Abstract
Prophylactic dietary intake of omega-3 polyunsaturated fatty acids (n-3 PUFAs) has been shown to remarkably ameliorate ischemic brain injury. However, the therapeutic efficacy of n-3 PUFA administration post-stroke, especially its impact on neurovascular remodeling and long-term neurological recovery, has not been fully characterized thus far. In this study, we investigated the effect of n-3 PUFA supplementation, as well as in combination with docosahexaenoic acid (DHA) injections, on long-term stroke outcomes. Mice were subjected to transient middle cerebral artery occlusion (MCAO) before randomly assigned to four groups to receive the following: (1) low dose of n-3 PUFAs as the vehicle control, (2) intraperitoneal DHA injections, (3) n-3 PUFA dietary supplement, or (4) combined treatment of (2) and (3). Neurological deficits and brain atrophy, neurogenesis, angiogenesis, and glial scar formation were assessed up to 28 days after MCAO. Results revealed that groups 2 and 3 showed only marginal reduction in post-stroke tissue loss and attenuation of cognitive deficits. Interestingly, group 4 exhibited significantly reduced tissue atrophy and improved cognitive functions compared to groups 2 and 3 with just a single treatment. Mechanistically, the combined treatment promoted post-stroke neurogenesis and angiogenesis, as well as reduced glial scar formation, all of which significantly correlated with the improved spatial memory in the Morris water maze. These results demonstrate an effective therapeutic regimen to enhance neurovascular restoration and long-term cognitive recovery in the mouse model of MCAO. Combined post-stroke DHA treatment and n-3 PUFA dietary supplementation thus may be a potential clinically translatable therapy for stroke or related brain disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke declines from the fourth to the fifth leading cause of death in the USA in 2013, yet remains a leading cause for long-term disability. Despite continuous and intensive efforts in stroke research to establish new therapeutic targets, current treatment to acute ischemic stroke is largely limited to the thrombolytic therapy using recombinant tissue-type plasminogen activator (tPA), which benefits only a small population of stroke patients due to its narrow therapeutic time window [1–5]. Physical or cognitive deficits often develop in stroke victims and are closely related to their life quality. In recent years, attentions have been gradually shifted from neuroprotective strategies aiming to rescue the injured tissue acutely to neurorestorative strategies with a focus on boosting post-stroke tissue repair [6]. Endogenous repair mechanisms of the neurovascular unit, including neurogenesis and angiogenesis, implement long-term structural and functional restoration of the injured brain and represent an opportunity to extend the therapeutic time window [7–9].
One promising candidate for the treatment of stroke is omega-3 polyunsaturated fatty acids (n-3 PUFAs). n-3 PUFAs are major components of dietary fish oil, of which docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are the two most involved in the regulation of human physiology [10]. The high ratio of n-6 PUFAs over n-3 PUFAs in the western diet is considered to contribute to the higher incidence of stroke in western societies [11]. Our group and others have demonstrated that n-3 PUFAs exert beneficial effect in an event of ischemic stroke [12–14]. n-3 PUFAs act on multiple cell types in the brain and function to attenuate pro-death [15] or pro-inflammatory [14] mechanisms or elicit cytoprotective responses [12]. n-3 PUFAs are also found to prevent blood-brain barrier breakdown and development of brain edema by preserving the endothelial tight junction proteins [16, 17]. Long-term elevation of n-3 PUFA levels in mice before stroke, achieved through either dietary supplementation or transgenic overproduction, robustly ameliorated the outcomes of ischemic brain injury and offered sustained improvement on post-stroke neurological recovery [18–20]. Furthermore, stroke-induced repair processes of the brain, such as neurogenesis, angiogenesis, and oligodendrogenesis, were shown to be enhanced by high n-3 PUFA levels [18–20], suggesting that n-3 PUFAs may offer long-term functional improvement through stimulation of multifaceted endogenous restorative mechanisms after brain injury. Although these studies demonstrate a protective role of prophylactic n-3 PUFA treatment, whether n-3 PUFAs can offer comparably effective and sustained protection when administered after stoke remains poorly investigated. Determining the effects of a delayed n-3 PUFAs treatment after stroke is therefore crucial for this therapy to be potentially translated from bench to bedside.
In order to fill the gap, the present study explored the therapeutic efficacy of n-3 PUFAs with the treatment being delivered after the stroke, using a mouse model of focal cerebral ischemia and reperfusion. We evaluated different treatment paradigms, consisting of intraperitoneal (i.p.) injections of DHA and/or dietary fish oil supplementation, on long-term histological and functional outcomes after stroke. Furthermore, we examined whether post-stroke n-3 PUFA treatment can enhance the endogenous repair processes triggered by ischemic/reperfusion brain injury.
Materials and Methods
Animals
C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed in a temperature- and humidity-controlled animal facility with a 12-h light-dark cycle. Food and water were available ad libitum. All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize animal suffering and the number of animals used.
Transient Focal Cerebral Ischemia Model
Transient focal cerebral ischemia was induced in adult C57BL/6J mice (male, 10–12 weeks old) by intraluminal occlusion of the left middle cerebral artery (MCA) for 60 min [21]. All procedures were performed following the Stroke Therapy Academic Industry Roundtable (STAIR) guidelines. Briefly, mice were anesthetized with 3 % isoflurane vaporized in 67:30 % N2O/O2 until they were unresponsive to the tail pinch test. A mouse nose cone continuously supplying 1.5 % isoflurane was used to maintain anesthesia during surgery. A suture (7-0) with a silicon-coated tip was introduced into the common carotid artery, advanced to the origin of the MCA, and left undisturbed for 60 min. Rectal temperature was maintained at 37.0 ± 0.5 °C during surgery using a temperature-controlled heating pad. To confirm the success of MCA occlusion and reperfusion, regional cerebral blood flow (rCBF) was measured using a laser-Doppler flowmeter before, during, and after MCA occlusion (MCAO). Animals that did not show a CBF reduction of at least 75 % of baseline levels or died immediately after ischemia induction or reperfusion (less than 10 %) were excluded from further experimentation.
Post-Stroke DHA Delivery and n-3 PUFA Dietary Supplementation
Immediately after the MCAO surgery, mice were randomly assigned to four groups with the use of a lottery-drawing box:
-
1.
Vehicle control group. Mice were fed a regular laboratory rodent diet (Prolab Isopro RMH 3000 5P76; LabDiet, St. Louis, MO, USA) with an inherently low n-3 PUFA concentration (0.36 %) and received injections of 0.9 % NaCl (300 μl per day, i.p. 2 h after MCAO, and then daily for 14 days).
