting its chemoprevention role of its phenolic compounds as a natural antioxidant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Narcissus tazetta (monocotyledon, Amaryllidaceae family), commonly known as Narjes, is widespread as an ornamental plant in the Mediterranean regions (Grey-Wilson and Mathew 1981). It is cultivated for its bulb trade, as well as for its essential oil and volatile compounds extracted from its fragrant white flowers (Chun Chen et al. 2013; Ruíz-Ramón et al. 2014). Its bulb extract was used in the Traditional Persian Medicine to treat internal ulcer, burns, nerve injury, skin stain, and inflammation. This plant also showed diverse pharmacological properties including anti-inflammatory, anti-cholinesterase, and anti-malaria activities (López et al. 2002; Sener et al. 2003). The study of Li et al. (2016) revealed an antioxidant activity of Narcissus tazetta var. chinensis bulb’s flavonoid compounds against hydrogen peroxide–induced human SH-SY5Y neuroblastoma cell impairment. Recently, Rameshk et al. (2018) evaluated the potential wound healing properties of Narcissus tazetta bulb extract and demonstrated anti-inflammatory and antioxidant activities of this species. Besides, Narcissus tazetta was shown to accumulate high amounts of cadmium in its roots, suggesting the possible use of this species not only in phytoremediation programs but also for protective effects against heavy metal–induced toxicity (Homeira Soleimani et al. 2019).
Cadmium, along with arsenic, chromium, lead, and mercury, is among the most toxic metals, presenting human health risks even at low concentrations, and they are categorized as human carcinogen by the International Agency for Research on Cancer. Cadmium is introduced in the environment through the anthropogenic actions following rapid progress of modern technologies and industries (Satarug 2019). It is commonly used in industrial procedures, for example, to protect steel from corrosion, stabilize polyvinyl chloride, and fabricate nickel-cadmium batteries, and it is used as a pigment and a neutron absorber in nuclear power plants (Godt et al. 2006). In the biosphere, it can reach the human body from industrial and manufacturing activities or from food, water, or air contamination and many other potential sources (Madejczyk et al. 2015). This metal is a multi-target cumulative toxicant, which mainly enters the body by inhalation or ingestion, causing severe toxicity in different organs including the kidney, liver, and skeletal system (Genchi et al. 2020). It induced toxicity through the overproduction of reactive oxygen species (ROS), via the Fenton reaction. Additionally, the toxic effect of cadmium ions, with high affinity to cysteine, is due to its binding to biological molecules with thiol groups, such as metallothioneins and reduced glutathione (Matovic et al. 2015). These alterations contribute to the impairment of biological membrane phospholipids, leading to protein and DNA damages, which in turn affect intrinsic pathways of apoptosis (Genchi et al. 2020). In this context, phenolic compounds have the ability to bind cadmium ions, owing to their structural richness in free hydroxyl groups. In addition, several flavonoids (catechin, quercetin, naringenin, and hesperetin) and phenolic acids (ferulic acid and gallic acid protocatechuate), extracted from plants, were shown to be able to protect against cadmium-induced hepatotoxicity via inhibition of inflammation and the enhancement of the antioxidant defense system (Li et al. 2018; Mężyńska and Brzóska 2018).
The pharmaceutical and therapeutic aspects of flavonoids and other phenolic compounds from medicinal plants are increasingly considered in both developed and developing countries, for alleviating xenobiotic-induced oxidative stress damages. Narcissus tazetta is widely grown in Tunisia flora; although to the best of our knowledge, there is no reports on polyphenol composition and protective effects of this species. Thus, this study identified, for the first time, polyphenol compounds and evaluated antioxidant and hepatoprotective potentials of Narcissus tazetta ethanolic flower extracts on CdCl2-intoxicated mice.
Materials and methods
In vitro study
Plant material collection
The flowers of Narcissus tazetta were harvested in February 2019 near Djedeida, Manouba (region located in the Medjerda valley at 25 km west of Tunis). Botanical identification was carried out by Chokri Messaoud, a professor at the National Institute of Applied Sciences and Technology. The plant was deposited at the herbarium of the Faculty of Tunis El-Manar; Tunisia, and its voucher specimen is not yet ready until now. The plant material was stored at room temperature and placed under dry conditions prior to use.
Preparation of Narcissus tazetta flower extracts
Five grams of air-dried flowers were extracted with 50-ml ethanol/water (4V/1V) under continuous stirring at room temperature for 30 min. The obtained aqueous ethanol extract was filtered under vacuum with Whatman filter paper (No. 1). The filtrate was concentrated to dryness under reduced pressure with a rotary evaporator (Stwartte RE 300) at 50 ± 1 °C. Residual aqueous fraction of the extract was further evaporated under nitrogen gas. The dried extract was kept at 4 °C prior to analysis.
Total phenolic content
The total phenolic content was determined using the Singleton and Rossi method (1965), modified by Dewanto et al. (2002). Briefly, an aliquot (5 μl) of flower extract (5 mg/ml) or standard solution of gallic acid was mixed with 20 μl of distilled water and 5 μl of Folin-Ciocalteu’s phenol reagent. After shaking, 52 μl of Na2CO3 (7%) and 41 μl of distilled water were added to the mixture. After an incubation period of 90 min at room temperature, the absorbance was measured at 760 nm with a microplate spectrophotometer reader (Multiskan Go Thermo, Life Sciences). The results were expressed as milligram gallic acid equivalent per gram of dry weight (mg GAE/g DW), through the calibration curve of gallic acid (0–500 mg/l).
Total flavonoid contents
The total flavonoid content was measured based on the aluminum chloride colorimetric analysis (Zhishen et al. 1999). An aliquot of plant extracts (16 μl) or standard solution of catechin was mixed with 75 μl of NaNO2 (7%). The mixture was shaken for 6 min before adding 10 μl of AlCl3 (10%). After 5 min, 33 μl of NaOH (1M) and 100 μl of distillated water were added. The absorbance of the mixture was determined at 510 nm, using a microplate spectrophotometer reader (Multiskan Go Thermo, Life Sciences). Using the calibration curve of catechin (0–500 mg/l), the results were expressed as milligram catechin equivalent per gram dry weight (mg CE/g DW).