-
2.
DHA treatment group. Mice were fed with a regular diet and received injections of DHA (10 mg/kg body weight, diluted with 300 μl of 0.9 % NaCl, i.p. 2 h after MCAO, and then daily for 14 days).
-
3.
n-3 PUFA dietary supplementation group. Mice were fed with a diet supplemented with n-3 PUFAs (DHA and EPA, triple strength n-3 fish oil, Puritan’s Pride, Oakdale, NY, USA; final n-3 PUFA concentration 4 %) [19], beginning at 5 days after MCAO for up to 28 days, and received injections of 0.9 % NaCl (300 μl per day, i.p. 2 h after MCAO, and then daily for 14 days).
-
4.
Combined treatment of (2) and (3). Mice were fed a diet with high concentration of n-3 PUFAs together with 14 days of DHA injections.
All outcome assessments were performed by investigators blinded to experimental group assignments.
Morris Water Maze
Long-term cognitive functions were assessed by the Morris water maze test at 23–28 days after MCAO, as we previously described [21]. Briefly, a circular pool (diameter 109 cm) containing opaque water was divided into four quadrants by phantom lines. A platform (diameter 11 cm) was submerged in the pool for 1 cm in one quadrant. Mice were placed into the pool from one of the three quadrants (except the one quadrant with the platform) with the platform present and were allowed to swim for 60 s to find the platform. Between each swim, mice were allowed to rest for 15 s on the platform to help them memorize the spatial cues around. The average latency for the mice to find the platform from the three quadrants was recorded at 23–27 days after MCAO to reflect the learning abilities of mice. At 28 days after MCAO, the platform was removed and each mouse was allowed to swim in the pool from the same starting position for 60 s. Spatial memory was assessed by measuring the time each mouse spent in the target quadrant where the platform was previously located and the time each mouse crossed the location of the platform. Swim speed was recorded on each testing day to evaluate the gross locomotor function of the mice.
Immunohistochemistry and Image Analysis
At 28 days after MCAO, mice were deeply anesthetized and transcardially perfused with 0.9 % NaCl followed by 4 % paraformaldehyde in phosphate-buffered saline (PBS). Brains were harvested and cryoprotected in 30 % sucrose in PBS, and frozen serial coronal brain sections (25 μm thick) were prepared on a cryostat (CM1900, Leica, Bensheim, Germany). Brain sections were blocked with 5 % donkey serum in PBS for 1 h, followed by incubation with primary antibodies for 1 h at room temperature and overnight at 4 °C. After a series of washing, sections were incubated with donkey secondary antibodies conjugated to DyLight 488 or Cy3 (1:1000, Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Alternate sections from each experimental condition were incubated in all solutions except the primary antibodies to assess non-specific staining. Sections were mounted and coverslipped with Fluoromount-G containing 4′,6-diamidino-2-phenylindole (DAPI; Southern Biotech, Birmingham, AL, USA). The following primary antibodies were used: rabbit anti-microtubule-associated protein 2 (MAP2; 1:200; Santa Cruz Biotechnology, Dallas, TX, USA), rabbit anti-NeuN (1:500; EMD Millipore, Billerica, MA, USA), rat anti-CD31 (1:200; BD Biosciences, San Jose, CA, USA), and rabbit anti-glial fibrillary acidic protein (GFAP; 1:500; Dako, Carpentaria, CA, USA). Images were acquired using an inverted Nikon Diaphot-300 fluorescence microscope equipped with a SPOT RT slider camera and Meta Series Software 5.0 (Molecular Devices, Sunnyvale, CA, USA). Alternatively, images were captured with an Olympus Fluoview FV1000 confocal microscope using FV10-ASW 2.0 software (Olympus America, Center Valley, PA, USA).
Chronic brain atrophy was measured on six equally spaced MAP2-stained sections, encompassing the MCA territory using ImageJ, as previously described [22]. The area of atrophy was measured by subtracting the none-lesioned area (MAP2 positive) of the ipsilateral hemisphere from that of the contralateral hemisphere. The volume of atrophy was calculated by multiplying the mean area of tissue atrophy by the thickness of the tissue evaluated.
The GFAP-stained sections were imaged with fixed exposure setting to quantify the astrocyte scar formation. After randomly selecting three regions of interest (ROIs) from the peri-infarct cortex (within 800 μm to the infarct core area), the average intensity of GFAP fluorescence was measured by the NIH ImageJ software. Alternatively, astrocyte scar area was assessed as previously described [23]. Briefly, GFAP immunofluorescence images were converted to black and white and threshold. The GFAP-positive areas were quantified with ImageJ.
Examination of Recently Proliferated Cells
Recently proliferated cells were labeled with the S-phase marker 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich, St. Louis, MO, USA) as previously described [19]. Briefly, BrdU was i.p. injected twice a day at a dose of 50 mg/kg body weight at 3–6 days after MCAO. At 28 days after MCAO, mice were sacrificed and coronal brain sections were prepared as described above. Sections were pretreated with 2N HCl for 1 h at 37 °C followed by 0.1 M boric acid (pH 8.5) for 10 min at room temperature. Sections were then blocked with M.O.M. Kit (Vector, Burlingame, CA, USA) for 1 h and incubated with purified mouse anti-BrdU antibody (1:1000; BD Biosciences) for 1 h at room temperature and then overnight at 4 °C. After a serial of washing, sections were incubated with the 488-AffiniPure donkey anti-mouse IgG (1:1000; Jackson ImmunoResearch Laboratories) for 1 h at room temperature. Fluorescence images were captured as described above.
BrdU immunopositive cells were counted using ImageJ and calculated as the number of cells in the designated fields divided by the area (mm2) of the fields. Neurogenesis was evaluated on BrdU/NeuN double-stained sections. Angiogenesis was assessed by counting BrdU immunopositive cells along the microvessels on BrdU/CD31 double-stained sections. At least four microscopic fields were randomly sampled in each section.
Statistical Analysis
All data are presented as mean ± SEM. The statistical differences among means of multiple groups were assessed by one- or two-way ANOVA followed by the Bonferroni’s post hoc test. The Pearson product linear regression analysis was used to correlate the multiple histological parameters with the mice’s spatial memory performance in the Morris water maze. A p value of less than 0.05 was deemed statistically significant.
Results
Post-Stroke DHA Treatment Combined with n-3 PUFA Dietary Supplement Reduces Chronic Brain Atrophy
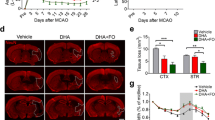
While the protective effects of n-3 PUFAs in ischemic/reperfusion brain injury have been largely studied based on treatments before the onset of ischemia/reperfusion, the therapeutic efficacy of a more clinically relevant paradigm of post-stroke n-3 PUFA treatment has not been thoroughly investigated. To our best knowledge, 1 h after the reperfusion is the latest for the post-stroke DHA injections to achieve protective effects in previous studies [16]; however, its impact on long-term histological and functional outcomes remains largely unknown. To test whether n-3 PUFAs have the potential to be translated to clinical use, we extended the first intervention time point to 2 h after post-MCAO reperfusion and examined long-term stroke outcomes. n-3 PUFAs were delivered by two methods: (1) i.p. injections of DHA, starting 2 h after MCAO and thereafter daily for 14 days; or (2) dietary supplement with triple-strength fish oil (FO), beginning 5 days after MCAO and lasting for 28 days. The dosage of DHA injection and FO supplementation was determined based on our previous studies [12, 19]. Furthermore, the FO supplementation did not start until 5 days after MCAO, when the food intake of mice was mostly recovered to the prestroke baseline levels [24]. Mice were subjected to 60 min of MCAO followed by reperfusion and randomly assigned to four groups as described in the “Materials and Methods” section: (1) vehicle control, (2) DHA only, (3) FO only, and (4) DHA + FO. At 28 days after MCAO, control mice developed prominent brain atrophy as evaluated on MAP2-immunostained coronal brain sections (Fig. 1a, b). Post-stroke treatment with DHA injections alone, or FO supplementation alone, did not rescue the brain from atrophy when compared to vehicle controls (Fig. 1c; DHA 19.52 ± 1.69 mm3 and FO 19.98 ± 2.95 mm3 vs. vehicle 25.23 ± 1.31 mm3, p = 0.20 and p = 0.37, respectively). Interestingly, brain atrophy was significantly reduced in mice receiving combined DHA and FO treatment (Fig. 1c; 13.32 ± 1.64 mm3, p < 0.01 vs. vehicle). These findings demonstrated the sustained protective effects of combined post-stroke treatment with DHA and FO, even when the delivery was delayed after ischemic injury.
Post-stroke DHA and FO combined treatment improves long-term histological and functional outcomes. a Representative images showing MAP2 immunofluorescence (green) on six equally-spaced coronal brain sections (bregma level 1.18 to −2.18 mm) at 28 days after 60 min MCAO. Dashed line: chronic brain infarct. Scale bar: 1 mm. b Areas of brain atrophy at 28 days after MCAO were measured on the six MAP2-stained sections encompassing the MCA territory in each mouse. c Total volume of atrophy calculated from b. n = 7 mice per group. *p ≤ 0.05, **p ≤ 0.01 DHA + FO vs. vehicle. DHA and FO combined treatment showed the most potent protective effect against MCAO-induced brain atrophy. d–h Cognitive functions were examined by the Morris water maze at 23–28 days after MCAO. d Representative images showing the swim paths of the mice from each group at the learning or memory phase of the test. e Average swimming speed at 23–28 days after MCAO was not different among all four groups. f Latency to locate the submerged platform at 23–27 days after MCAO. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 FO or DHA + FO vs. vehicle by one-way ANOVA (individual time point) or two-way ANOVA (bracket). g, h Spatial memory was assessed at 28 days after MCAO by measuring the times the mouse crossed the previous location of the platform (g) or the percentage of time the mouse spent in the target quadrant (h) when the platform was removed. *p ≤ 0.05, **p ≤ 0.01 FO or DHA + FO vs. vehicle group by one-way ANOVA. n = 7 mice per group
DHA and FO Combined Treatment Improves Long-Term Cognitive Functions After Ischemic Brain Injury
Cognitive impairment is a prevalent deficit among stroke victims and an important parameter to evaluate the effectiveness of therapeutic interventions [25]. To determine whether post-stroke n-3 PUFA treatment offers long-term improvement in cognitive functions after ischemic brain injury, we performed Morris water maze, a widely used test for spatial cognitive deficits in rodents [26–28], at 23–28 days after MCAO (Fig. 1d). All four groups showed comparable swimming speed at 23–28 days after MCAO (Fig. 1e), excluding the possibility that any observed difference in the performance of learning and memory tasks resulted from varied gross motor functions. The latency for the mice to find the submerged platform decreased during the 5 days of training in all groups (Fig. 1f), reflecting spatial learning. Compared to control, the combined treatment of DHA and FO significantly decreased this escape latency, suggesting a substantially improved spatial learning ability (Fig. 1f; p ≤ 0.001 DHA + FO vs. vehicle by two-way ANOVA; p ≤ 0.001 on day 26 and p ≤ 0.05 on day 27 DHA + FO vs. vehicle by one-way ANOVA). The spatial memory deficits were also ameliorated in mice receiving combined DHA and FO treatments, as shown by more numbers of incidents that the mice passed the previous location of the platform and longer time the mice spent in the target quadrant during the memory phase of the test at 28 days after MCAO (Fig. 1g, h). Interestingly, FO supplementation alone resulted in significant improvement in spatial learning and memory (Fig. 1f–h), suggesting an effect from the non-DHA n-3 PUFA components in the fish oil, such as EPA. In summary, these data suggest that FO dietary supplementation is partially effective in improving post-stroke spatial cognitive functions and that this beneficial effect was further boosted when FO supplementation is combined with DHA injections.
DHA and FO Combined Treatment Promotes Hippocampal Neurogenesis After Ischemic Brain Injury
We further investigated the underlying mechanisms which account for the persistent protective effects of DHA and FO combined treatment on histology and cognitive functions after MCAO. A remarkably positive correlation between hippocampal integrity and spatial cognitive functions has been confirmed by previous studies [29, 30]. The significantly improved spatial learning and memory observed in the Morris water maze led us to suspect that there might be more intact hippocampal neurons in post-ischemic mice receiving n-3 PUFAs. Indeed, the numbers of NeuN-positive neurons in the hippocampal CA1, CA3, and dentate gyrus (DG) areas were all dramatically elevated at 28 days after MCAO in mice with FO dietary supplementation, alone, or combined with DHA injections (Fig. 2a–d). After ischemic stroke, neural stem cells and progenitor cells from the subventricular zone and subgranular zone of DG migrate to the ischemic zone and contribute to the endogenous repair process of the injured brain [31, 32]. Promoting neurogenesis after ischemic brain injury has been identified as a therapeutic strategy for stroke treatment [33–36]. Twenty-eight days after stroke, there was barely any detectable neurogenesis in the non-injured contralateral hippocampus (Supplementary Fig. 1). In the ipsilateral hemisphere, however, we observed significantly more newly generated neurons (BrdU/NeuN double-positive cells) in the DHA + FO group compared to vehicle controls at 28 days after MCAO, in all examined areas in the hippocampus including CA1, CA3, and DG (Fig. 2e–g). Despite this prominent neurogenesis, DHA + FO treatment did not increase the total number of viable neurons (NeuN+ cells) in the hippocampus of the ischemic hemisphere to a level above that in the non-injured contralateral hemisphere (Fig. 2; Supplementary Fig. 1). In contrast, DHA injections alone or FO supplementation alone did not show a positive influence on neurogenesis in the hippocampus.