Total antioxidant activity
The total antioxidant activity was determined using a colorimetric method as described by Prieto et al. (1999). In short, an aliquot of flower extracts (100 μl) was mixed with 1-ml reagent solution (sulfuric acid (H2SO4, 0.6 M), sodium phosphate (NaH2PO4H2O, 28 mM), and ammonium heptamolybdate (NH46Mo7O244H2O, 4 mM)). After an incubation period of 90 min at 95 °C, the absorbance was measured at 695 nm. Using the calibration curve of gallic acid (0–500 mg/l), the results were expressed as mg GAE/g DW.
DPPH scavenging activity assay
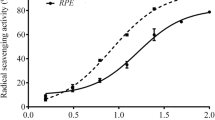
The DPPH free radical scavenging activity was determined following the method of Hatano et al. (1988). The sample was diluted in the solvent of extraction at different concentrations (10, 20, 100, and 200 μg/ml), and then 20 μl of sample was added to 180 μl of DPPH (0.2 mM/l) methanolic solution. After incubation in dark at room temperature for 30 min, the absorbance was read at 517 nm. The ability to scavenge DPPH radical was calculated using the following equation: DPPH= (A0-A1)/A0 ×100, where A0 and A1 stand for the absorbance at 30 min of control and sample, respectively. The scavenging activity was expressed as IC50 (μg/ml), which is the antiradical dose required to cause a 50 % inhibition.
Identification of phenolic compounds using HPLC-MS
The phenolic compounds present in Narcissus tazetta ethanolic flower extract were carried out using HPLC (Jasco-LC-Net II ADC; LC-2000Plus Series) equipped with a diode array detector and coupled with mass spectrometry (MS-ZMD4, Micromass, Waters Inc., Manchester, UK). A C18 reverse-phase analytical column (25 cm 202 length × 4.6 mm i.d., 5 μm particle size; Teknokroma, Barcelona, Spain) was used. Samples were eluted with a linear gradient of solvents A (0.1% aqueous formic acid solution) and B (0.1% formic acid in acetonitrile) at a flow rate of 1 ml/min at 30°C (Hamdi et al. 2017).
The MS was recorded in the 100 to 1000 m/z range within negative- and positive-electrospray ionization (ESI), which were obtained at ionization energies of 50 and 100 eV (negative mode) and 50 eV (positive mode). For analysis, the ESI MS parameters were as follow: capillary voltage (3 Kv), dissolving temperature (200 °C), source temperature (100 °C), and extractor voltage (12 V). Results were calculated from the means of three replicates. Phenolic compound structures were proposed by comparison of the retention time, UV spectrum, and product ion spectra in negative [M-H]− and positive [M+H]+ mode, with those of authentic standards.
Standards of rutin and chlorogenic acid were purchased from Sigma-Aldrich Quimica (Madrid, Spain; CAS Number: 153-18-4 and 9-97-327, respectively). Nicotiflorin and narcissin were purchased from Extrasynthese (Genay, France) with CAS Number 17650-84-9 and 604-80-8, respectively. All solvents were of HPLC grade purity (Romyl and Teknokroma, Barcelona, Spain); ethanol (CAS Number: 5-17-64), formic acid (96%) (CAS Number: 64-18-6), and acetonitrile (CAS Number: 75-05-08) were purchased from Sigma Chemical Co. (St. Louis, MO).
Quantification of individual phenolic compounds were expressed as mg/kg crude extract, through the calibration curve of authentic standards (0–250 μg for the flavonoids and 0–100 μg for phenolic acids). When standards were not available, tentative characterization was carried out by comparison of the experimental mass spectra with data from the scientific literature.
In vivo study
Animals and experimental design
Female Swiss albino mice, weighing approximately 30 g at their reception, were obtained from the Central Pharmacy (SIPHAT, Tunisia). They were maintained in a controlled condition: temperature (22 ± 2°C), humidity (40%), and photoperiod (12 h light-dark cycle). A commercial standard pellet diet (SNA, Sfax, Tunisia) and drinking water were provided to mice ad libitum. Animal care and all the experimental procedures were conducted with strict adherence to the Ethical Committee of Sciences Faculty of Sfax, with ethics approval (date: 18.05.2017, no: 1204), and were in accordance with the International Guidelines for Animal Care.
After 1 week of acclimatization, mice were randomly divided into six groups of six each: group 1, serving as negative controls, received by intraperitoneal way only distilled water; group 2 received by intraperitoneal way CdCl2 (0.16 mg/kg bw); groups 3 and 4 received CdCl2 at the same dose of group 2 and 100 or 200 mg/kg bw of Narcissus tazetta flower extracts via oral route; groups 5 and 6, serving as positive controls, received only Narcissus tazetta flower extracts for 8 days. Cadmium chloride anhydrous (puriss. p.a. > 99.0%) was purchased from Sigma-Aldrich (CAS number: 10108-64-2 04-008-00-3; St. Louis, MO, USA). The dose of this metal (0.16 mg/kg bw, corresponding to 1/20 of LD50), used in the present study, was selected on the basis of a previous study conducted by Smalinskiene et al. (2008).
At the end of the experimental period (8 days), animals of the aforementioned groups were killed by decapitation to avoid stress and suffering. The blood samples were immediately collected in heparinized tubes and centrifuged at 2200 × g for 10 min. Plasma samples were drawn and stored at −80 °C until analysis. Liver tissues were dissected out, cleaned from adipose tissue, and weighed. Some samples were homogenized in frozen Tris-HCl buffer (100 mM; pH 7.4). After centrifugation, the obtained supernatants were collected and maintained at −80 °C until biochemical analysis. Meanwhile, other samples were either fixed in 10 % of buffered formalin solution for histopathological studies or frozen at −80 °C for DNA assays.
Protein determination
Total protein content of the liver homogenates was measured according to Lowry et al. (1951), using bovine serum albumin as standard.
Liver malondialdehyde (MDA) measurement
The concentration of MDA, an index of lipid peroxidation, was determined according to the method described by Draper and Hadley (1990), and the results were expressed as nmol/mg protein.