Post-stroke DHA and FO combined treatment enhances hippocampal neurogenesis. a Representative images of double-label immunostaining of BrdU (green) and NeuN (red) in the peri-infarct hippocampal CA1, CA3, and DG areas at 28 days after MCAO. Arrows: BrdU+/NeuN+ cells (yellow). Arrowheads: BrdU+ newly generated cells that are negative for NeuN signal. Scale bar: 50 μm. b–d Quantification of total cells that are positive for NeuN immunosignal in CA1, CA3, and DG. e–g Quantification of NeuN+/BrdU+ cells in CA1, CA3, and DG. n = 7 mice per group. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 vs. vehicle group
A large number of the BrdU-labeled cells surprisingly expressed the mature neuronal marker NeuN in the DHA + FO group. Although this phenomenon suggested that the newly generated cells may fully differentiate during the 28 days of post-MCAO period, whether these cells functionally integrated into the existing neural circuits remains unknown. Importantly, the number of viable hippocampal neurons in CA1 and CA3 exhibited a moderate but statistically significant positive correlation with mice spatial memory performance in the Morris water maze at 28 days after dMCAO (Fig. 3a, b; CA1 r = 0.489, p = 0.011; CA3 r = 0.448, p = 0.022). The number of neurons in the DG displayed an even stronger correlation with spatial memory (Fig. 3c; r = 0.616, p = 0.001). These results indicate that the dramatic increase of hippocampal viable neurons in the FO and DHA + FO groups might be an important factor, dictating long-term spatial memory recovery in post-stroke mice.
Viable hippocampal neurons significantly correlate with spatial memory after stroke. Pearson product linear regression analysis was performed to correlate the performance of mice in the spatial memory test of the Morris water maze (presented as time spent in the target quadrant) to the numbers of viable neurons (NeuN+ cells) in hippocampal CA1 (a), CA3 (b), DG (c) areas after MCAO. n = 7 mice per group
DHA and FO Combined Treatment Promotes Neurogenesis in the Cortex and Striatum After Ischemic Brain Injury
Spatial learning and memory, which are essential abilities for properly navigating the Morris water maze, not only depend on the hippocampus but also require the coordinated actions of multiple different brain regions to produce a functionally integrated neural network [37–39]. When examining the peri-infarct cortex and striatum of mice in different treatment groups (Fig. 4a), we found a similar pattern of effects on neurogenesis from all groups compared to the hippocampus. Significantly higher number of newly generated neurons was observed from the DHA + FO group than that from the vehicle controls in both cortex (90.69 ± 25.29 cells/mm2 vs. 25.02 ± 6.95 cells/mm2, p < 0.01) and striatum (107.04 ± 22.01 cells/mm2 vs. 37.76 ± 10.16 cells/mm2, p < 0.01) (Fig. 4b–d). In summary, combined DHA and FO treatment after stroke, but neither treatment alone, promotes neurogenesis in various brain regions, which could partly account for the improved cognitive functions.
Post-stroke DHA and FO combined treatment enhances neurogenesis in the peri-infarct cortex and striatum. a Representative images of NeuN immunofluorescence (red) in coronal brain sections at 28 days after MCAO. White boxes illustrate the peri-infarct cortical and striatal areas where images in b were taken. Scale bar: 1 mm. b Double-labeling immunostaining of BrdU (green) and NeuN (red) in the peri-infarct cortex and striatum at 28 days after MCAO. Boxes indicate areas enlarged in high-power images (the second and fourth rows). Arrows: BrdU+/NeuN+ cells (yellow). Arrowheads: BrdU+ newly generated cells that are negative for NeuN signal. Scale bar: 50 μm. c, d Quantification of BrdU+/NeuN+ cells in the cortex and striatum. n = 7 mice per group. **p ≤ 0.01 vs. vehicle group
Post-Stroke Combined Treatment of DHA and FO Enhances Angiogenesis in the Peri-Infarct Area
Angiogenesis is an important endogenous repair mechanism after brain injury, which may improve tissue perfusion, support the activity of neurons and neural progenitor cells, and collectively, promote long-term functional improvement [40–44]. The potent effect of DHA and FO combined treatment in promoting post-stroke neurogenesis suggests the potential development of new blood vessels, which provided a vascular niche that is required during neurogenesis [7]. We therefore examined the impact of post-stroke n-3 PUFA treatment on angiogenesis in the peri-infarct cortical and striatal area, by double-label immunostaining of BrdU (marker for recently proliferated cells) and CD31 (marker for microvessels) at 28 days after MCAO (Fig. 5a, b). Mice receiving combined DHA and FO treatment showed the greatest number of BrdU-positive cells on CD31-positive microvessels (Fig. 5c). BrdU and CD31 double-stain on the new blood vessels (Fig. 5d, e) showed that DHA injection or FO dietary supplement alone did not significantly affect angiogenesis compared to the vehicle control group, in both cortex (Fig. 5d; 59.10 ± 3.08 cells/mm2 in DHA group, 58.96 ± 2.88 cells/mm2 in FO group vs. 55.44 ± 6.94 cells/mm2 in vehicle group, p > 0.05) and striatum (Fig. 5e; 94.24 ± 3.82 cells/mm2 in DHA group, 95.63 ± 3.50 cells/mm2 in FO group vs. 93.02 ± 3.50 cells/mm2 in vehicle group, p > 0.05). In comparison, the number of BrdU+/CD31+ cells is markedly increased in mice from combined treatment group (Fig. 5d, e; 85.96 ± 13.07 cells/mm2 in cortex and 117.12 ± 9.35 cells/mm2 in striatum, p < 0.05) than that from the control group. Furthermore, the number of BrdU+/CD31+ cells demonstrated significant positive correlation with spatial memory performance in mice in the Morris water maze (Fig. 5f, g; cortex r = 0.596, p = 0.006; striatum r = 0.602, p = 0.001). These findings indicate that DHA and FO combined treatment promotes angiogenesis after ischemia, which could support neural development in the peri-infarct area and partially account for long-term functional improvement.
Post-stroke DHA and FO combined treatment promotes angiogenesis. a A representative image of the brain at 28 days after MCAO illustrating the infarct (red dashed line), and peri-infarct cortical and striatal areas where images in c were taken (yellow boxes). Scale bar: 1 mm. b A representative image taken from the peri-infarct cortex showing a BrdU immunopositive cell on a CD31+ microvessel (in yellow). c Double-label immunostaining of CD31 (red) and BrdU (green) in peri-infarct cortex and striatum at 28 days after MCAO. White boxes indicate areas that were enlarged in the high-power images (the fifth column). Arrows: BrdU+/NeuN+ cells (yellow). Arrowheads: BrdU+ newly generated cells that are negative for NeuN signal. Scale bar: 50 μm. A significantly higher number of BrdU+/CD31+ cells was observed in the DHA + FO group, compared to either treatment alone. d, e Quantification of CD31+/BrdU+ cells in the cortex and striatum. *p ≤ 0.05 vs. vehicle group. f, g Pearson correlation between spatial memory and CD31+/BrdU+ cells in the cortex (f) and striatum (g). n = 7 mice per group
DHA and FO Combined Treatment Ameliorates the Formation of Glial Scars After Ischemia
Astrocytes compose of a large number of brain cells and rapidly react upon ischemic insults, a process characterized by increased GFAP expression and hypertrophy of processes. Extensive reactive astrogliosis leads to glial scarring [45, 46], which may exert distinct functions on tissue injury and repair in a context-dependent manner. For example, scar-forming astrocytes may play a protective role at the acute stage after injury, acting as shields to keep neurons from oxidative stress and limiting the propagation of inflammation [45, 47]. However, at later stages after injury, glial scars may negatively affect axonal regeneration and tissue repair [48]. Whether n-3 PUFAs inhibit astroglial activation, thus benefit the survival or proliferation of neurons is unknown. We performed immunostaining of the astrocyte marker GFAP on brain slices at 28 days after MCAO (Fig. 6a, b). GFAP expression was attenuated by dietary FO supplement, alone, or combined with DHA injections (Fig. 6b). The fluorescence intensity of GFAP was also remarkably decreased in mice receiving FO supplement alone or combined with DHA injections when compared to vehicle controls (Fig. 6c). The GFAP fluorescence intensity negatively correlated with the spatial memory performance of the mice in the Morris water maze (Fig. 6d; r = −0.562, p = 0.005). The area covered by scar-forming astrocytes was also significantly reduced in FO supplement and combined treatment groups (Fig. 6e). These results suggested that FO and DHA combined treatment suppressed glial scar formation in the peri-infarct area, which may be associated with a favorable microenvironment for post-ischemic tissue regeneration and repair.
DHA and FO combined treatment attenuates astrocyte glial scar formation. a Representative images of the lateral view of the brains (upper panel) or GFAP (red)-stained coronal brain sections (lower panel) showing the formation of glial scars at 28 days after MCAO. Boxes indicate the peri-infarct cortical regions where images in b were taken. Scale bar: 1 mm. b Double-label immunostaining of GFAP (red) and DAPI (blue) in the cortex. Scale bar: 50 μm. c Quantification of relative GFAP intensity in the peri-infarct cortex. d Pearson correlation between spatial memory and GFAP fluorescence intensity (arbitrary unit). e Quantification of the areas occupied by scar-forming astrocytes in the peri-infarct cortex. FO supplementation alone or combined with DHA injections significantly reduced GFAP intensity and astrocyte scar area compared to vehicle group. n = 7 mice per group. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 vs. vehicle group
Discussion
The present study is the first to comprehensively characterize the therapeutic efficacy of n-3 PUFAs, delivered 2 h after the onset of ischemic stroke and reperfusion in a mouse model. We demonstrated an effective therapeutic regimen, during which FO dietary supplement combined with DHA injection for an extended period of time after stroke provided potent and sustained protection against ischemia-induced tissue loss and functional deficiencies. Furthermore, the underlying mechanism by which n-3 PUFAs improve long-term stroke outcomes may involve in the promotion of endogenous brain repair, including neurogenesis, angiogenesis, and reduction of scar formation.
Epidemiologic analyses have suggested that the morbidity of many neurological disorders, such as ischemic stroke and Alzheimer’s disease, is much lower in the population with high intake of n-3 PUFAs [49, 50]. High ratio of n-6 to n-3 fatty acids is associated with poor neural development and retardation of functional recovery after injury [51]. n-3 PUFAs have long been shown to be protective against ischemic brain injury [13, 18–20]; however, the majority of these studies introduced n-3 PUFAs before the onset of stroke, which provided limited information on whether n-3 PUFAs are suitable to be used clinically. Although some studies using a post-stroke delivery strategy have provided invaluable information on the possible protective effects of n-3 PUFAs [16, 52–54], the long-term consequences, especially functional recovery, still remain largely unknown. The novelty of the present study lies in the extension of the treatment time window and the evaluation of long-term functional outcomes after post-stroke n-3 PUFA treatment. We delayed the first injection of DHA to 2 h after reperfusion, and the FO dietary supplement started 5 days after MCAO. The present study is the first to report that post-stroke treatment with n-3 PUFAs will provide long-term neurological improvements, up to 28 days after stroke. This protective effect is boosted significantly when the mice were treated with FO and DHA together, which is perhaps due to the elevated level of n-3 PUFAs in the brain that was maintained for a long period of time. DHA was previously reported to offer neuroprotection against ischemic brain injury for up to 21 days after MCAO, even when it was administered as late as 1 h after reperfusion [55]. In our study, we did not observe significant beneficial effects of DHA injections alone on histological and functional outcomes at 28 days after MCAO. This data suggests that although DHA may elicit some neuroprotection, treatment with acute DHA injection alone may not be sufficient to sustain this protection. In contrast, mice given only FO supplement showed significantly improved cognitive functions, probably accredited to enhanced neurorestorative processes. We previously reported that long-term dietary FO supplementation resulted in significant and sustained elevation in the ratio of n-3/n-6 fatty acids [19]. In the current project, FO was not started until 5 days after MCAO, when the food intake of post-stroke mice had fully recovered. We may have thereby missed the optimal therapeutic window for acute protection from n-3 PUFAs, which partially explains why a combination of acute DHA injection with long-term supplementation of FO was able to boost the beneficial effects of FO alone. Furthermore, the different effects of FO and DHA treatments may be associated with acute protection against cell death versus chronic tissue repair and restoration, as discussed below. With long-term tissue restoration as a target, n-3 PUFAs have a prolonged treatment time window, making them an appropriate candidate to be used in combination with the current tPA therapy.