Liver hydrogen peroxide (H2O2) measurement
The determination of H2O2 was carried out according to the method of Ou and Wolff (1996), using the ferrous ion oxidation xylenol orange reagent. The results were expressed as μmol/mg protein.
Liver advanced oxidation protein product content (AOPP)
Liver AOPP levels were determined according to the method of Witko et al. (1992), using the extinction coefficient 261 cm−1 mM−1. The results were expressed as nmol/mg protein.
Assessment of DNA degradation
The extraction of the total DNA from liver tissue (50 mg) was carried out as previously described by the Clark and Melki (2002) method using cetyltrimethyl ammonium bromide buffer. After loading onto an agarose gel (1 %), the DNA content in liver of each treatment was recorded at 260 nm according to Sambrook and Russell (2001). The DNA content was expressed as mg/g of tissue.
Liver non-enzymatic antioxidant levels
Reduced glutathione (GSH) levels were estimated based on the reduction of 5,5′dithiobis(2-nitrobenzoic acid) with glutathione (Ellman 1959; Jollow et al. 1974). The values of GSH were expressed as nmol/mg protein.
Nonprotein thiols (NPSH) levels were determined by the method of Ellman (1959). Absorbance of the colorimetric reaction mix was measured at 412 nm. NPSH content was expressed as nmol/mg protein.
Vitamin C (Vit C) levels were determined by the dinitrophenyl hydrazine method, following Jacques-Silva et al. (2001). The data were expressed as nmol/mg protein.
Metallothioneins (MTs) content in the liver was assayed according to Viarengo et al. (Viarengo 1997)) as modified by Petrovic et al. (2001). Absorbance was measured at 412 nm. The data were expressed as μmol/mg protein.
Liver enzymatic antioxidant activities
Superoxide dismutase (SOD) activity was determined at 560 nm by the Beauchamp and Fridovich method (1971), and the enzyme activity was expressed as U/mg protein.
Catalase (CAT) activity in liver tissue was measured at 240 nm by the Aebi method (1984), and the values were expressed as μmol H2O2 consumed/min/mg protein.
Glutathione peroxidase (GPx) activity was estimated according to the method described by Flohe and Günzler (1984). Enzyme activity was expressed as nmol GSH oxidized/min/mg protein.
Biomarkers of liver toxicity in plasma
Plasma activities of hepatic enzymatic markers aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma glutamyl transpeptidase (γGT) were estimated using the commercially available diagnostic kits (Biomaghreb, Ariana, Tunisia, Ref. 20046, 20042, and 20021). The plasma activities of AST, ALT, and γGT were expressed as IU/l.
Determination of lactate dehydrogenase in plasma and liver
Plasma and liver activities of lactate dehydrogenase (LDH) were measured using the commercially available diagnostic kit (Biomaghreb, Ariana, Tunisia, Ref: 20011), and the results were expressed as IU/l and IU/g of tissue, respectively.
Histological examination
The samples of liver tissue were fixed in a 10 % formalin solution, dehydrated through a graded series of alcohol (70 to 90 %), cleaned in toluene, and then embedded in paraffin. A Leica Microsystems microtome was used to prepare tissue sections, at a thickness of 5 μm. Each tissue section was deparaffinized with toluene, rehydrated using a series of alcohol, and lastly stained with hematoxylin-eosin (Suvarna et al. 2013). Each liver slide was examined under a light microscope. Liver sections were examined and assigned for severity of changes using scores on a scale of none (−), mild (+), moderate (++), high (+++), and severe (++++) damages.
Statistical analysis
All the data presented the mean values of three and six repeats in plant extracts and different animal groups, respectively. The data were represented by means ± standard deviation, and one-way analysis of variance (ANOVA, CoStat software, version 6.4, CoHort Software, Monterey, CA) was applied. Statistical assessments of differences between mean values were analyzed using Duncan’s multiple range test at a probability of P ≤u0.05.
Results
Evaluation of levels of total polyphenols, flavonoids, antioxidant, and free radical scavenging activities
In this study, phytochemical characterization of Narcissus tazetta flower ethanolic extracts was evaluated, relative to its total phenolic and flavonoid contents. Results showed that the extracts contained total polyphenols at 64.14 mg GAE/g DW and flavonoids at 70.79 mg CE/g DW (Table 1). In addition, the results revealed that the flower extracts of this species exhibited 134.57 mg GAE/g DW of the total antioxidant activity. Its IC50 values to quench DPPH radical were 29 μg/ml, indicative of an important aptitude for its phenolic fraction to scavenge free radicals (Table 1).
HPLC-MS identification of polyphenolic compounds
A typical HPLC-MS profile of polyphenol constituents of Narcissus tazetta flower ethanolic extracts was presented in Figure 1. The analysis showed the presence of three phenolic acids (two mono-caffeoylquinic acid isomers (1 and 2) and one chlorogenic acid (5-O-caffeoylquinic acid (5-CQA)) and five flavonols (kaempferol-di-O-glucoside, rutin (quercetin-3-O-rutinoside), kaempferol-3-O-rutinoside, nicotiflorin (kaempferol-3-O-rutinoside), and narcissin (isorhamnetin-3-O-rutinoside)) (Fig. 1).
The quantitative analysis revealed that rutin (47109.5 mg/kg crude extract) was the most abundant flavonol, followed by nicotiflorin (2046.7 mg/kg crude extract), kaempferol-di-O-glucoside (515.9 mg/kg crude extract), narcissin (384.3 mg/kg crude extract), and kaempferol-3-O-rutinoside (91.6 mg/kg crude extract). Concentrations of chlorogenic acid isomers 1 and 2 and those of chlorogenic acid (5-O-caffeoylquinic acid (5-CQA)) were 1766.5, 8307.5, and 257.7 mg/kg crude extract, respectively (Table 2).
Liver oxidative stress biomarkers and DNA degradation
In CdCl2-treated mice, MDA, H2O2 and AOPP levels, and DNA degradation increased by 52, 80, 159, and 58 % respectively, as compared with controls (Table 3). Co-administration of Narcissus tazetta flower extracts significantly reduced the levels of studied oxidative stress marks and repaired DNA degradation relative to CdCl2-treated mice. There was no differences between controls and mice treated only by Narcissus tazetta flower extracts (Table 3).