One interesting finding of the present study is that FO treatment alone provided some protection against stroke-induced spatial memory deficits (Fig. 1g, h) or neuronal loss (Fig. 2a–d), while DHA treatment alone had no noticeable effect. We thereby suspect that these improvements were due to the non-DHA components in the FO, i.e., EPA. DHA is the most enriched n-3 fatty acid in the brain, while the level of EPA is about 250–300 times lower [56]. The triple strength omega-3 FO used in this study has an EPA/DHA ratio of 3:1. In our recent study, we have shown that a 3-month continuous supply of dietary FO, using the same dosage as in the current study, would increase the content of multiple n-3 PUFAs in the brain in a non-proportional manner [19]. Specifically, DHA content was increased by less than 5 %, likely due to its already high baseline concentrations in the brain. However, the brain EPA level reached an approximately sixfold increase after 3 months [19]. Although the beneficial effects of n-3 PUFAs have been traditionally accredited to all components without discrimination [57], it is possible that the dramatically elevated EPA played a major role in the current treatment paradigm. To date, the effect of EPA treatment in stroke was much less studied compared to that of DHA, although it was reported previously that EPA attenuated post-ischemic inflammation and brain injury in part due to its function as a peroxisome proliferator-activated receptor gamma (PPARγ) agonist [58]. Future studies are warranted to elucidate the unique effects of different n-3 PUFAs, such as EPA.
Improving long-term neurological recovery has been a major focus in rehabilitation for stroke victims. In the present study, we used the Morris water maze to assess the cognitive deficits in post-ischemic mice. To properly navigate in the Morris water maze, spatial learning and memory require not only the integrity of the hippocampus but also the coordinated actions of multiple different brain regions to produce a functionally integrated neural network. Ischemic insults trigger proliferation and migration of neuroblasts [8, 35, 59], which may differentiate into mature neurons and contribute to neurological recovery [32]. Consistent with the improved cognitive functions, mice receiving DHA and FO combined treatment showed enhanced neurogenesis after stroke. Although a large number of BrdU-positive recently proliferated cells were also observed to express NeuN, a marker for mature neurons (Figs. 2 and 3), we are yet to confirm whether these cells were fully functional. Hippocampal pyramidal neurons regenerated from endogenous progenitor cells after ischemic brain injury were previously reported to be integrated into the existing brain circuitry and contributed to improved neurological functions [59, 60]. Using electrophysiological approaches, an important future study could focus on whether the regenerated neurons, triggered by the post-stroke n-3 PUFA treatment, could be integrated into an existing neural network. Besides the enhancement of neurogenesis, DHA and FO combined treatment promoted post-stroke angiogenesis, thereby providing the vascular niche that is required for the survival and migration of newborn neurons [61]. While the acute protective effects from DHA may support the survival of progenitor cells, the differentiation of these cells into mature neurons may require the sustained supplementation of n-3 PUFAs, which together positively correlate with the recovery of post-stroke spatial memory.
Another crucial regulator of the post-injury brain repair is reactive astrogliosis and formation of glial scars. Astrocytes, the most abundant cell population in the brain, rapidly respond to brain injury and undergo heterogeneous molecular and morphological alterations, a process defined as reactive astrogliosis [62, 63]. The role in which reactive astrogliosis and glial scar formation play after the brain injury is still controversial and may be context-dependent as determined by specific molecular signaling cascades. Generation of scar tissues at an early stage after injury provides a barrier to block the infiltration of inflammatory cells and other harmful factors, thereby limiting the progression of the injury [64–66], whereas at later stages, glial scars may impede tissue repair. Scar-forming astrocytes are generally held responsible for the failure of axonal regrowth [67]. In the present study, we observed ameliorated astrocytic scar formation in mice treated with DHA and FO together. It is likely that the reduced scar formation permitted the regeneration of white matter axons and facilitated the reestablishment of neural circuits in the peri-infarct area, accounting for the improved functional outcomes. At this point of the study, we do not know whether the reduced gliosis resulted from a direct action of n-3 PUFAs on astrocytes or from a secondary effect of reduced tissue injury. Although our results implicated a detrimental role of glial scars in post-stroke tissue restoration, a recent study based on a spinal cord injury model suggested that astrocyte scar formation may aid, rather than prevent, central nervous system axonal regeneration [23]. Future cell-type specific and mechanistic studies are warranted to further elucidate the role of astrocytes under pathological conditions.
In summary, our study demonstrates that chronic administration of n-3 PUFAs, starting as late as 2 h after post-ischemic reperfusion, could achieve long-lasting neurological and histological protection against ischemic brain injury and has significant potential to be translated into future clinical use. DHA and FO combined treatment promoted post-stroke neurogenesis and angiogenesis and also reduced the formation of glial scars, thereby facilitating the restoration of the neurovascular unit. Some components of the neurovascular unit were not covered in the present study, e.g., the activation of microglia and inflammatory responses [68–72], remyelination, and white matter repair [73, 74]. Therefore, we will investigate in future studies whether these responses contribute to n-3 PUFAs-mediated tissue repair. Another limitation of the present study is the use of a transient MCA occlusion model by intraluminal monofilament. Despite being one of the most widely used stroke models, the intraluminal suture model does not fully mimic human ischemic stroke and could lead to severe reperfusion injury [75]. Future preclinical research using models involving tPA-induced reperfusion and/or permanent MCAO [76, 77] is warranted to facilitate the translation of therapeutic interventions. Finally, stroke risk factors, aging, and comorbid diseases may alter the progression of injury and interfere with the efficacy of therapeutics, which should be taken into consideration in further studies.
References
Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020–35.
Lapchak PA. Critical early thrombolytic and endovascular reperfusion therapy for acute ischemic stroke victims: a call for adjunct neuroprotection. Transl Stroke Res. 2015;6(5):345–54.
Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl Stroke Res. 2014;5(4):442–53.
Hafez S, Hoda MN, Guo X, Johnson MH, Fagan SC, Ergul A. Comparative analysis of different methods of ischemia/reperfusion in hyperglycemic stroke outcomes: interaction with tPA. Transl Stroke Res. 2015;6(3):171–80.