Non-enzymatic antioxidant status in the liver
The levels of non-enzymatic antioxidants such as GSH, NPSH, Vit C, and MTs were determined in the liver of experimental animals (Table 3). Results showed that CdCl2 treatment decreased significantly the levels of GSH (−44 %), NPSH (−36 %), and Vit C (−34 %), while the liver MTs content was increased by 87 % after CdCl2 intoxication, relative to control. These alterations were corrected following co-administration of Narcissus tazetta flower ethanolic extracts, compared to CdCl2-treated mice (Table 3).
Enzymatic antioxidant stress biomarkers
The CdCl2 treatment increased the activities of SOD and GPx by 47 and 40 %, respectively, and decreased those of CAT by 42 % as compared to controls (Fig. 2). Co-administration of Narcissus tazetta flower ethanolic extracts restored the activities of SOD and GPx (Fig. 2A and C) and upregulated those of CAT (Fig. 2B), near to the control values, relative to those of CdCl2-treated mice.
Enzymatic antioxidant activities of superoxide dismutase (A), catalase (B), and glutathione peroxidase (C) in the liver of control and treated mice with CdCl2, CdCl2 with Narcissus tazetta ethanolic flower extracts (100 or 200 mg/kg), or Narcissus tazetta flower extracts (100 or 200 mg/kg), during 8 days. Means of six animals in each group ± standard deviation. Bars labeled with different letters are significantly different according to Duncan’s multiple range test at P ≤ 0.05
Plasma and liver biochemical parameters
Plasma AST, ALT, and γGT activities increased in CdCl2-treated mice by 59, 90, and 180 %, respectively, when compared to controls. In addition, LDH activity increased by 140 % in the plasma and decreased by 42 % in the liver of CdCl2-treated mice, in comparison with controls. Co-administration of Narcissus tazetta flower extracts decreased the leakage of these hepatic enzymes (Table 4).
Histopathological analysis
Histological analysis showed normal structure of liver in controls and in those treated only by Narcissus tazetta flower extracts (Fig. 3A, E, and F). After 8 days of CdCl2 treatment, there was marked disorganization of hepatic architecture. In fact, central and portal veins were dilated and surrounded with leukocytes infiltration (Fig. 3B1 and B2). Most of the hepatocytes appeared fused together or extensively deteriorated with necrosis. Cadmium chloride induced also the progress of numerous micro-vesicular steatosis (Fig. 3B3) and the appearance of granulomatous inflammation (Fig. 3B4). In animals co-administered with Narcissus tazetta flower extracts, the central and portal veins regained their normal structure. Inflammatory cells progressively decreased with the flower extracts mainly at 200 mg/kg bw. The hepatocytes appeared to regain their normal organization and structure with the disappearance of the micro-vesicular steatosis (Fig. 3C and D). The histopathological changes are graded and summarized in Table 5.
Liver histological sections of control (A), treated mice with CdCl2 (B1, B2, B3, and B4), CdCl2 co-treated with Narcissus tazetta ethanolic flower extracts at 100 or 200 mg/kg (C and D, respectively), or treated only with Narcissus tazetta ethanolic flower extract at 100 and 200 mg/kg (E and F, respectively). Coloration hematoxylin and eosin. Magnification ×400. B1 and B2 Marked dilatation of center and portal veins surrounded with leucocytes infiltration; B3 extensive degeneration of hepatocytes with necrosis and micro-vesicular steatosis (black arrows); B4 marked granuloma inflammatory disorders (yellow arrows); C normal central and portal veins and mild organization of hepatocytes; D normal appearance of the liver histoarchitecture
Discussion
Based on their richness in bioactive components including polyphenols, medicinal and aromatic plants have been largely used in traditional medicine as remedies to treat heavy metal–induced liver damages. In this study, Narcissus tazetta flower extracts contained considerable amounts of total polyphenols and flavonoids compounds, confirming that bioactive molecules of the Amaryllidaceae family are not just limited to alkaloids, but they are able to synthesize a diverse range of polyphenols, with well-known antioxidant activities properties (Bati Ay et al. 2018; Benedec et al. 2018). Actually, the antioxidant capacity of plant extracts containing polyphenols might be evaluated through several techniques. Evaluation of this study revealed that Narcissus tazetta flower extracts exhibited a strong total antioxidant activity and a low IC50 scavenging ability of DPPH, which can be explained by the presence of phenolic compounds in its flower extracts. Indeed, the scavenging properties of polyphenols are owned to the presence of one or more hydroxyl groups and phenolic ring, which are able to donate an electron and/or a hydrogen atom to free radicals (Erkan et al. 2011). These properties may be principally attributed to flavonols (rutin, nicotiflorin, narcissin, kaempferol-di-O-glucoside, and kaempferol-3-O-rutinoside) and/or chlorogenic acids derivatives (two mono-caffeoylquinic acid isomers and one 5-O-caffeoylquinic acid) identified in Narcissus tazetta ethanolic flower fraction. In the same context, Li et al. (2015) identified various glycosides forms of quercetin, kaempferol, and isorhamnetin and one chlorogenic acid derivative in different cultivars of Narcissus extracts, suggesting that this genus of Amaryllidaceae plants would offer important biological activities. Benedec et al. (2018) reported also that Amaryllidaceae plants, including Narcissus poeticus, Narcissus pseudonarcissus, Galanthus nivalis, and Leucojum vernum, were rich in chlorogenic and p-coumaric acids, with important anti-staphylococcal and antifungal activities.
The anti-peroxidative properties of Narcissus tazetta flower extracts, at tested doses, on CdCl2 toxicity was assessed by a significant decrease in MDA and H2O2 levels, remembering its richness in aforementioned flavonol and chlorogenic acid compounds. In particular, rutin, the most abundant compound identified in its flower extracts, was described as potential molecule to alleviate cell disorders induced by oxidative stress. This flavonol may interact with the polar head of membrane phospholipids, resulting in the improvement and protection against oxidative stress–induced membrane damages (Erlejman et al. 2004). It may protect cells through attenuating H2O2-induced apoptosis (Gong et al. 2010). In addition, glycoside kaempferols, recognized in Narcissus tazetta flower extracts, might be involved in the alleviation of CdCl2-induced hepatotoxicity in mice (Zang et al. 2017). For instance, nicotiflorin, the most abundant glycoside kaempferol of Narcissus tazetta flower extracts, has probably an important role in attenuating CdCl2-induced liver injuries. In fact, according to Zhao et al. (2017), the extraction of this flavonol from Nymphaea candida has antioxidant activity on concanavalin A and D-galactosamine–induced liver injuries in mice via MDA level reduction. Chlorogenic acid constituents, identified in Narcissus tazetta flower extracts by HPLC-MS, was also revealed to have significant ability to mitigate carbon tetrachloride (CCl4)–induced hepatotoxicity in rats (Shi et al. 2016). These properties would be owned to their one-electron oxidation products formed after interaction with free radicals, resulting in their broken down (Shibata et al. 1999).
Toxicity of CdCl2 may also induce protein oxidation and damage to DNA structure, giving rise to mutations and/or cell death (Jadoon and Malik 2017). In this study, it is reasonable to conclude that Narcissus tazetta flower extracts could modulate liver AOPP levels and DNA degradation. This was again referred to the presence of rutin, as this compound was reported to prevent increased DNA fragmentation and inhibit inflammation and apoptosis (Gong et al. 2010). It is noteworthy to not exclude the importance of the other identified flavonols and chlorogenic acid isomers in the protection of the liver against CdCl2, through targeting pathway of ROS activation, protein oxidation, and DNA degradation of hepatocytes.
Chemo-protective effects of natural antioxidant components were reported to regenerate and/or preserve intracellular non-enzymatic antioxidants to their normal levels. In particular, Narcissus tazetta flower extracts restored the hepatic GSH, NPSH, and Vit C levels of CdCl2-treated mice, which became comparable to normal values. It may be suggested that phenolic constituents of this plant either acted synergistically or separately to prohibit the binding of Cd to –SH groups of GSH and NPSH and to increase Vit C content via activating the activity of gulonooxidase (Girja and Chandrat 1998). Consideration on rutin’s role to regenerate GSH levels was reported by Gong et al. (2010), proposing that this molecule might rise up the intracellular GSH under oxidative stress conditions. According to Mirani et al. (2012), co-administration of rutin restored GSH and Vit C contents to normal values in the liver of cadmium-intoxicated mice. Related studies on other xenobiotic intensified liver injury confirmed that kaempferol (Xu et al. 2019; Zang et al. 2017) and chlorogenic acid (Shi et al. 2016) significantly restored liver glutathione consumption.
Metallothioneins act as antioxidants and free radical scavengers due to their (–SH) groups presents in their cysteine residues. They may play a protective role against the cytotoxicity of cadmium and other heavy metals through ROS detoxification under oxidative stress conditions (Jurczuk et al. 2006; Genchi et al. 2020). In our study, enhanced hepatic levels of MTs following CdCl2 exposure contradicted with the aforementioned disturbances. In fact, these antioxidants would further increase cadmium retention in hepatocytes, exceeding their ability to bind with this metal. The reduction of its level after co-administration of Narcissus tazetta flower extracts might be accredited to phenolic compounds, essentially to rutin’s ability to decrease cadmium absorption from the gastrointestinal tract (Genchi et al. 2020).
The promising therapeutic effects of plant extracts on liver injuries may be involved in the amelioration of antioxidative defense enzymes. For instance, enzymes like SOD, CAT, and GPx are crucial in the defense of living organs against ROS, and the alteration in their activities might restrict cell protection against oxidative damages (Chater et al. 2008). In our study, CdCl2 increased liver activities of SOD and GPx enzymes but reduced those of CAT. These results would be probably due to enhanced H2O2 production through SOD activity, resulting in the exhaustion of CAT detoxification capacity (Jurczuk et al. 2004). Co-administration of Narcissus tazetta flower extracts modulated SOD, CAT, and GPx activities, designating the involvement of its phenolic constituents, most likely rutin, in harmonizing activities of these enzymes. In this way, Mirani et al. (2012) assumed that rutin was able to restore hepatic SOD, CAT, and GST activities to normal values in cadmium-intoxicated mice. In other studies, rutin was proposed to restore the activities of these enzymes under nonalcoholic fatty liver disease and CCl4 exposure (Liu et al. 2017; Ravi et al. 2018). Besides, kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside, isolated from Carthamus tinctorius, restored the SOD and CAT activities, indorsing great antioxidant properties of these compounds on CCl4-induced oxidative liver injury (Wanga et al. 2018). According to Shi et al. (2016), restoration of hepatic activities of SOD and CAT was accredited to chlorogenic acid importance to increase expression levels of nuclear Nrf2 and Nrf2-regulated anti-oxidant genes in treated mice with CCl4.
Liver injuries, including lipid peroxidation and imbalances in the antioxidant system, may induce the release of hepatic enzymes, an indicator of cell membranes integrity loss (Paul et al. 2013). In the current study, co-administration of Narcissus tazetta flower extracts reduced the release of AST, ALT, γGT, and LDH enzymes, proposing the involvement of flavonols and/or chlorogenic acids of this plant in the stabilization of hepatocyte membranes. Similarly, aqueous extracts of Raphanus sativus and Monodora myristica reduced serum activities of ALT, AST, LDH, and γGT in CdCl2-induced liver damage in mice (Emmanuel Oyinloye et al. 2016; He et al. 2019). Shehab et al. (2015) highlighted that the presence of abundant rutin concentration in Zygophyllum hamiense prompted the downregulation of AST activity in blood of CCl4-intoxicated mice. Kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside prevented CCl4-induced elevation of AST and alkaline phosphatase activities in blood, restoring therefore hepatic membrane integrity (Wanga et al. 2018). Isorhamnetin 3-O-glucoside extracted from Atsumi-kabu leaves significantly reduced plasma activities of ALT and AST, supported the effective role of this metabolite to alleviate CCl4 induced liver injury in mice (Igarashi et al. 2008). In this regard, the tendency of liver enzymatic biomarker to return to near normalcy may indicate hepatocyte tissue regeneration as described in our study. Indeed, the non-expression of histological alterations after co-administration of Narcissus tazetta flower extracts was revealed mainly at the dose 200 mg/kg, suggesting that the chemopreventive role of its phenolic compounds was in a dose-dependent manner.