Mandava P, Shah SD, Sarma AK, Kent TA. An outcome model for intravenous rt-PA in acute ischemic stroke. Transl Stroke Res. 2015;6(6):451–7.
Hermann DM, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012;11(4):369–80.
Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler RA, Lawton MT, et al. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol. 2014;115:138–56.
Ruan L, Lau BW, Wang J, Huang L, Zhuge Q, Wang B, et al. Neurogenesis in neurological and psychiatric diseases and brain injury: from bench to bedside. Prog Neurobiol. 2014;115:116–37.
Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, et al. Cell based therapies for ischemic stroke: from basic science to bedside. Prog Neurobiol. 2014;115:92–115.
Gupta S. Brain food: clever eating. Nature. 2016;531(7592):S12–3.
Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365–79.
Zhang M, Wang S, Mao L, Leak RK, Shi Y, Zhang W, et al. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J Neurosci. 2014;34(5):1903–15.
Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke. 2009;40(9):3121–6.
Lalancette-Hebert M, Julien C, Cordeau P, Bohacek I, Weng YC, Calon F, et al. Accumulation of dietary docosahexaenoic acid in the brain attenuates acute immune response and development of postischemic neuronal damage. Stroke. 2011;42(10):2903–9.
Shi Z, Ren H, Luo C, Yao X, Li P, He C et al. Enriched endogenous omega-3 polyunsaturated fatty acids protect cortical neurons from experimental ischemic injury. Mol Neurobiol. 2015. [Epub ahead of print]
Hong SH, Khoutorova L, Bazan NG, Belayev L. Docosahexaenoic acid improves behavior and attenuates blood-brain barrier injury induced by focal cerebral ischemia in rats. Exp Transl Stroke Med. 2015;7(1):3.
Zhang W, Zhang H, Mu H, Zhu W, Jiang X, Hu X, et al. Omega-3 polyunsaturated fatty acids mitigate blood-brain barrier disruption after hypoxic-ischemic brain injury. Neurobiol Dis. 2016;91:37–46.
Wang J, Shi Y, Zhang L, Zhang F, Hu X, Zhang W, et al. Omega-3 polyunsaturated fatty acids enhance cerebral angiogenesis and provide long-term protection after stroke. Neurobiol Dis. 2014;68:91–103.
Zhang W, Wang H, Zhang H, Leak RK, Shi Y, Hu X, et al. Dietary supplementation with omega-3 polyunsaturated fatty acids robustly promotes neurovascular restorative dynamics and improves neurological functions after stroke. Exp Neurol. 2015;272:170–80.
Hu X, Zhang F, Leak RK, Zhang W, Iwai M, Stetler RA, et al. Transgenic overproduction of omega-3 polyunsaturated fatty acids provides neuroprotection and enhances endogenous neurogenesis after stroke. Curr Mol Med. 2013;13(9):1465–73.
Shi Y, Zhang L, Pu H, Mao L, Hu X, Jiang X, et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun. 2016;7:10523.
Han L, Cai W, Mao L, Liu J, Li P, Leak RK, et al. Rosiglitazone promotes white matter integrity and long-term functional recovery after focal cerebral ischemia. Stroke. 2015;46(9):2628–36.
Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200.
Springer J, Schust S, Peske K, Tschirner A, Rex A, Engel O, et al. Catabolic signaling and muscle wasting after acute ischemic stroke in mice: indication for a stroke-specific sarcopenia. Stroke. 2014;45(12):3675–83.
Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57(2):202–7.
Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab. 2004;24(7):805–13.
Stetler RA, Cao G, Gao Y, Zhang F, Wang S, Weng Z, et al. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci. 2008;28(49):13038–55.
Chen Q, Zhang J, Guo J, Tang J, Tao Y, Li L, et al. Chronic hydrocephalus and perihematomal tissue injury developed in a rat model of intracerebral hemorrhage with ventricular extension. Transl Stroke Res. 2015;6(2):125–32.
Zuloaga KL, Zhang W, Yeiser LA, Stewart B, Kukino A, Nie X, et al. Neurobehavioral and imaging correlates of hippocampal atrophy in a mouse model of vascular cognitive impairment. Transl Stroke Res. 2015;6(5):390–8.
Wang G, Jiang X, Pu H, Zhang W, An C, Hu X, et al. Scriptaid, a novel histone deacetylase inhibitor, protects against traumatic brain injury via modulation of PTEN and AKT pathway: scriptaid protects against TBI via AKT. Neurotherapeutics. 2013;10(1):124–42.
Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–61.
Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22(3):629–34.
Zhao S, Qu H, Zhao Y, Xiao T, Zhao M, Li Y, et al. CXCR4 antagonist AMD3100 reverses the neurogenesis and behavioral recovery promoted by forced limb-use in stroke rats. Restor Neurol Neurosci. 2015;33(6):809–21.
Kuptsova K, Kvist E, Nitzsche F, Jolkkonen J. Combined enriched environment/atipamezole treatment transiently improves sensory functions in stroke rats independent from neurogenesis and angiogenesis. Rom J Morphol Embryol. 2015;56(1):41–7.
Zhang W, Cheng J, Vagnerova K, Ivashkova Y, Young J, Cornea A, et al. Effects of androgens on early post-ischemic neurogenesis in mice. Transl Stroke Res. 2014;5(2):301–11.
Pena I, Borlongan CV. Translating G-CSF as an adjunct therapy to stem cell transplantation for stroke. Transl Stroke Res. 2015;6(6):421–9.
Okada M, Nakanishi H, Tamura A, Urae A, Mine K, Yamamoto K, et al. Long-term spatial cognitive impairment after middle cerebral artery occlusion in rats: no involvement of the hippocampus. J Cereb Blood Flow Metab. 1995;15(6):1012–21.
Yonemori F, Yamaguchi T, Yamada H, Tamura A. Spatial cognitive performance after chronic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19(5):483–94.
Block F, Kunkel M, Schwarz M. Quinolinic acid lesion of the striatum induces impairment in spatial learning and motor performance in rats. Neurosci Lett. 1993;149(2):126–8.
Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8(5):491–500.
Wang L, Wang X, Su H, Han Z, Yu H, Wang D, et al. Recombinant human erythropoietin improves the neurofunctional recovery of rats following traumatic brain injury via an increase in circulating endothelial progenitor cells. Transl Stroke Res. 2015;6(1):50–9.
Soliman S, Ishrat T, Fouda AY, Patel A, Pillai B, Fagan SC. Sequential therapy with minocycline and candesartan improves long-term recovery after experimental stroke. Transl Stroke Res. 2015;6(4):309–22.