To this end, it might be hypothesized that phenolic constituents of Narcissus tazetta ethanolic flower extracts either acted synergistically or separately to inhibit lipid peroxidation; prevent GSH, NPSH, and Vit C depletion; and decrease transaminases, LDH, and GGT leakage to plasma, therefore playing a protective role against CdCl2-mediated liver injury. Additional investigations are required to understand the action mode of the identified phenolic compounds to challenge liver enzymes leakage and histoarchitecture disorders. Investigations, evaluating the impact of Narcissus tazetta flower extracts on cell membrane fatty acid composition, will be also of great importance due to its involvement to maintain membrane integrity.
Conclusion
The current study indicated that CdCl2 induces hepatotoxicity resulting in the rise of oxidative stress markers and the disturbance of biochemical and histopathological parameters. Ethanol flower extracts of Narcissus tazetta revealed potential defensive effects against this toxicant. Indeed, HPLC-MS analysis identified many phenolic compounds including flavonols and chlorogenic acid isomers, which may have synergetic antioxidant, radical scavenging activity, and hepatoprotective properties. Our study on Narcissus tazetta flowers, collected from Tunisia flora, may be a prominent finding through offering new friendly alternative drug against CdCl2-induced hepatotoxicity.
Data availability
Not applicable
Abbreviations
- AOPP:
-
Advanced oxidation protein products
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- CdCl2 :
-
Cadmium chloride
- CAT:
-
Catalase
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- γGT:
-
Gamma glutamyl transpeptidase
- GPx:
-
Glutathione peroxidase
- HPLC:
-
High-performance liquid chromatograph
- H2O2 :
-
Hydrogen peroxide
- LDH:
-
Lactate dehydrogenase
- MDA:
-
Malondialdehyde
- MS:
-
Mass spectrometry
- MTs:
-
Metallothioneins
- NPSH:
-
Nonprotein thiols
- ROS:
-
Reactive oxygen species
- GSH:
-
Reduced glutathione
- SOD:
-
Superoxide dismutase
- Vit C:
-
Vitamin C
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Bati Ay E, Gül M, Açikgöz AM, Yarilgaç T, Kara ŞM (2018) Assessment of antioxidant activity of giant snowdrop (Galanthus elwesii Hook) extracts with their total phenol and flavonoid contents. Indian J Pharm Educ Res 52(4):128–132. https://doi.org/10.5530/ijper.52.4s.88
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gel. Anal Biochem 44(1):276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Benedec D, Oniga I, Hanganu D, Gheldiu AM, Pușcaș C, Silaghi-Dumitrescu R, Duma M, Tiperciuc B, Vârban R, Vlase L (2018) Sources for developing new medicinal products: biochemical investigations on alcoholic extracts obtained from aerial parts of some Romanian Amaryllidaceae species. BMC Complement Altern Med 18(226):1–12. https://doi.org/10.1186/s12906-018-2292-8
Chater S, Douki T, Garrel C, Favier A, Sakly M, Abdelmelek H (2008) Cadmium-induced oxidative stress and DNA damage in kidney of pregnant female rats. C R Biol 331(6):426–432. https://doi.org/10.1016/j.crvi.2008.03.009
Chun Chen H, Shan Chi H, Yun Lin L (2013) Headspace solid-phase microextraction analysis of volatile components in Narcissus tazetta var. chinensis Roem. Molecules 18:13723–13734. https://doi.org/10.3390/molecules181113723
Clark SJ, Melki J (2002) DNA methylation and gene silencing in cancer: which is the guilty party? Oncogene 21(35):5380–5387. https://doi.org/10.1038/sj.onc.1205598
Dewanto V, Wu X, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014. https://doi.org/10.1021/jf011558
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431. https://doi.org/10.1016/0076-6879(90)86135-I
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Emmanuel Oyinloye B, Adenowo AF, Osunsanmil FO, Ogunyinka BI, Nwozo SO, Paul Kappol A (2016) Aqueous extract of Monodora myristica ameliorates cadmium-induced hepatotoxicity in male rats. Springer Plus 5:641. https://doi.org/10.1186/s40064-016-2228-z
Erkan N, Akgonen S, Ovat S, Goksel G, Ayranci E (2011) Phenolic compounds profile and antioxidant activity of Dorystoechas hastata L. Boiss et Heldr. Food Res Int 44:3013–3020. https://doi.org/10.1016/j.foodres.2011.07.015
Erlejman AG, Verstraeten SV, Fraga CG, Oteiza PI (2004) The interaction of flavonoids with membranes: potential determinant of flavonoid antioxidant effects. Free Radic Res 38(12):1311–1320. https://doi.org/10.1080/10715760400016105
Flohe L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121. https://doi.org/10.1016/S0076-6879(84)05015-1
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17(11):3782. https://doi.org/10.3390/ijerph17113782
Girja SS, Chandrat SV (1998) Cadmium toxicity and bioantioxidants: status of vitamin E and ascorbic acid of selected organs in rat. J Appl Toxicol 9(2):119–122. https://doi.org/10.1002/jat.2550090209
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, Groneberg DA (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 1(22):1–6. https://doi.org/10.1186/1745-6673-1-22
Gong G, Qin Y, Huang W, Zhou S, Yang X, Li D (2010) Rutin inhibits hydrogen peroxide-induced apoptosis through regulating reactive oxygen species mediated mitochondrial dysfunction pathway in human umbilical vein endothelial cells. Eur J Pharmacol 628(1-3):27–35. https://doi.org/10.1016/j.ejphar.2009.11.028
Grey-Wilson C, Mathew B (1981) The bulbous plants of Europe and their allies. Collins, London