Li Q, Khatibi N, Zhang JH. Vascular neural network: the importance of vein drainage in stroke. Transl Stroke Res. 2014;5(2):163–6.
Zhang JH. Vascular neural network in subarachnoid hemorrhage. Transl Stroke Res. 2014;5(4):423–8.
Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, et al. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 2016;131(3):323–45.
Chen D, Yu SP, Wei L. Ion channels in regulation of neuronal regenerative activities. Transl Stroke Res. 2014;5(1):156–62.
Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29(37):11511–22.
Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–56.
He K, Rimm EB, Merchant A, Rosner BA, Stampfer MJ, Willett WC, et al. Fish consumption and risk of stroke in men. JAMA. 2002;288(24):3130–6.
Mohajeri MH, Troesch B, Weber P. Inadequate supply of vitamins and DHA in the elderly: implications for brain aging and Alzheimer-type dementia. Nutrition. 2015;31(2):261–75.
King VR, Huang WL, Dyall SC, Curran OE, Priestley JV, Michael-Titus AT. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci. 2006;26(17):4672–80.
Eady TN, Khoutorova L, Anzola DV, Hong SH, Obenaus A, Mohd-Yusof A, et al. Acute treatment with docosahexaenoic acid complexed to albumin reduces injury after a permanent focal cerebral ischemia in rats. PLoS One. 2013;8(10):e77237.
Eady TN, Khoutorova L, Obenaus A, Mohd-Yusof A, Bazan NG, Belayev L. Docosahexaenoic acid complexed to albumin provides neuroprotection after experimental stroke in aged rats. Neurobiol Dis. 2014;62:1–7.
Eady TN, Khoutorova L, Atkins KD, Bazan NG, Belayev L. Docosahexaenoic acid complexed to human albumin in experimental stroke: neuroprotective efficacy with a wide therapeutic window. Exp Transl Stroke Med. 2012;4(1):19.
Hong SH, Belayev L, Khoutorova L, Obenaus A, Bazan NG. Docosahexaenoic acid confers enduring neuroprotection in experimental stroke. J Neurol Sci. 2014;338(1-2):135–41.
Chen CT, Liu Z, Ouellet M, Calon F, Bazinet RP. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins Leukot Essent Fatty Acids. 2009;80(2-3):157–63.
Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52.
Sumiyoshi M, Satomi J, Kitazato KT, Yagi K, Shimada K, Kurashiki Y, et al. PPARgamma-dependent and -independent inhibition of the HMGB1/TLR9 pathway by eicosapentaenoic acid attenuates ischemic brain damage in ovariectomized rats. J Stroke Cerebrovasc Dis. 2015;24(6):1187–95.
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–70.
Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110(4):429–41.
Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–94.
Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–47.
Rossi D. Astrocyte physiopathology: at the crossroads of intercellular networking, inflammation and cell death. Prog Neurobiol. 2015;130:86–120.
Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28(28):7231–43.
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24(9):2143–55.
Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44.
Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390(6661):680–3.
An C, Shi Y, Li P, Hu X, Gan Y, Stetler RA, et al. Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair. Prog Neurobiol. 2014;115:6–24.
Hu X, Liou AK, Leak RK, Xu M, An C, Suenaga J et al. Neurobiology of microglial action in CNS injuries: receptor-mediated signaling mechanisms and functional roles. Prog Neurobiol. 2014;119-120:60-84.
Seifert HA, Pennypacker KR. Molecular and cellular immune responses to ischemic brain injury. Transl Stroke Res. 2014;5(5):543–53.
Franco R, Fernandez-Suarez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131:65–86.
Li L, Tao Y, Tang J, Chen Q, Yang Y, Feng Z, et al. A cannabinoid receptor 2 agonist prevents thrombin-induced blood-brain barrier damage via the inhibition of microglial activation and matrix metalloproteinase expression in rats. Transl Stroke Res. 2015;6(6):467–77.
Mallucci G, Peruzzotti-Jametti L, Bernstock JD, Pluchino S. The role of immune cells, glia and neurons in white and gray matter pathology in multiple sclerosis. Prog Neurobiol. 2015;127-128:1-22.
Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, et al. Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol. 2014;117:54–72.
Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317.
Jin R, Zhu X, Li G. Embolic middle cerebral artery occlusion (MCAO) for ischemic stroke with homologous blood clots in rats. J Vis Exp. 2014;91:51956.
Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766(1-2):83–92.
Acknowledgments
*H.P. and *X.J. contributed equally to this research. This project was supported by the US Department of Veterans Affairs (VA) RR&D Merit Review RX000420; the US National Institutes of Health grants NS045048, NS091175, and NS095671; the American Heart Association grant 13SDG14570025; and the Chinese Natural Science Foundation grants 81529002, 81171149, 81371306, 81571285, and 81100978. J.C. is a recipient of the VA Senior Research Career Scientist Award. The authors are indebted to Pat Strickler for excellent administrative support. The present address of J.X. is Cerebrovascular Center, Henan Provincial People’s Hospital, Zhengzhou University, Zhengzhou 450003, China.
Authors’ Contributions
Y.S., Y.G., X.H., and J.C. designed the research. H.P., X.J., J.X., and W.Z. performed the research. X.J. and Y.S. analyzed the data. H.P., X.J., D.H., J.C., and Y.S. wrote the manuscript. All authors reviewed and edited the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Hongjian Pu and Xiaoyan Jiang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Post-stroke DHA and FO treatments do not change hippocampal neurogenesis in the non-injured contralateral hemisphere. a Representative images of double-label immunostaining of BrdU (green) and NeuN (red) in the hippocampal CA1, CA3 and DG areas of the non-injured contralateral hemisphere at 28 days after MCAO. There was barely any BrdU immunosignal in all regions. Scale bar: 50 μm. b-d Quantification of total cells that are positive for NeuN immunosignal in CA1, CA3, and DG of the contralateral hippocampus. There was no statistical difference among all groups. e-g Quantification of BrdU+/NeuN+ cells in CA1, CA3, and DG of the contralateral hippocampus. Few BrdU+/NeuN+ cells were observed in all regions. There was no statistical difference among all groups. n = 7 mice per group. (DOCX 2039 kb)
Rights and permissions
About this article
Cite this article
Pu, H., Jiang, X., Hu, X. et al. Delayed Docosahexaenoic Acid Treatment Combined with Dietary Supplementation of Omega-3 Fatty Acids Promotes Long-Term Neurovascular Restoration After Ischemic Stroke. Transl. Stroke Res. 7, 521–534 (2016). https://doi.org/10.1007/s12975-016-0498-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-016-0498-y