Hamdi A, Jaramillo-Carmona S, Srairi Beji R, Tej R, Zaoui S, Rodríguez-Arcos R, Jiménez-Araujo A, Kasria M, Lachaal M, Karray Bouraoui N, Guillén-Bejarano R (2017) The phytochemical and bioactivity profiles of wild Asparagus albus L. plant. Food Res Int 99(Pt 1):720–729. https://doi.org/10.1016/j.foodres.2017.06.027
Hatano T, Kagawa H, Yasuhara T, Okuda T (1988) Two new flavonoids and other constituents in licorice root their relative astringency and radical scavenging effect. Chem Pharm Bull 36:1090–1097. https://doi.org/10.1248/cpb.36.2090
He Q, Luo Y, Zhang P, An C, Zhang A, Li X, You L, Liu C (2019) Hepatoprotective and antioxidant potential of radish seed aqueous extract on cadmium-induced hepatotoxicity and oxidative stress in mice. Phcog Mag 15:283–289. https://doi.org/10.4103/pm.pm_365_18
Homeira Soleimani S, Bernard F, Amini M, Khavari-nezhad RA (2019) Cadmium accumulation and alkaloid production of Narcissus tazetta plants grown under in vitro condition with cadmium stress. Plant Physiol Rep 25:51–57. https://doi.org/10.1007/s40502-019-00476-6
Igarashi K, Mikami T, Takahashi Y, Sato H (2008) Comparison of the preventive activity of isorhamnetin glycosides from Atsumi-kabu (red turnip, Brassica, campestris L.) leaves on carbon tetrachloride-induced liver injury in mice. Biosci. Biotechnol. Biochem. 72(3):856–860. https://doi.org/10.1271/bbb.70558
Jacques-Silva MC, Nogueira CW, Broch LC (2001) Diphenyldiselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. Pharmacol Toxicol 88(33):119–125. https://doi.org/10.1034/j.1600-0773.2001.d01-92.x
Jadoon S, Malik A (2017) DNA Damage by heavy metals in animals and human beings: an overview. Biochem Pharmacol 6(3):1000235. https://doi.org/10.4172/2167-0501.1000235
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11(3):151–169. https://doi.org/10.1159/000136485
Jurczuk M, Brzóska MM, Moniuszko-Jakoniuk J, Gäazyn-Sidorczuk M, Kulikowska-Karpinska E (2004) Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem Toxicol 42(3):429–438. https://doi.org/10.1016/j.fct.2003.10.005
Jurczuk M, Moniuszko-Jakoniuk J, Brzoska MM (2006) Involvement of some low-molecular thiols in the peroxidative mechanisms of lead and ethanol action on rat liver and kidney. Toxicology 219(1-3):11–21. https://doi.org/10.1016/j.tox.2005.10.022
Li X, Lu M, Tang D, Shi Y (2015) Composition of carotenoids and flavonoids in Narcissus cultivars and their relationship with flower Color. PLoS One 10(11):1–14. https://doi.org/10.1371/journal.pone.0142074
Li FK, Li X, Ye J, Lu L, Ke Xu X, Liang Li H, Dong Zhang W, Heng Shen Y (2016) Chemical constituents of Narcissus tazetta var. chinensis and their antioxidant activities. Fitoterapia 113:110–116. https://doi.org/10.1016/j.fitote.2016.07.013
Li S, Yue Tan H, Wang N, Cheung F, Hong M, Feng Y (2018) The potential and action mechanism of polyphenols in the treatment of liver diseases. Oxidative Med Cell Longev 2018:1–25. https://doi.org/10.1155/2018/8394818
Liu Q, Pana R, Dinga L, Zhanga F, Hua L, Dinga B, Zhue L, Xiad Y, Doua X (2017) Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid induced oxidative injuries. Int Immunopharmacol 49:132–141. https://doi.org/10.1016/j.intimp.2017.05.026
López S, Jaume Francesc B, Carles VC (2002) Acetylcholinesterase activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci 71(21):2521–2529. https://doi.org/10.1016/S0024-3205(02)02034-9
Lowry OH, Rosenbrough NJ, Farr AL (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Madejczyk MS, Baer CE, Dennis WE, Minarchick VC, Leonard SS, Jackson DA, Stallings JD, Lewis JA (2015) Temporal changes in rat liver gene expression after acute cadmium and chromium exposure. PLoS One 10:e0127327. https://doi.org/10.1371/journal.pone.0127327
Matovic V, Buha A, Dukic-Cosic D, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol 78:130–140. https://doi.org/10.1016/j.fct.2015.02.011
Mężyńska M, Brzóska MM (2018) Review of polyphenol-rich products as potential protective and therapeutic factors against cadmium hepatotoxicity. J Appl Toxicol 39(1):117–145. https://doi.org/10.1002/jat.3709
Mirani N, Ashraf J, Siddique J, Rub A (2012) Protective effect of rutin against cadmium induced hepatotoxicity in Swiss albino mice. J Pharmacol Toxicol 7(3):150–157. https://doi.org/10.3923/jpt.2012.150.157
Ou P, Wolff SP (1996) A discontinuous method for catalase determination at near physiological concentrations of H2O2 and its application to the study of H2O2 fluxes within cells. J Biochem Biophys Methods 31:59–67. https://doi.org/10.1016/0165-022X(95)00039-T
Paul A, Das J, Das S, Samadder A, Khuda-Bukhsh Poly AR (2013) Poly (lactide-co-glycolide) nano-encapsulation of chelidonine, an active bioingredient of greater celandine (Chelidonium majus), enhances its ameliorative potential against cadmium induced oxidative stress and hepatic injury in mice. Environ Toxicol Pharmacol 36(3):937–947. https://doi.org/10.1016/j.etap.2013.08.008
Petrovic S, Ozretic B, Krajnovic-Ozretic M, Bobinac D (2001) Lysosomal membranestability and metallothioneins in digestive gland of mussels (Mytilusgallo provincialis Lam.) as biomarkers in a field study. Mar Pollut Bull 42(12):1373–1378. https://doi.org/10.1016/S0025-326X(01)00167-9
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341. https://doi.org/10.1006/abio.1999.40199
Rameshk M, Sharififar F, Mehrabani M, Pardakhty A, Farsinejad A, Mehrabani M (2018) Proliferation and in vitro wound healing effects of the microniosomes containing Narcissus tazetta L. Bulb Extract on Primary Human Fibroblasts (HDFs). DARU J Pharm Sci 26:31–42. https://doi.org/10.1007/s40199-018-0211-7
Ravi GS, Narayana Charyulu R, Dubey A, Prabhu P, Hebbar S, Candida Mathias A (2018) Nano-lipid complex of rutin: development, characterisation and in vivo investigation of hepatoprotective, antioxidant activity and bioavailability study in rats. AAPS PharmSciTech 19(8):3631–3649. https://doi.org/10.1208/s12249-018-1195-9
Ruíz-Ramón F, Águila DJ, Egea-Cortines M, Weiss J (2014) Optimization of fragrance extraction: daytime and flower age affect scent emission in simple and double narcissi. Ind Crop Prod 52:671–678. https://doi.org/10.1016/j.indcrop.2013.11.034
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. CSHL Press, New York, pp 577–581
Satarug S (2019) Cadmium sources and toxicity. Toxics 7(2):25. https://doi.org/. https://doi.org/10.3390/toxics7020025
Sener B, Orhan I, Satayavivad J (2003) Antimalarial activity screening of some alkaloids and the plant extracts from Amaryllidaceae. Phytother Res 17(10):1220–1223. https://doi.org/10.1002/ptr.1346
Shehab NG, Abu-Gharbieh E, Bayoumi FA (2015) Impact of phenolic composition on hepatoprotective and antioxidant effects of four desert medicinal plants. BMC Complement Altern Med 15(401):1–12. https://doi.org/10.1186/s12906-015-0919-6
Shi H, Shi A, Dong L, Lu X, Wang Y, Zhao J, Dai F, Guo X (2016) Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin Nutr 35(6):1366–1373. https://doi.org/10.1016/j.clnu.2016.03.002
Shibata H, Sakamoto Y, Oka M, Kono Y (1999) Natural antioxidant, chlorogenic acid, protects against DNA breakage caused by monochloramine. Biosci Biotechnol Biochem 63(7):1295–1297. https://doi.org/10.1271/bbb.63.1295
Singleton VL, Rosi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Smalinskiene A, Lesauskaite V, Savickiene N, Zitkevicius V, Savickas A, Ryselis S, Kregzdyte R, Abdrakhmanov O, Sadauskiene I, Ivanov L (2008) The relationship of Echinacea purpurea to the toxicity of cadmium. Pharm Biol 43(9):797–802. https://doi.org/10.1080/13880200500408590
Suvarna SK, Layton C, Bancroft JD (2013) Bancroft’s theory and practice of histological techniques, 7th edn. Churchill Livingston, NewYork, p 637
Viarengo A, Ponzano E, Dondero F, Fabbri R (1997) A simple spectrophotometric methodfor metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic mollusks. Mar Environ Res 44:69–84. https://doi.org/10.1016/S0141-1136(96)00103-1
Wanga H, Chena L, Zhanga X, Xua L, Xiea B, Shia H, Duana Z, Zhangc H, Rena F (2018) Kaempferol protects mice from D-GalN/LPS-induced acute liver failure by regulating the ER stress-Grp78-CHOP signaling pathway. Biomed Pharmacother 111:468–475. https://doi.org/10.1016/j.biopha.2018.12.105
Willems J, Khamis MM, Mohammed Saeid W, Purves RW, Katselis G, Low N, El-Aneed A (2016) Analysis of a series of chlorogenic acid isomers using differential ion mobility and tandem mass spectrometry. Anal Chim Acta 933:164–174. https://doi.org/10.1016/j.aca.2016.05.041
Witko V, Nguyen AT, Descamps-Latscha B (1992) Microtiter plate assay for phagocyte-derived taurine chloramines. J Clin Lab Anal 6(1):47–53. https://doi.org/10.1002/jcla.1860060110
Xu T, Huang S, Huang Q, Ming Z, Wang M, Li R, Zhao Y (2019) Kaempferol attenuates liver fibrosis by inhibiting activin receptor–like kinase 5. J Cell Mol Med 23:6403–6410. https://doi.org/10.1111/jcmm.14528
Zang Y, Zhang D, Yu C, Jin C, Igarashi K (2017) Antioxidant and hepatoprotective activity of kaempferol 3-O-b-D- (2,6-di-O-a-L-rhamnopyranosyl) galactopyronoside against carbon tetrachloride-induced liver injury in mice. Food Sci Biotechnol 26(4):1071–1076. https://doi.org/10.1007/s10068-017-0170-7
Zhao J, Zhang S, You S, Liu T, Xu F, Ji T, Gu Z (2017) Hepatoprotective Effects of nicotiflorin from Nymphaea candida against concanavalin A-induced and D-galactosamine-induced liver injury in mice. Int J Mol Sci 18(3):587. https://doi.org/10.3390/ijms18030587
Zhishen J, Tang M, Wu J (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64(4):555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Acknowledgements
The current work was supported by the Ministry of Higher Education and Laboratory of Plant Productivity and Environmental Constraints (LR18ES04). The authors are grateful to Dr. Abdelali Hannoufa, research scientist-adjunct professor at Agriculture and Agri-Food Canada, for critical reading of the manuscript and Mr. Mejri Hssan for his skillful technical assistance.
Funding
This work received financial assistance from the Ministry of Higher Education and Scientific Research of Tunisia.
Author information
Authors and Affiliations
Contributions
SBA and MS were responsible for most of the work; NS contributed to data analysis, AH contributed to the biochemical assays and data analysis; SB contributed to the molecular assays; SBA wrote the manuscript draft; MEL and NKB edited and revised the manuscript and supervised the research project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The animals received humane care in accordance with the International Guidelines for Animal Care and with strict adherence to the Ethical Committee of Faculty of Sciences, Sfax, Tunisia, under the ethical approval no. 1204.
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ben-Abdallah, ., Sefi, M., Soudani, N. et al. Potential antioxidant effects of Narcissus tazetta phenolic compounds against cadmium chloride–induced hepatotoxicity in Swiss albino mice. Environ Sci Pollut Res 28, 66193–66205 (2021). https://doi.org/10.1007/s11356-021-15497-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15497-8